Abstract
Human sperm centrosome reconstitution and the parental contributions to the zygotic centrosome are examined in mammalian zygotes and after exposure of spermatozoa to Xenopus laevis cell-free extracts. The presence and inheritance of the conserved centrosomal constituents γ-tubulin, centrin, and MPM-2 (which detects phosphorylated epitopes) are traced, as is the sperm microtubule-nucleating capability on reconstituted centrosomes. γ-Tubulin is biparentally inherited in humans (maternal >> than paternal): Western blots detect the presence of paternal γ-tubulin. Recruitment of maternal γ-tubulin to the sperm centrosome occurs after sperm incorporation in vivo or exposure to cell-free extract, especially after sperm “priming” induced by disulfide bond reduction. Centrin is found in the proximal sperm centrosomal region, demonstrates expected calcium sensitivity, but appears absent from the zygotic centrosome after sperm incorporation or exposure to extracts. Sperm centrosome phosphorylation is detected after exposure of primed sperm to egg extracts as well as during the early stages of sperm incorporation after fertilization. Finally, centrosome reconstitution in cell-free extracts permits sperm aster microtubule assembly in vitro. Collectively, these results support a model of a blended zygotic centrosome composed of maternal constituents attracted to an introduced paternal template after insemination.
INTRODUCTION
Although the molecular characterization of the centrosome is progressing swiftly (see reviews by Brinkley et al., 1980; Schliwa et al., 1982; Kuriyama et al., 1986; McIntosh and Koonce, 1989; Rose et al., 1993; see also Davis, 1997; Stearns and Winey, 1997; Doxsey, 1998; Zimmerman et al., 1999), the precise manner in which the conserved proteins interact to form a fully functional centrosome capable of duplication is still largely unclear. γ-Tubulin appears to be an essential, invariant centrosomal protein serving both to nucleate microtubules and to define the microtubule’s intrinsic polarity (Oakley and Oakley, 1989; Horio et al., 1991; Stearns et al., 1991; Zheng et al., 1991; Joshi et al., 1992; Palacios et al., 1993). In lower vertebrates such as Xenopus and in mice, γ-tubulin appears to be strictly acquired from the maternal cytoplasm after insemination, being lost from the mature spermatozoa probably during the latter stages of spermatogenesis (Gard, 1994; Stearns and Kirschner, 1994; Manandhar et al., 1998). Centrin, a ubiquitous, calcium-sensitive, biparentally contributed centrosomal component, has been reported to sever axonemal microtubules from their associated basal bodies and may also be involved in centrosome duplication (Baum et al., 1986; Sanders and Salisbury, 1989, 1994; Biggins and Rose, 1994; Stearns and Kirschner, 1994; reviewed by Salisbury, 1995). Phosphorylation of centrosomal components during meiosis or mitosis is a well-conserved mechanism for regulating centrosome activity, as observed in studies in a variety of cell types with the phosphoprotein mAb MPM-2 (Davis et al., 1983; Vandréet al., 1986).
Centrosome reconstitution during fertilization, an essential process for the initiation of development, is a unique model for exploring the molecular components necessary to determine centrosome parental origin and function (reviewed by Schatten, 1994). Classic studies (Boveri, 1901) suggested that the sperm in most animals contributes the dominant centrosome structure, because a sperm aster composed of a radial array of microtubules is focused on the cytoplasmic site adjacent to the sperm pronucleus during monospermic fertilization. Although the presence of multiple sperm asters during polyspermy supports the hypothesis that the centrosome is of paternal origin, studies on parthenogenesis and murine fertilization demonstrate that oocytes possess mechanisms to reconstitute a maternal centrosome that is capable of duplication and of forming a functional bipolar mitotic spindle (Schatten et al., 1986, 1991; reviewed by Schatten, 1994).
The cell-free system to explore the molecular events leading to centrosome reconstitution and microtubule assembly has been developed using demembranated Xenopus laevis sperm exposed to X. laevis cytostatic factor (CSF) arrested egg extracts (Doxsey et al., 1994; Félix et al., 1994; Stearns and Kirschner, 1994). These studies demonstrated that frog sperm have centrin but no detectable quantities of γ-tubulin or the phosphorylated epitopes recognized by the mAb MPM-2. After exposure to egg extracts, γ-tubulin is bound to the sperm centrosome; this binding is independent of microtubule or microfilament assembly (Stearns and Kirschner, 1994). The sperm exposed to egg extracts also became immunoreactive for MPM-2, suggesting that a phosphorylation reaction occurred. Moreover, these centrosomes were competent for nucleating microtubule growth into sperm asters.
This study explores centrosomal molecules in human and bovine gametes to understand the molecular basis of zygotic centrosomal reconstitution during nonrodent mammalian fertilization. The experimental approaches used provide a means to identify the inheritance characteristics of conserved centrosomal proteins and their fates after either in vitro fertilization or exposure to CSF-arrested Xenopus cell-free extracts. Surprisingly, γ-tubulin is observed to be biparentally inherited in nonrodent mammalian gametes: both human and bovine mature sperm retain γ-tubulin, as detected by Western blots. Paternal γ-tubulin is largely inaccessible in the mature spermatozoa, however, until after disulfide bond reduction. Exposure of disulfide-reduced sperm to egg cytoplasm dramatically increases γ-tubulin detection at the sperm centrosome and is a principal step in sperm aster formation in vitro. Centrin is detected in both human and bovine gametes, as demonstrated by immunostaining in mature spermatozoa and Western blots of bovine oocytes. Centrin localization at the neck region in spermatozoa is calcium sensitive and consistently modified upon exposure to oocyte cytoplasm, either after fertilization or after exposure to cell-free extracts. Centrin is predicted to be important in the reorganization of the sperm centrosomal complex after insemination and perhaps in the subsequent splitting of the early zygotic centrosome. The phosphorylation of the human and bovine sperm centrosome is apparent after cytoplasmic exposure, as detected by the MPM-2 antibody. Taken together, the results of this work help to characterize the parental origins of specific centrosomal molecules, the process of reconstitution, and the microtubule-organizing ability of the reconstituted sperm centrosome in vitro.
MATERIALS AND METHODS
Preparation and Handling of Human, Bovine, and X. laevis Sperm
Human sperm were obtained from fertile donors at in vitro fertilization clinics (University of Wisconsin-Madison or Rush Medical Center, Chicago, IL) or from a sperm bank (Follas Labs, West Lafayette, IN, or Cryobiology, Columbus, OH). Bovine sperm were obtained from American Breeders Service (DeForest, WI). Frozen sperm specimens were thawed and selected for viable sperm by either a swim-up procedure or a 90/45% discontinuous Percoll gradient, adjusted to a concentration of 1 × 107/ml, and sedimented onto coverslips. The sperm were treated with 0.05% lysophosphatidylcholine (lysolecithin; Sigma Chemical, St. Louis, MO) in KMT buffer (100 mM KCl, 2 mM MgCl2, 10 mM Tris-HCl, pH 7.0, and 5 mM EGTA) followed by 3% BSA in KMT. For male pronuclear decondensation in vitro (also referred to as “priming”), the methods of Ohsumi et al. (1986) were followed. Sperm were incubated in 5 mM DTT (pH 8.2) followed by 1 mM N-ethylmaleimide (pH 8.2) to irreversibly block thiol groups by alkylation. X. laevis sperm were prepared according to the methods of Félix et al. (1994).
Preparation of X. laevis Egg Extracts
Concentrated CSF-arrested cytoplasmic frog extract was prepared according to the methods of Stearns and Kirschner (1994). Before experimental use, the extract was fortified with an “energy mix” containing 150 mM creatine phosphate, 20 mM ATP, pH 7.4, 2 mM EGTA, pH 7.7, and 20 mM MgCl2 (5 μl/100-μl extract), plus the addition of 10 μg/ml each of cytochalasin B and a protease inhibitor cocktail (leupeptin, chymostatin, and pepstatin A; completed extract). In addition, 2 μg/ml nocodazole (Sigma) was added to the thawed extract before incubation with sperm in all cases except those in which in vitro microtubule aster growth was desired.
Immunodepletion of γ-tubulin from CSF-arrested extracts was performed as reported previously by Stearns and Kirschner (1994)
Incubation of Sperm in Egg Extract
For immunocytochemical experiments, ∼1000 sperm per microliter were added to 10 μl of complete extract and incubated at 37°C for 40 min. After extensive washing in KMT buffer, sperm were affixed to clean 12-mm-round coverslips, fixed, and then processed for immunocytochemistry as described below.
For aster formation in vitro, sperm were treated with 1 μM ionomycin for 5 min and then washed in KMT buffer. Sperm were permeabilized in 0.05% lysolecithin in KMT buffer for 10 min at ambient temperature before subsequent exposure to 5 mM DTT in KMT buffer (pH 8.2) for 1 h at 37°C. After several washes in KMT buffer, 1 μl of the treated sperm was added to 8 μl of completed extract containing 0.08 mg/ml rhodamine-labeled tubulin prepared from bovine brains (a gift from G. Borisy, University of Wisconsin, Madison, or purchased from Cytoskeleton, Denver, CO). The mixture was incubated for 60–90 min at 29°C. Nucleation and growth of the sperm asters were analyzed according to the methods of Stearns and Kirschner (1994).
Immunocytochemical Detection of Centrosomes, Microtubules, and Phosphorylated Epitopes
Polyspermically inseminated human oocytes that failed to develop normally and monospermically fertilized bovine oocytes were extracted and fixed according to protocols from Simerly and Schatten (1993) and Navara et al. (1994), respectively. Microtubules and sperm tails were labeled for 1 h with mAb raised against β-tubulin (E-7, 1:5 dilution; Developmental Studies Hybridoma Bank, Iowa City, IA) and acetylated α-tubulin 6-11B-1 (Sigma) or glutamate α-tubulin (a gift of C. Bulinsky, Columbia University, New York, NY), respectively. γ-Tubulin was detected with an affinity-purified rabbit polyclonal antibody raised against the entire sequence of X. laevis γ-tubulin (XG-1-4; Stearns et al., 1991; Stearns and Kirschner, 1994).
Bovine and human sperm were fixed in either absolute methanol (−20°C for 10 min) or 2% formaldehyde in KMT buffer. Fixed spermatozoa were immunostained with antibodies that recognize centrin (20H5), a mouse mAb that was raised against bacterially derived Chlamydomonas reinhardtii centrin (Errabolu et al., 1994; Salisbury, 1995); MPM-2, a mAb raised against mitotic HeLa cell extracts that detects phosphorylated epitopes; or the γ-tubulin antibody XG-1-4. After fixation, sperm were treated with 10% goat serum in PBS, pH 7.2, for 30 min at 37°C. The primary antibody was applied for 30 min at 37°C, then rinsed in PBS containing 0.1% Triton X-100 and 5% goat serum for 10 min. To detect the primary antibodies, either FITC goat anti-mouse (1:50; Sigma) or goat anti-rabbit secondary antibodies (1:50; Zymed Laboratories, South San Francisco, CA) were applied for 30 min at 37°C, followed by a 10-min wash in PBS containing 0.1% Triton X-100 and 5% goat serum. DNA was fluorescently detected, with 5 μg/ml Hoechst 33342 (Sigma) added to the penultimate rinse. Coverslips were mounted in an anti-fade medium (Vectashield H-1000, Vector Laboratories, Burlingame, CA) to retard photobleaching. Conventional fluorescence microscopy was performed using a Carl Zeiss (Thornwood, NY) Axiophot microscope with high-numerical-aperture objectives. For each experiment, a count of at least three microscopic fields with a minimum total of 100 spermatozoa was recorded. Black-and-white negatives were obtained using Tri-X film and digitized using a Nikon (Melville, NY) Coolscan LS-10. All images were archived on a magneto-optical disk. Oocytes were further analyzed with a laser-scanning confocal microscope (MRC-600, Bio-Rad, Hercules, CA). Digital data were downloaded to a dye-sublimation printer (Sony, Parkridge, NJ) using Adobe Photoshop software (Adobe Systems, Mountain View, CA).
Immunogold Electron Microscopy
Immunogold electron microscopy was performed using a modification of the method described previously by Sutovsky et al. (1993). Briefly, sperm were pelleted by centrifugation at 700 × g and fixed for 2 h at 4°C in 3% glutaraldehyde in 0.05 M piperazine-N,N′-bis[2-ethanesulfonic acid] buffer (pH 7.3) containing 2 mM CaCl2 and 0.5 mM MgCl2. After washing in piperazine-N,N′-bis[2-ethanesulfonic acid] buffer, the sperm pellet was embedded in hot 1% agar and the fixed sperm were dehydrated in an ice-cold graded ethanol series up to 90%, then embedded in LR White resin (Electron Microscopy Sciences, Fort Washington, PA) at −20°C. Polymerization was performed by the addition of 1 μl/ml LR White accelerator (Electron Microscopy Sciences, Newtown, CT). Ultrathin sections were cut using a Sorvall MT2B ultramicrotome and collected on formvar-coated 100-mesh nickel grids. Colloidal gold labeling was performed by sequential incubation in 5% normal goat serum (Sigma) in PBS with 0.1% BSA (Sigma) for 1 h, 20H5 centrin (1:50) in PBS with 0.1% BSA and 1% normal goat serum for 90 min, and 5-nm colloidal gold–conjugated anti-mouse immunoglobulin G plus immunoglobulin M for 1 h (British BioCell International, Cardiff, UK; purchased from Ted Pella, Redding, CA). Grids were stained with uranyl acetate for 10 min and examined with a Phillips CM 120 transmission electron microscope (Phillips, Eindhoven, Netherlands). Negatives were scanned with an Eastman Kodak (Rochester, NY) Leafscan 35 image scanner, recorded on a magneto-optical disk, and printed using Adobe Photoshop 4.0 software. Negative controls were performed by the omission of 20H5 from the labeling protocol.
SDS-PAGE and Western Blotting
Sperm proteins were separated on linear gradient SDS-PAGE gels (4–20%; Bio-Rad, Hercules, CA) for 2.5 h at 80 V. The amounts loaded per lane were determined by standard protein determinations using the bicinchonimic acid (BCA) protein assay (Pierce, Rockford, IL). After electrophoresis, the gels were placed into Towbins transfer buffer (25 mM Tris, 192 mM glycine, 20% methanol, 0.037% SDS) for 5 min. Proteins were transferred onto a nitrocellulose membrane (Bio-Rad) using a SemiPhor apparatus (Hoefer Scientific Instruments, San Francisco, CA) at a current of 0.8 A/cm2 for 4 h. The membrane was blocked with Tris-buffered saline–Tween (25 mM Tris, 137 mM NaCl, 2.7 mM KCl, and 0.2% Tween) supplemented with 5% dry milk for 1 h on a rotating platform. The membrane was briefly washed with Tris-buffered saline and incubated with a 1:2000 dilution of primary antibody (mouse monoclonal anti-centrin 20H5 and rabbit polyclonal XG-1-4 anti-γ-tubulin) in Tris-buffered saline–Tween supplemented with 5% dry milk and 5% fetal calf serum for 1 h. The membrane was washed four times (15 min for each wash) with Tris-buffered saline and incubated with a 1:2000 dilution of a horseradish peroxidase–conjugated secondary antibody in Tris-buffered saline supplemented with 5% dry milk for 1 h. The membrane was washed again as described above. To induce the enzymatic reaction, the membrane was incubated with the chemiluminescence reagents (ECLPlus, Amersham, New York, NY) for 5 min, wrapped in plastic wrap, and immediately exposed to X-Omat film (Eastman Kodak). For control experiments, preimmune serum was used in place of the primary antibody incubations.
In some cases, Western blots were reprobed with different primary antibodies. The membrane was first stripped of primary and secondary antibodies by incubation in 0.1 M glycine (pH 2.7) for 30 min at room temperature. To ensure that primary antibody was removed from the membrane, the membrane was incubated only with the secondary antibody (as described above) and exposed to chemiluminescence reagents. The film obtained after this control procedure showed the absence of any immunostaining. The same membrane was then subjected to immunostaining with a different primary antibody.
RESULTS
γ-Tubulin Detection in the Mature Human and Bovine Sperm, and Recruitment from Cytoplasmic Sources
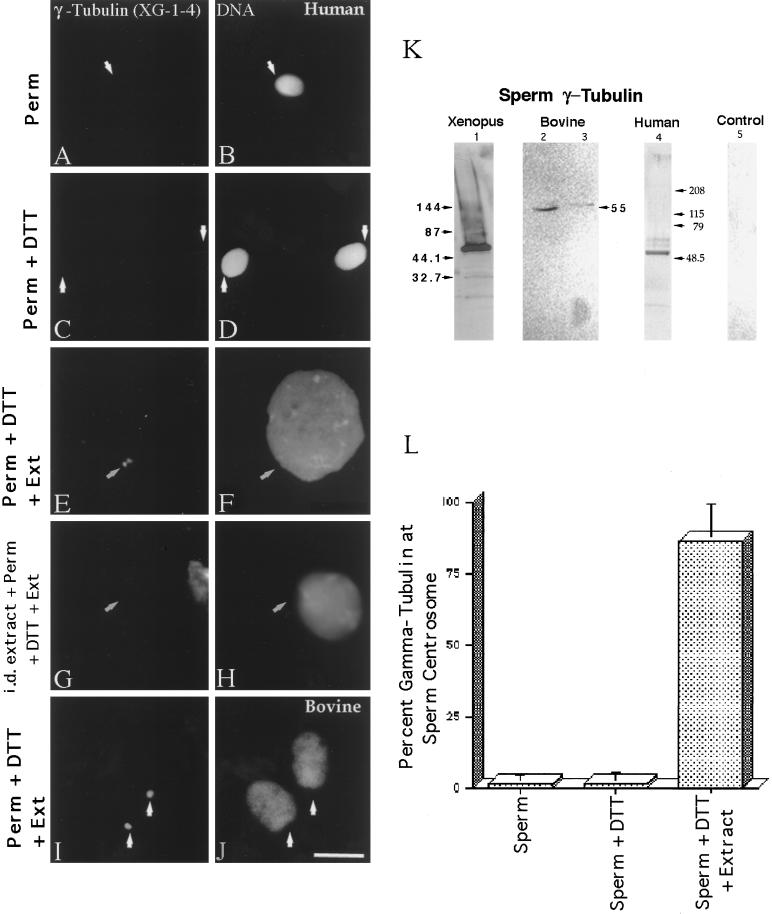
Exposure of lysolecithin-permeabilized Xenopus sperm to CSF-arrested cell-free extract leads to the accumulation of γ-tubulin at the sperm centrosome, as previously reported (Doxsey et al., 1994; Félix et al., 1994; Stearns and Kirschner, 1994; our unpublished results). In mature human sperm permeabilized with lysolecithin and fixed in methanol, <5% of the sperm demonstrated XG-1-4 γ-tubulin immunostaining at the centrosomal region by indirect immunofluorescence (Figure 1, A and B, and L, left bar). Similar low levels of detectable γ-tubulin staining at the base of the sperm head were observed after permeabilized sperm were treated with 5 mM DTT, which reduces disulfide bonds and permits sperm nuclear decondensation in cell-free extracts (Figure 1, C and D, arrows, and L, middle bar). However, exposure of permeabilized human sperm to CSF-arrested extract significantly increased XG-1-4 γ-tubulin detection at the sperm centrosome, especially after priming with 5 mM DTT (Figure 1, E and F, and L, right bar). The majority of γ-tubulin observed at the base of permeabilized, DTT-treated human sperm appears to be maternally derived, in that immunodepletion of the CSF extract with the XG-1-4 γ-tubulin antibody before sperm addition did not demonstrate γ-tubulin at the base of the sperm head after anti-γ-tubulin immunofluorescence staining (Figure 1, G and H). Similar evidence of the acquisition of maternal γ-tubulin in bovine sperm was also seen (Figure 1, I and J).
Figure 1.
γ-Tubulin in human and bovine sperm centrosomes. More than 98% of human sperm do not immunostain with the XG-1-4 γ-tubulin antibody (A; arrow points to the sperm centrosomal region) after lysolecithin permeabilization and methanol fixation (B, DNA). Likewise, no significant increase in the detection of γ-tubulin at the human sperm centrosome is observed after 5 mM DTT priming treatment (C; arrows point to the sperm centrosomal region), although some DNA decondensation occurs in vitro (D). Very similar observations have been observed in bovine spermatozoa treated in exactly the same manner. However, both human (E and F) and bovine (I and J) spermatozoa treated with 5 mM DTT followed by CSF-arrested cell-free extract exposure demonstrated extensive DNA decondensation after 1 h (F and J) and XG-1-4 γ-tubulin immunolocalization at the sperm centrosomal regions (E and I; arrows point to the sperm centrosomal region).Immunodepletion of γ-tubulin from the CSF-arrested extracts by the XG-1-4 γ-tubulin abolishes detection of γ-tubulin at the base of permeabilized, DTT-treated human spermatozoa, demonstrating that the vast majority of γ-tubulin is maternally derived (G and H). (K) Western blot analysis of Xenopus, human, and bovine sperm demonstrates prominent bands at ∼55 kDa with the XG-1-4 antibody, indicating the presence of paternal γ-tubulin in these sperm. Lane 1, Xenopus sperm, 1.25 × 106 sperm per lane; lane 2, bovine sperm subjected to Percoll density centrifugation, at ∼2.6 × 106 sperm per lane; lane 3, washed bovine sperm without Percoll separation, at ∼2.6 × 106 sperm per lane; lane 4, human sperm subjected to Percoll density separation and labeled with XG-1-4 γ-tubulin antibody, ∼2.5 × 106 sperm per lane; lane 5, 0.5 μg of purified α- and β-tubulin, demonstrating no cross-reactivity of these tubulin superfamily members with the XG-1-4 γ-tubulin antibody. (L) Graphic representation of permeabilized human spermatozoa immunostained with γ-tubulin XG-1-4 antibody after permeabilization, DTT priming, and CSF-arrested cell-free extract. By immunofluorescence, very little paternal γ-tubulin is observed in permeabilized human sperm (left bar) or permeabilized human sperm primed by exposure to 5 mM DTT (middle bar). However, a significant increase in the detection of γ-tubulin is observed when permeabilized and primed sperm are treated with CSF-arrested cell-free extract (right bar). All images were double labeled for γ-tubulin and Hoechst DNA. (Arrows) Sperm centrosomal region as observed with phase or differential interference contrast optics. Bar in J, 10 μm.
Interestingly, a 55-kDa band was detected in Western blots of Xenopus, bovine, and human sperm using XG-1-4 γ-tubulin antibody (Figure 1K: Xenopus sperm, lane 1; bull sperm, lanes 2 and 3; human sperm, lane 4). Purified α- and β-tubulin protein (Figure 1K, lane 5) was not detected with the XG-1-4 antibody in Western blots.
Presence and Calcium Ion Sensitivity of Centrin in Human and Bovine Sperm
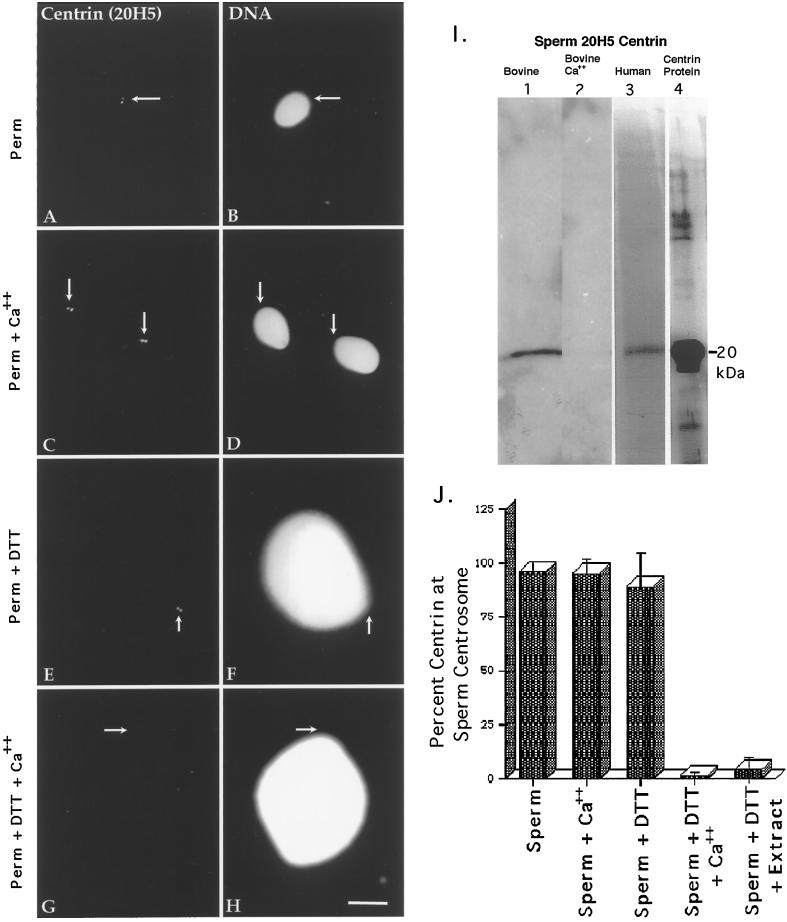
Human and bovine sperm probed with antibodies against centrin are shown in Figure 2. The centrin antibody 20H5 bound preferentially to the centrosomal region in human sperm after permeabilization and methanol fixation, with sperm typically demonstrating one or two punctate spots (Figure 2, A and B, arrows, and J, first bar). This staining pattern was not affected in lysolecithin-permeabilized sperm containing 2 mM CaCl2 (Figure 2, C and D, arrows, and J, second bar).
Figure 2.
Centrin in human sperm centrosomes. Centrin is localized exclusively as either a pair of punctate sources or a single spot (depending on orientation) at the centrosome in permeabilized human sperm (A, centrin antibody 20H5; B, DNA) or permeabilized spermatozoa exposed to 2 mM CaCl2 (C and D). This staining is not dependent on calcium exposure. Sperm exposed to 5 mM DTT (E and F) for 40 min show no change in the centrin-staining pattern from controls. However, spermatozoa exposed to DTT and 2 mM CaCl2 show a dissipation of centrin staining (G and H). Arrows indicate the point of tail attachment to the sperm head as observed with phase or differential interference contrast optics. Identical results were found with bovine sperm. (I) Western blots of bovine and human sperm, demonstrating a single band at ∼20 kDa that comigrates with bacterially expressed centrin and the calcium sensitivity of sperm centrosomal centrin. Lane 1, DTT-primed bovine sperm (60 μg of total protein per lane); lane 2, DTT-primed bovine sperm treated for 30 min with 2 mM CaCl2, showing the loss in 20H5 centrin detection after high external calcium treatment (43 μg of total protein per lane); lane 3, human sperm, immunoprecipitated with 20H5 anti-centrin and immunostained with anti-centrin serum 24/14-1; lane 4, purified bacterially expressed centrin protein immunostained with anti-centrin serum 24/14-1. (J). Graphic representation of human spermatozoa immunostained with 20H5 centrin after permeabilization, DTT priming, and elevated external calcium exposure. Analysis reveals that DTT-primed spermatozoa treated with either high external calcium (fourth bar) or CSF-arrested extract (fifth bar) demonstrate a significant reduction in the detection of centrin at the sperm centrosome. Bar in H, 10 μm.
After 5 mM DTT treatment, sperm decondensation ensued (Figure 2F) and centrin antibody localization was retained at the base of the sperm head (Figure 2E, arrow, and J, third bar). Occasionally, the sperm heads and tails separated during this DTT priming step and centrin antibody localization remained exclusively with the sperm tails, further demonstrating that the paternal centrin protein resides within the pericentriolar region in human and bull spermatozoa. In contrast to 5 mM DTT treatment alone, however, DTT-primed sperm subsequently exposed to 2 mM CaCl2 lost detectable centrin immunostaining at the sperm centrosome (Figure 2G, arrow, and J, fourth bar). Identical results were found with bovine sperm (our unpublished results).
Bull and human DTT-primed sperm subjected to Western blot analysis with the centrin mAb 20H5 and the use of techniques designed for the transfer of low-molecular- mass calcium-binding proteins (Hulen et al., 1991) demonstrated distinct proteins migrating at ∼20 kDa (Figure 2I: lane 1, bovine; lane 3, human). The bands observed in bovine and human sperm were similar in molecular mass to that observed after 20H5 anti-centrin immunoblotting of bacterially expressed centrin protein (Figure 2I, lane 4; immunoblotted with the polyclonal antibody 24/14-1). A Western blot of DTT-primed bovine sperm exposed to 2 mM CaCl2 showed the loss of 20H5 detection, suggesting that high external calcium removes paternal centrin from the sperm centrosome (Figure 2I, lane 2).
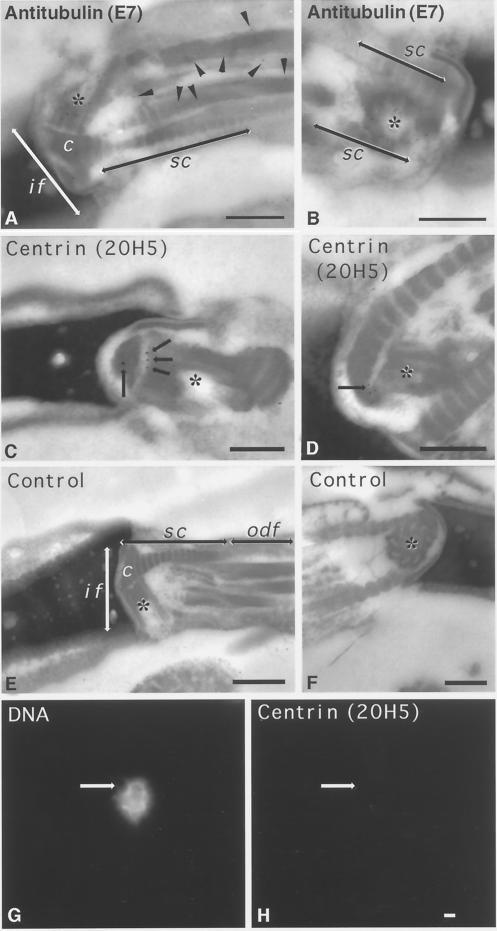
Electron micrographs of mature nonrodent mammalian sperm often showed the presence of an intact proximal centriole but only a remnant of the distal centriole (Zamboni and Stefanini, 1971). Control immunogold labeling with anti-β-tubulin antibody demonstrated extensive decoration of the proximal centriole (Figure 3, A and B, asterisks) and microtubule outer doublets (Figure 3A, arrowheads) in bovine sperm. In contrast, immunogold labeling with 20H5 anti-centrin antibody demonstrated centrin association with the ends of the centriolar cylinder in the proximal centriole only (Figure 3, C and D, arrows and asterisks). Human sperm labeled with secondary antibody alone showed no immunogold labeling of the implantation fossa, including the proximal centriole (Figure 3, E and F, asterisks).
Figure 3.
Ultrastructural detection of centrin in the bovine mature sperm centrosome and its sensitivity to CSF-arrested cell-free extract. (A and B) Immunogold labeling of mature bovine spermatozoa with anti-β-tubulin antibody, demonstrating extensive immunolabeling of the sperm proximal centriole (asterisks) and outer microtubule doublets of the sperm axoneme (A, arrowheads). (C and D) Immunogold labeling of mature bovine sperm with anti-centrin antibody 20H5. In C, a longitudinal section of proximal centriole in the sperm tail connecting piece is observed, with centrin localized to its capitulum-attached end (arrows). (D) Oblique sections of the proximal centriole demonstrating centrin detection in an area of the centriole adjacent to the striated columns of the connecting piece. (E and F) Control bovine spermatozoa immunolabeled with colloidal gold–conjugated secondary antibody only. No labeling is observed in the connecting piece structures (E, longitudinal section of the centriole) or in the proximal centriole (F, cross-section). (G and H) Human permeabilized spermatozoa treated sequentially with 5 mM DTT and CSF-arrested cell-free extract and then immunostained with the 20H5 centrin antibody. The primed sperm has begun to decondense in the presence of egg extract (G), but 20H5 centrin is no longer detected at the base of the sperm head (H, arrow). if, implantation fossa; c, capitulum; sc, striated columns; odf, outer dense fibers; asterisks, proximal centriole. Bars in A, B, D, E, and F, 0.2 μm; bar in C, 0.5 μm; bar in H, 1 μm.
Human and bovine lysolecithin-permeabilized sperm exposed to 5 mM DTT treatment and CSF-arrested cell-free extracts demonstrated a significant reduction in the detection of 20H5 antibody labeling at the sperm centrosome after 1 h of incubation (Figure 3, G and H, arrows; Figure 2J, fifth bar). Similar observations were made after immunolabeling with 13A1 and 3C10 mouse anti-centrin mAbs, indicating that the centrin was either lost or masked after exposure to egg cytoplasm. These observations are in agreement with human sperm centrin immunolabeling after in vitro fertilization (see Figure 6C, inset).
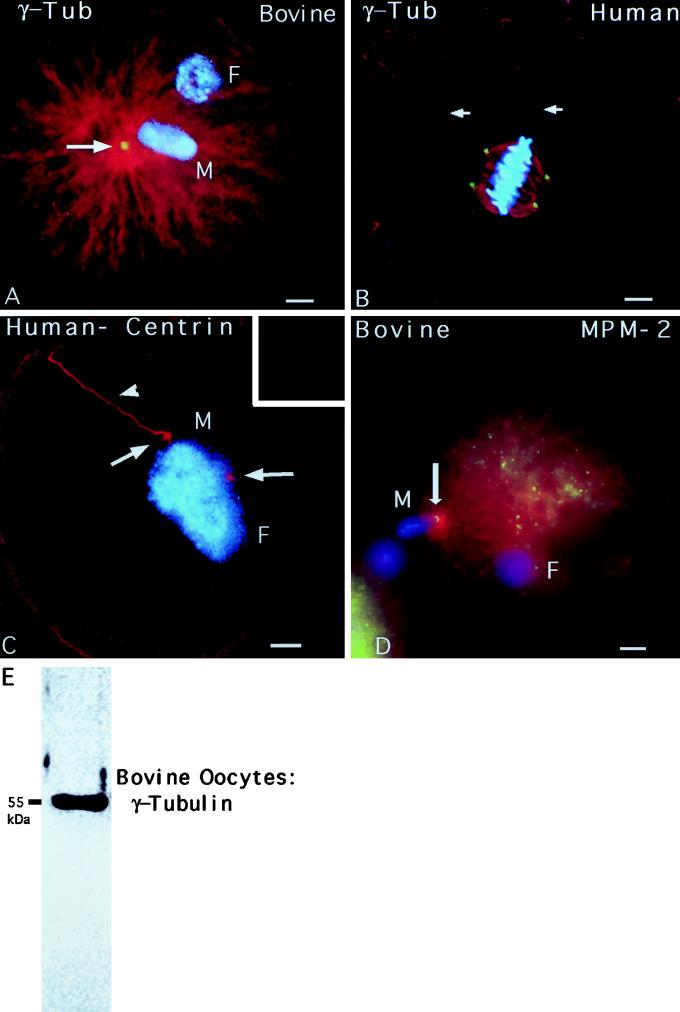
Figure 6.
γ-Tubulin, centrin, and MPM-2 detection in human and bovine zygotes. (A) Fertilized bovine oocyte fixed 12 h after insemination and immunostained with γ-tubulin antibody (red), XG-1-4 γ-tubulin antibody (green), and Hoechst DNA (blue). The sperm aster is a radially symmetrical array of microtubules emanating from the base of the sperm head (M). γ-Tubulin is detectable as a bright dot at the focal point of the sperm aster (arrow). (B) A dispermic human zygote fixed 26.5 h after insemination and triple labeled for microtubules (red, costained with β-tubulin and acetylated α-tubulin antibodies), XG-1-4 γ-tubulin antibody (green), and Hoechst DNA (blue). Each pole of the bipolar mitotic spindle has a sperm axoneme (red, arrows). γ-Tubulin is detected as four bright dots at the spindle poles (green), two of which are associated with the incorporated sperm axonemes (red, arrows). (C) An arrested, monospermically inseminated human oocyte fixed 48 h after insemination and immunostained with antibodies to microtubules (red, glutamate α-tubulin antibody), 20H5 mAb to centrin (inset), and Hoechst DNA (blue). A replicated and split centrosome, indicated by the two small microtubule asters (red, arrows) around the adjacent male (M) and female (F) pronuclei, is shown. The incorporated sperm axoneme (arrowhead) is associated with one of the two microtubule asters, but no centrin is detected at the centrosome (inset). (D) A bovine oocyte fertilized in vitro and fixed 10 h after insemination demonstrating sperm aster formation (red) and a pair of MPM-2 immunoreactive foci within the assembled astral microtubules (green). After activation of bovine oocytes, cortical microtubule assembly increases (red) and other cytoplasmic MPM-2 reactive foci (green) can be seen within this polymerizing array. (E) A 55-kDa band is prominently detected in 50 mature bovine oocytes after Western blotting with the rabbit poly-clonal XG-1-4 antibody, indicating the presence of abundant maternal γ-tubulin protein. All images were triple labeled for microtubules (red), γ-tubulin, centrin, or MPM-2 (green), and DNA (blue). Bars, 10 μm.
Human Sperm Centrosome Phosphorylation
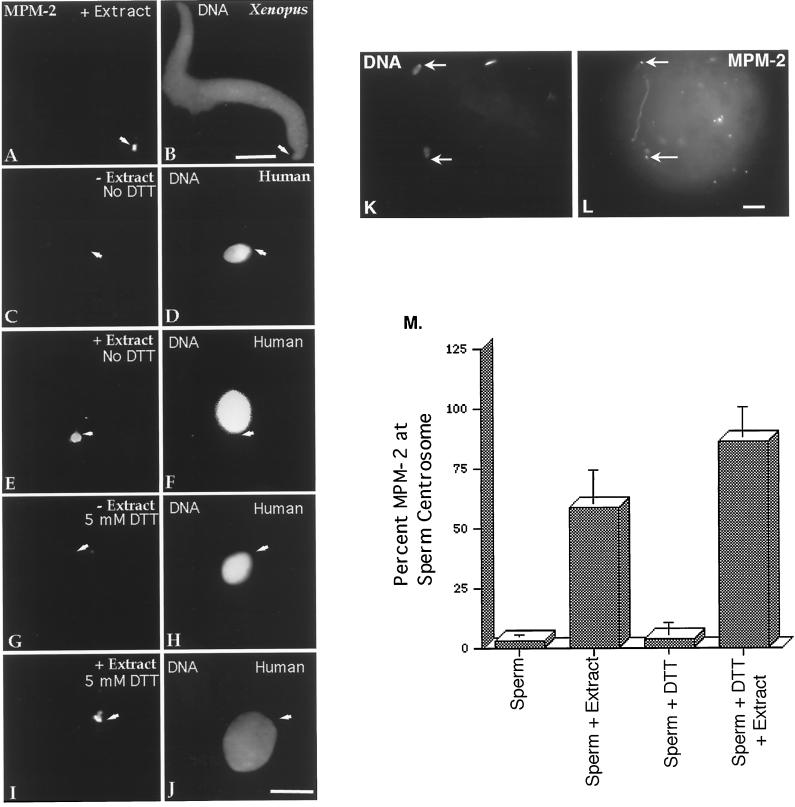
MPM-2, which recognizes phosphorylated epitopes (Davis et al., 1983), has been used successfully to demonstrate that Xenopus sperm centrosomes are phosphorylated after exposure to Xenopus egg extracts (Figure 4, A and B) (Doxsey et al., 1994; Félix et al., 1994; Stearns and Kirschner, 1994). The centrosomes from human and bovine sperm displayed a similar response. Only 3% of human sperm permeabilized in lysolecithin and fixed in methanol demonstrated positive MPM-2 immunostaining at the base of the sperm head (Figure 4, C and D, arrows, and M, first bar). In contrast, more than half of the human sperm permeabilized in lysolecithin and subsequently incubated for 1 h in CSF-arrested extract were found to be positive for MPM-2 labeling (Figure 4, E and F, arrows, and M, second bar). Priming sperm first by 5 mM DTT treatment did not increase the detection of MPM-2 staining at the base of the human sperm (Figure 4, G and H, arrows, and M, third bar) until after CSF-arrested extract exposure (Figure 4, I and J, arrows, and M, fourth bar). Although a punctate MPM-2 immunostaining pattern of the sperm head, midpiece, and principal piece of the sperm tail was sometimes observed in mature spermatozoa, the immunostaining pattern was clearly more pronounced at the junction between the sperm head and tail after extract exposure. This observation was in good agreement with the pronounced MPM-2 decoration of the sperm centrosomal area after bovine sperm incorporation in vivo (Figure 4, K and L, arrows; see Figure 6D, arrow).
Figure 4.
MPM-2 detection in human sperm and early bovine sperm penetration. X. laevis sperm become phosphorylated after incubation in X. laevis CSF-arrested cell-free extract (A, MPM-2; B, DNA). The mature human sperm centrosome does not immunostain with MPM-2 antibody after methanol fixation (C, MPM-2; D, DNA), although punctate staining is occasionally observed in the head, midpiece, and principal sperm tailpiece (not shown). Human spermatozoa permeabilized in lysolecithin and exposed to CSF-arrested cell-free extract demonstrate MPM-2 immunostaining at the sperm centrosomal region (E, MPM-2; F, DNA). Sperm priming with 5 mM DTT does not significantly increase the detection of centrosome phosphorylation (G, MPM-2; H, DNA) until after exposure to CSF-arrested cell-free extract (I, MPM-2; J, DNA). Arrows depict sperm tail attachment to the sperm head as observed with phase or differential interference contrast optics. Similar results were observed in mature bovine sperm exposed to CSF-arrested extracts. (K and L) Dispermic penetration in a bovine oocyte after in vitro fertilization (8 h after insemination). MPM-2 phosphorylation of the sperm centrosomes (L, arrows) is observed, suggesting that the positive MPM-2 staining of the assembling zygotic centrosome observed after exposure to frog extracts is mimicked in vivo. (M) Graphic representation of human spermatozoa immunostained with MPM-2 antibody after permeabilization, DTT treatment, and exposure to cell-free extract. The analysis demonstrates that significant MPM-2 immunostaining at the sperm centrosome is observed only when permeabilized or DTT-primed spermatozoa are exposed to CSF-arrested cell-free extract (second and fourth bars). Bars in B, J, and L, 10 μm.
Microtubule Nucleation and Human Sperm Aster Formation In Vitro
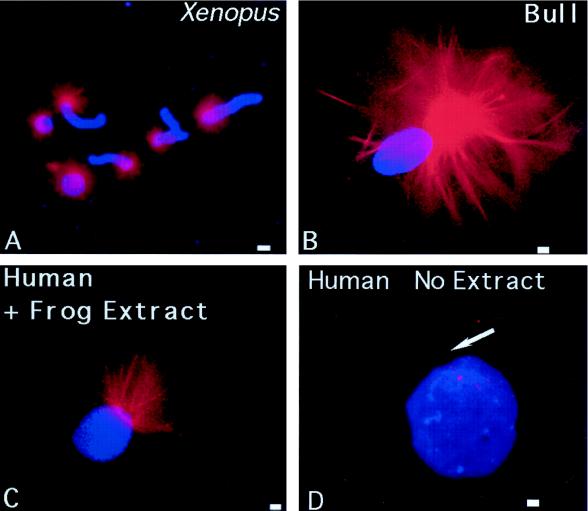
Microtubule nucleation and assembly into the sperm aster, using human or bovine centrosomes as templates, is demonstrated in Figure 5. Xenopus sperm, permeabilized in lysolecithin and incubated in a CSF-arrested extract containing rhodamine-conjugated tubulin, assembled microtubules in vitro after a 10-min incubation at room temperature (Figure 5A, red). In both human and bovine sperm, in vitro microtubule assembly was observed at the base of the sperm head, but only after membrane permeabilization with lysolecithin, 5 mM DTT treatment, and exposure to CSF-arrested egg extract for up to 1 h (Figure 5, B and C, red). Extended incubation periods of human or bovine sperm resulted in extensive random microtubule polymerization and stabilization onto the decondensing nuclei, as observed by Ohsumi et al. (1986). However, the possibility of unanchored microtubules assembling and secondarily associating with the sperm chromatin was not observed in these CSF extracts, because very few free microtubules were assembled within 1 h of extract treatment (note the background of Figure 5) (Verde et al., 1990; Stearns and Kirschner, 1994). Exposure of permeabilized, DTT-treated human sperm to rhodamine tubulin in Pipes buffer without previous CSF extract treatment did not lead to microtubule assembly in vitro from the sperm centrosome (Figure 5D, arrow).
Figure 5.
Microtubule assembly in vitro nucleated by X. laevis, human, and bovine sperm. (A) Lysolecithin-permeabilized Xenopus sperm incubated in CSF-arrested cell-free extract containing 0.08 mg/ml rhodamine-conjugated bovine brain tubulin. Microtubule assembly (red) is radially symmetric and tightly focused at the sperm centrosome (blue, DNA). Bull (B) and human (C) sperm (blue), exposed to 5 μM ionomycin and primed with 5 mM DTT, also demonstrate assembly of microtubules in vitro (red) from the centrosomal region after 40–60 min of incubation in CSF-arrested cell-free extract. No free asters or assembled microtubules are present in the background, suggesting that microtubule nucleation, as opposed to microtubule capture, has occurred. Primed human sperm (blue) that was not exposed to CSF extract did not nucleate microtubules when exposed to rhodamine-conjugated bovine brain tubulin in Pipes buffer alone (D, red; arrow points to sperm axoneme). Bar in A, 30 μm; bars in B, C, and D, 1 μm.
γ-Tubulin, Centrin, and MPM-2 Localization in Inseminated Human and Bovine Oocytes
The sperm aster, a radially symmetrical array of microtubules nucleated from the sperm centrosome, assembles within hours of sperm entry in bovine (Navara et al., 1994) and human (Simerly et al., 1995) oocytes. Astral microtubules (Figure 6A, red) emanated from the base of the incorporated, decondensed bovine sperm head (Figure 6A, blue; M, male pronucleus; F, female pronucleus), which coincided with γ-tubulin immunoreactivity (Figure 6A, arrow, green). Similar microtubule and γ-tubulin immunostaining patterns have been observed in early human zygotes (Simerly et al., 1995).
In a dispermic human oocyte at first mitotic metaphase (Figure 6B, blue), each sperm axoneme terminated at one pole of the bipolar mitotic spindle (Figure 6B, red, arrows). γ-Tubulin was detected as four bright punctate sources, two at each of the spindle poles (Figure 6B, green). In contrast to fertilized oocytes, however, γ-tubulin was undetectable at meiotic spindle poles in mature human or bovine oocytes (not shown), although a prominent 55-kDa band was observed on Western blots using unfertilized bovine oocytes (Figure 6E).
Consistent with the observation that centrin immunostaining is reduced at the base of the human sperm head after exposure to cell-free extracts in vitro (Figure 3, G and H), 20H5 centrin antibody localization (Figure 6C, inset) was not observed in microtubule-containing asters (Figure 6C, red, arrows; incorporated sperm tail, arrowhead) of a pronucleate-stage human zygote (Figure 6C, blue; M, male pronucleus; F, female pronucleus).
In inseminated bovine oocytes, MPM-2 immunostaining of two bright dots (Figure 6D, green, arrow) within the developing sperm aster (Figure 6D, red) was observed after sperm incorporation (Figure 6D, blue; M, male pronucleus; F, female pronucleus), consistent with the observation that zygotic centrosomal phosphorylation occurs during the early stage of sperm aster formation in vivo.
DISCUSSION
This study explores the gametic centrosomal contributions to the zygote and the process by which they form a complete, functional, and replicative microtubule-organizing center during fertilization. Although each gamete contributes equal amounts of genetic information at fertilization, the egg provides the stockpiles of proteins and the energy, cellular machinery, and environment needed for the initial phase of embryonic development. Among the crucial events necessary for early development is the reconstitution of the sperm centrosome (Schatten, 1994). The “procentrosome” (Stearns, 1995) of the mature nonrodent mammalian sperm is the paternally contributed component that must attract and organize maternal centrosomal proteins capable of microtubule nucleation and duplication into the zygotic centrosome. The implication is that each gamete contains critical, complementary protein components but does not possess a fully functional centrosome without gametic union. A reconstituted zygotic centrosome, composed of a blend of maternal and paternal centrosomal proteins, has additional functions unique from somatic cell centrosome functions: it must choreograph the union of the parental genomes and establish the initial cleavage axis, thereby greatly influencing the distribution of organelles, cytoplasm, and all subsequent cell divisions.
Questions might be posed as to the relevance of extracts prepared from Xenopus oocytes as a reliable indicator for exploring early fertilization events in mammalian oocytes. Concerns regarding the use of frog cell-free extracts might include intracellular species specificity for the mammalian zygotic centrosome reconstitution, including the inability of maternal centrosomal proteins from Xenopus to associate with the human sperm centrosome, as well as the presence of disulfide bonds in the mammalian sperm heads. However, centrosomal fractions from diverse species such as sea urchins and Tetrahymena will nucleate asters after microinjection into Xenopus eggs (Heidermann and Kirschner, 1975; Maller et al., 1976; Karsenti et al., 1984). In addition, a high degree of evolutionary conservation of centrosomal proteins, such as γ-tubulin, centrin, and pericentrin, argue against this concern (Oakley, 1992; Zimmerman et al., 1999). Both human and Xenopus sperm added to extracts simultaneously result in both sperm centrosomes attracting γ-tubulin.
Past studies have shown that cycling frog extracts mimic many aspects of the cell cycle in vivo, including events such as semiconservative DNA replication, nuclear envelope breakdown and reformation, cell cycle alterations in microtubule dynamics, and membrane vesicle fusion (Murray, 1991). Recent evidence has also shown that the human sperm genome can be completely replicated in Xenopus extracts (Xu et al., 1998). Both Xenopus and mammalian oocytes are arrested at metaphase of second meiosis by cytostatic factor, and both species are fertilized at this stage. At fertilization, sperm fusion with the oocyte induces an increase in intracytoplasmic calcium that presumably inactivates both CSF and maturation-promoting factor, allowing these oocytes to pass from metaphase II arrest into interphase. The first mitotic cycle in frogs is only 75 min, whereas those of primate and bovine oocytes are 24–36 h, and Xenopus egg cytoplasm does not efficiently reduce the cysteine-rich disulfide bonds in protamines in mature mammalian sperm (Brown et al., 1987; Lohka and Maller, 1988). Nevertheless, this study is focused only on the very early events occurring in CSF-arrested frog extracts that permit the assembly of the zygotic centrosome, including the ability to bind maternal γ-tubulin, the calcium sensitivity of centrin localized to the sperm proximal centriole, and the phosphorylation of centrosomal epitopes in M-phase extracts as might occur during the early stages of sperm penetration in mammalian oocytes.
γ-Tubulin Is Retained in Human and Bovine Mature Spermatozoa but Is Significantly Increased after Exposure to the Cytoplasmic Environment
Immunofluorescence and Western blotting with human, bovine, and Xenopus sperm suggest that paternally derived γ-tubulin is present in modest amounts before exposure to egg cytoplasm (Figure 1), albeit at concentrations nearing detection thresholds (Félix et al., 1994; Stearns and Kirschner, 1994). The variability in detection of γ-tubulin in mature spermatozoa by immunofluorescence suggests that paternal γ-tubulin may be largely inaccessible until after cytoplasmic exposure. In addition, the presence of any paternal γ-tubulin in the mature spermatozoa is not sufficient to assemble microtubules in vitro without previous exposure to cytoplasmic extract (Figure 5D). The introduced paternal γ-tubulin may be necessary for attracting larger amounts of maternal γ-tubulin protein to the centrosome, a step critical for nucleation of a small sperm astral array. The unveiling of γ-tubulin at the sperm centrosome may be a direct consequence of sperm head decondensation, in which the loosening of the tightly compacted implantation fossa is linked to the loosening of the tightly compacted sperm chromatin by disulfide bond reduction. Alternatively, disulfide bond reduction may directly unfold the highly condensed procentrosomal structure, thereby exposing hidden γ-tubulin and γ-tubulin–binding sites. This would enable the reconstituting zygotic centrosome to acquire additional maternal γ-tubulin for nucleation of microtubules, which, in turn, would promote the attraction of yet more maternal γ-tubulin for building the microtubule sperm astral array. Support for this sequence of events is provided by the detection of γ-tubulin in mature bovine oocytes in Western blots (Figure 6E) and by the finding that the mature Xenopus oocyte is enriched in γ-tubulin in the cortical region (Gard, 1994), suggesting that any newly incorporated sperm has immediate access to an oocyte’s centrosomal protein pool. The nature of the γ-tubulin–binding protein’s effect on the sperm centrosome is unknown, although it may be linked to the presence of the γ-tubulin ring complexes (Moritz et al., 1995; Zheng et al., 1995). If this model is correct, there are serious clinical implications for patients being treated for infertility by intracytoplasmic sperm injection, in which, typically, a single spermatozoan is microinjected deep into the oocyte’s center rather than into the cell cortical region.
Centrin Is a Paternal Centrosomal Protein Demonstrating Ca2+ Sensitivity in Bovine and Human Spermatozoa
Centrin is a paternal centrosomal component in human and bovine sperm that appears to reside in the proximal centriole (Figure 3). Interestingly, centrin is also strongly detected by Western blots in bovine oocytes, although no centrin is detected in the assembled sperm asters by immunofluorescence in either human (Figure 6C, inset) or bovine (our unpublished results) zygotes. This observation agrees with cellular fractionation experiments, which demonstrate that the vast majority of cytoplasmic centrin is not associated with the centrosome (Paoletti et al., 1996). Three human centrin genes, Hcen1p, Hcen2p, and Hcen3p, have recently been cloned (Lee and Huang, 1993; Errabolu et al., 1994; Middendorp et al., 1997) and are localized in several cell types with the anti-centrin mAb 20H5. Although Hcen2p and Hcen3p stain the centrosomes of ciliated and nonciliated cell types, Hcen1p stains only the centriolar region of ciliated cells (Salisbury, 1995). Perhaps the Hcen2 and Hcen3 gene products are found in both gametes, whereas the Hcen1 gene product is exclusively parceled to the sperm, so that the epitope recognized by 20H5 more closely resembles that of a specific region on the Hcen1 gene product, thereby explaining the differential staining between gametes. Alternatively, centrin could be functionally and immunologically hidden within the oocyte by forming complexes with other proteins. Although no 20H5 centrin detection has been observed in early zygotes, egg activation events could induce a disassembly of this complex and result in the liberation of centrin for participation in later mammalian embryonic development after the first cell cycle.
Centrin has been localized to the stellate fibers of the transition zone between the basal body and the axoneme (Sanders and Salisbury, 1989; Baron et al., 1992) and to the distal lumen of the centrioles in animal cells (Paoletti et al., 1996; Middendorp et al., 1997). Indirect and direct evidence implicates centrin as a calcium-binding protein that undergoes ultrastructural and distributional changes upon alteration of calcium levels (Sanders and Salisbury 1989, 1994; Baron et al., 1994; Errabolu et al., 1994). In Chlamydomonas, it has been suggested that calcium induces centrin proteins to “contract” and exert shear force and torsional load on the axonemal doublets, resulting in flagellar severing (Sanders and Salisbury, 1994). In human and bovine sperm, centrin also displays a calcium sensitivity after DTT priming and exposure to >1 mM CaCl2, as observed by both immunofluorescence (Figure 2G) and Western blots (Figure 2I). Fertilization is also accompanied by an increase in intracellular calcium (reviewed by Whitaker and Swann, 1993). One of the proposed functions for an increase in intracellular calcium might be a centrin-induced uncoupling of the sperm tail axoneme from the basal body, although clearly the sperm axoneme is not severed from the proximal centriole in the human or bovine sperm during the first cell cycle. This loosening of the sperm centrosomal region might initiate a functional and structural conversion of the sperm basal body to that of a mature centriole. Perhaps the increase in intracellular calcium also aids in breaking the tether between mother and daughter centrioles before replication (Bornens et al., 1987) or in the separation of centrioles at anaphase. In frogs, the microinjection of recombinant heterologous centrin into one blastomere of a two-cell frog embryo impaired early amphibian development by disrupting cytoplasmic microtubules, nuclear segregation, and cytokinesis (Paoletti et al., 1996).
Disulfide Bond Reduction and Formation in Centrosomal Function
This study suggests that the human sperm centrosome contains γ-tubulin, albeit inaccessible as a result of centrosomal protein folding or compaction induced by disulfide bond formation. The oxidation state of mammalian sperm has also been well characterized. It has been reported that the oxidation of sulfhydryl groups occurs in sperm heads and tails as they mature during their passage through the epididymis (Calvin et al., 1973; Kosower and Kosower, 1987; Shalgi et al., 1989). Mammalian sperm chromatin, in contrast to that from X. laevis, contains cysteine-rich protamines in the oxidized state that become reduced by endogenous reductases shortly after sperm penetration, allowing for the exchange of protamines for histones (Rodman et al., 1981; Perreault et al., 1984, 1987; Ward and Coffey, 1991; Bellvéet al., 1993). The reducing environment of the mammalian oocyte also provides the means for breaking disulfide bonds and allowing pronuclear decondensation, centrosomal exposure, and possibly centrosomal decondensation. Oxidizing and reducing compounds will affect microtubule stability, presumably by acting on the centrosome (Mazia and Zimmerman, 1958; Mellon and Rebhun, 1976; Oliver et al., 1976). Interestingly, these observations on the oxidative cycles in mammalian gametes are supported by research into thiol cycles, which have been shown to correspond precisely with other cell cycle events, such as DNA decondensation, and the state of centrosomal condensation during mitotic spindle formation (Mazia and Zimmerman, 1958; Mazia, 1961). It is interesting to speculate that these cyclical changes in the centrosome oxidation state could account for the expansion and contraction observed in this structure as it progresses through the cell cycle.
Centrosomes Phosphorylation Is Observed after Exposure to CSF Extract or during Early Sperm Incorporation In Vivo
The MPM-2 results reported in this study differ slightly from the observations of Pinto-Correia et al. (1994) and Long et al. (1997). In those studies, the phosphoprotein antibody was detected in bull, rabbit, boar, and mouse sperm in the outer dense fibers and connecting piece within the neck region before insemination. Dephosphorylation of these midpiece components occurred after the calcium-induced maturation-promoting factor decline associated with sperm penetration, resulting in sperm aster microtubule assembly in vivo. Although we occasionally observed MPM-2 punctate staining in the head, midpiece, and principal piece in bovine and human spermatozoa, a positive detection of MPM-2 antibody at the sperm centrosome was found in <5% of the mature spermatozoa, whether permeabilized or exposed to disulfide bond reduction (Figure 4M). However, a significant increase in phosphorylation of the human sperm centrosomal region occurred after exposure to CSF-arrested egg extract, which is in agreement with the detection of MPM-2 in the decondensing sperm head after rabbit fertilization in vitro (Pinto-Correia et al., 1994). In addition, we also observed MPM-2 immunostaining at the sperm centrosome during early bovine sperm incorporation after in vitro fertilization (Figure 4L) and during the early stages of sperm aster formation in vivo (Figure 6D).
Many kinases are present and functional in CSF-arrested X. laevis egg extracts, including p34cdc2 and MAPKs (reviewed by Murray and Hunt, 1993). In mammalian oocytes, sperm incorporation and sperm aster formation overlap with the completion of second meiosis. Maturation-promoting factor activity, as measured by H1 kinase activity, decreases after sperm incorporation, although microtubule and chromatin configurations remain in a metaphase-like configuration for several hours after oocyte activation, probably as a result of high MAPK activity (Choi et al., 1991; Verlhac et al., 1994). This unique transition period between the completion of second meiosis and the first interphase in mammals may be important for sperm nuclear remodeling events, including reconstitution of the zygotic sperm centrosome. Nevertheless, pronuclear migration occurs strictly during interphase, requiring continued microtubule nucleation, organization, and interaction with chromatin or nuclear envelopes (Harrouk and Clarke, 1993; Steffen-Zoran et al., 1993). For this degree of microtubule dynamics, some kinase activity would be expected.
Centrosome Reconstitution In Vitro
Centrosome reconstitution appears to be a multistep process occurring between the end of second meiosis and the transition into interphase of the first cell cycle. Microtubule nucleation and organization capabilities must function properly and quickly to form the sperm aster, the structure responsible for pronuclear migration. After pronuclear apposition, the centrosomes must replicate and split to provide the correct number of microtubule organizing centers necessary to form the bipolar mitotic spindle apparatus. Analysis of human and bovine sperm in cell-free extracts has provided clues to the milestones that must be reached before a zygotic centrosome is functional to organize microtubules in vitro. In lower animals such as amphibians, zygotic centrosome formation is microtubule and microfilament independent but egg extract and ATP dependent (Stearns and Kirschner, 1994). Mammalian sperm, exposed to increased levels of calcium and with the plasma membrane destabilized, must be treated to disulfide bond reduction to extricate the sperm mitochondria, outer dense fibers, and fibrous sheath structures. This may expose the sperm centrosome to the maternal cytoplasmic environment, and, concomitant with the onset of pronuclear decondensation also initiated by disulfide bond reduction, the sperm centrosome unveils γ-tubulin and other centrosomal protein-binding sites. A large, cortically derived maternal γ-tubulin pool, which can accumulate into spindle poles during parthenogenesis, is typically attracted and bound to the sperm procentrosome after insemination and, with phosphorylation, shifts the microtubule dynamics to a state of nucleation and polymerization (reviewed by Schatten, 1994). Although speculative, this study characterizes the presence of centrin, γ-tubulin, and the state of centrosomal phosphorylation in nonrodent gametes, demonstrating steps in the normal sequence of events that transform the mature mammalian sperm into an active participant in the zygote, i.e., the intracellular priming of the sperm centrosome induced by endogenous disulfide bond reduction within the mammalian oocyte’s cytoplasm.
ACKNOWLEDGMENTS
The authors thankfully acknowledge Drs. P. Rao for the generous donation of MPM-2, G. Borisy for rhodamine-derivatized bovine brain tubulin, G. Gundersen for detyrosinated α-tubulin, T. Stearns for the XG-1-4 γ-tubulin, P. Schoff for preliminary Western blotting results, and G. Scott at the electron microscopy facility of the University of Wisconsin, Madison. We extend our gratitude to the anonymous patients who have provided informed consent and donated excess, discarded oocytes and zygotes for this research. We thank Drs. S. Shapiro, O. Khorran, and Jeff Jones (University of Wisconsin, Madison, Infertility Clinic) for helpful discussions. The protocols were approved by the universities’ human subjects institutional review boards and research animal review committees. The sperm research was supported by grants from the National Institutes of Health (to G.S. and J.L.S.) and the U.S. Department of Agriculture (to G.S.). P.S. was supported by a Fogarty International Research Fellowship from the National Institutes of Health.
REFERENCES
- Baron AT, Greenwood TM, Bazinet CW, Salisbury JL. Centrin is a component of the pericentriolar lattice. Biol Cell. 1992;76:383–388. doi: 10.1016/0248-4900(92)90442-4. [DOI] [PubMed] [Google Scholar]
- Baron AT, Suman VJ, Nemeth E, Salisbury JL. The pericentriolar lattice of PtK2 cells exhibits temperature and calcium-modulated behavior. J Cell Sci. 1994;107:2993–3003. doi: 10.1242/jcs.107.11.2993. [DOI] [PubMed] [Google Scholar]
- Baum P, Furlong C, Byers B. Yeast gene required for spindle pole body duplication: homology of its product with Ca2+-binding proteins. Proc Natl Acad Sci USA. 1986;83:5512–5516. doi: 10.1073/pnas.83.15.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellvé AR, Zheng W, Martinova YS. Recovery, capacitation, acrosome reaction, and fractionation of sperm. Methods Enzymol. 1993;225:113–136. doi: 10.1016/0076-6879(93)25010-y. [DOI] [PubMed] [Google Scholar]
- Biggins S, Rose MD. Direct interaction between yeast spindle pole body components: Kar1p is required for Cdc31p localization to the spindle pole body. J Cell Biol. 1994;125:843–852. doi: 10.1083/jcb.125.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornens M, Paintrand M, Berges J, Marty MC, Karsenti E. Structural and chemical characterization of isolated centrosomes. Cell Motil Cytoskeleton. 1987;8:238–249. doi: 10.1002/cm.970080305. [DOI] [PubMed] [Google Scholar]
- Boveri T. Zellen-studien: Uber die Natur der Centrosomen. IV. Jena, Germany: Fisher; 1901. [Google Scholar]
- Brinkley BR, Cox SM, Fistel S. Organizing centers for cell processes. Neurosci Res Program Bull. 1980;19:108–124. [Google Scholar]
- Brown DB, Blake EJ, Wolgemuth DJ, Gordon K, Ruddle FH. Chromatin decondensation and DNA synthesis in human sperm activated in vitro by using Xenopus laevis egg extracts. J Exp Zool. 1987;242:215–231. doi: 10.1002/jez.1402420213. [DOI] [PubMed] [Google Scholar]
- Calvin HI, Yu CC, Bedford JM. Effects of epididymal maturation, zinc (II) and copper (II) on the reactive sulfhydryl content of structural elements in rat spermatozoa. Exp Cell Res. 1973;81:333–341. doi: 10.1016/0014-4827(73)90523-5. [DOI] [PubMed] [Google Scholar]
- Choi T, Aoki F, Mori M, Yamashita M, Nagahama Y, Kohmoto K. Activation of p34cdc2 protein kinase activity in meiotic and mitotic cell cycles in mouse oocytes and embryos. Development. 1991;113:789–795. doi: 10.1242/dev.113.3.789. [DOI] [PubMed] [Google Scholar]
- Davis FM, Tsao TY, Fowler SK, Rao PN. Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci USA. 1983;80:2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TN. The centrosome on center stage. Trends Cell Biol. 1997;7:508–510. doi: 10.1016/S0962-8924(97)01180-X. [DOI] [PubMed] [Google Scholar]
- Doxsey S. The centrosome: a tiny organelle with big potential. Nat Genet. 1998;20:104–106. doi: 10.1038/2392. [DOI] [PubMed] [Google Scholar]
- Doxsey SJ, Stein P, Evans L, Calarco PD, Kirschner M. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell. 1994;76:639–650. doi: 10.1016/0092-8674(94)90504-5. [DOI] [PubMed] [Google Scholar]
- Errabolu R, Sanders MA, Salisbury JL. Cloning of a cDNA encoding human centrin, an EF-hand protein of centrosomes and mitotic spindle poles. J Cell Sci. 1994;107:9–16. doi: 10.1242/jcs.107.1.9. [DOI] [PubMed] [Google Scholar]
- Félix MA, Antony C, Wright M, Maro B. Centrosome assembly in vitro: role of γ-tubulin recruitment in Xenopus sperm aster formation. J Cell Biol. 1994;124:19–31. doi: 10.1083/jcb.124.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DL. γ-Tubulin is asymmetrically distributed in the cortex of Xenopus oocytes. Dev Biol. 1994;161:131–140. doi: 10.1006/dbio.1994.1015. [DOI] [PubMed] [Google Scholar]
- Harrouk W, Clarke HJ. Sperm chromatin acquires an activity that induces microtubule assembly during residence in the cytoplasm of metaphase oocytes of the mouse. Chromosoma. 1993;102:279–286. doi: 10.1007/BF00352402. [DOI] [PubMed] [Google Scholar]
- Heidermann SR, Kirschner MW. Aster formation in egg of Xenopus laevis. J Cell Biol. 1975;67:105–117. doi: 10.1083/jcb.67.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio T, Uzawa S, Jung MK, Oakley BR, Tanaka K, Yanagida M. The fission yeast γ-tubulin is essential for mitosis and is localized at microtubule organizing centers. J Cell Sci. 1991;99:693–700. doi: 10.1242/jcs.99.4.693. [DOI] [PubMed] [Google Scholar]
- Hulen D, Baron A, Salisbury J, Clarke M. Production and specificity of monoclonal antibodies against calmodulin from Dictyostelium discoideum. Cell Motil Cytoskeleton. 1991;18:113–122. doi: 10.1002/cm.970180206. [DOI] [PubMed] [Google Scholar]
- Joshi HC, Palacios MJ, McNamara L, Cleveland DW. γ-Tubulin is a centrosomal protein required for cell cycle-dependent microtubule nucleation. Nature. 1992;356:80–83. doi: 10.1038/356080a0. [DOI] [PubMed] [Google Scholar]
- Karsenti E, Newport J, Hubble R, Kirschner M. Interconversion of metaphase and interphase microtubule arrays as studied by the injection of centrosomes and nuclei into Xenopus eggs. J Cell Biol. 1984;98:1730–1745. doi: 10.1083/jcb.98.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosower NS, Kosower EM. Thiol labeling with bromobimanes. Methods Enzymol. 1987;143:76–84. doi: 10.1016/0076-6879(87)43015-2. [DOI] [PubMed] [Google Scholar]
- Kuriyama R, Borisy GG, Masui Y. Microtubule cycles in oocytes of the surf clam, Spisula solidissima: an immunofluorescence study. Dev Biol. 1986;114:151–160. doi: 10.1016/0012-1606(86)90391-x. [DOI] [PubMed] [Google Scholar]
- Lee VD, Huang B. Molecular cloning and centrosomal localization of human caltractin. Proc Natl Acad Sci USA. 1993;90:11039–11043. doi: 10.1073/pnas.90.23.11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohka MJ, Maller JL. Induction of metaphase chromosome condensation in human sperm by Xenopus egg extracts. Exp Cell Res. 1988;179:303–309. doi: 10.1016/0014-4827(88)90370-9. [DOI] [PubMed] [Google Scholar]
- Long CR, Suncan RP, Robl JM. Isolation and characterization of MPM-2 reactive sperm proteins: homology to components of the outer dense fibers and segmented columns. Biol Reprod. 1997;57:246–254. doi: 10.1095/biolreprod57.2.246. [DOI] [PubMed] [Google Scholar]
- Maller J, Poceia D, Nishioka D, Gerhart J, Hartman H. Spindle formation and cleavage in Xenopus eggs injected with centriole-containing fractions from sperm. Exp Cell Res. 1976;99:285–294. doi: 10.1016/0014-4827(76)90585-1. [DOI] [PubMed] [Google Scholar]
- Manandhar G, Sutovsky P, Joshi HC, Stearns T, Schatten G. Centrosome reduction during mouse spermiogenesis. Dev Biol. 1998;203:424–434. doi: 10.1006/dbio.1998.8947. [DOI] [PubMed] [Google Scholar]
- Mazia D. Mitosis and the physiology of cell division. In: Bracket J, Mirsky AE, editors. The Cell. III. New York: Academic Press; 1961. pp. 78–412. [Google Scholar]
- Mazia D, Zimmerman AM. SH compounds in mitosis. II. The effect of mercaptoethanol on the structure of the mitotic apparatus in sea urchin eggs. Exp Cell Res. 1958;15:138–153. doi: 10.1016/0014-4827(58)90070-3. [DOI] [PubMed] [Google Scholar]
- McIntosh JR, Koonce MP. Mitosis. Science. 1989;246:622–628. doi: 10.1126/science.2683078. [DOI] [PubMed] [Google Scholar]
- Mellon MG, Rebhun LI. Sulfhydryls and the in vitro polymerization of tubulin. J Cell Biol. 1976;70:226–238. doi: 10.1083/jcb.70.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middendorp S, Paoletti A, Schiebel E, Bornens M. Identification of a new mammalian centrin gene, more closely related to Saccharomyces cerevisiae CDC31 gene. Proc Natl Acad Sci USA. 1997;94:9141–9146. doi: 10.1073/pnas.94.17.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M, Braunfeld MB, Sedat JW, Alberts B, Agard DA. Microtubule nucleation by γ-tubulin-containing rings in the centrosome. Nature. 1995;378:638–640. doi: 10.1038/378638a0. [DOI] [PubMed] [Google Scholar]
- Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–595. [PubMed] [Google Scholar]
- Murray AW, Hunt T. The Cell Cycle: An Introduction. New York: W.H. Freeman; 1993. [Google Scholar]
- Navara CS, First NL, Schatten G. Microtubule organization in the cow during fertilization, polyspermy, parthenogenesis, and nuclear transfer: the role of the sperm aster. Dev Biol. 1994;162:29–40. doi: 10.1006/dbio.1994.1064. [DOI] [PubMed] [Google Scholar]
- Oakley BR. γ-Tubulin: the microtubule organizer. Trends Cell Biol. 1992;2:1–5. doi: 10.1016/0962-8924(92)90125-7. [DOI] [PubMed] [Google Scholar]
- Oakley CE, Oakley BR. Identification of γ-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans. Nature. 1989;338:662–664. doi: 10.1038/338662a0. [DOI] [PubMed] [Google Scholar]
- Ohsumi K, Katagiri C, Yanagimachi R. Development of pronuclei from human spermatozoa injected microsurgically into frog (Xenopus) eggs. J Exp Zool. 1986;237:319–325. doi: 10.1002/jez.1402370304. [DOI] [PubMed] [Google Scholar]
- Oliver JM, Albertini DF, Berlin RD. Effects of glutathione oxidizing agents on microtubule assembly and microtubule-dependent surface properties of human neutrophils. J Cell Biol. 1976;71:921–932. doi: 10.1083/jcb.71.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios MJ, Joshi HC, Simerly C, Schatten G. Dynamic reorganization of γ-tubulin during murine fertilization. J Cell Sci. 1993;104:383–389. doi: 10.1242/jcs.104.2.383. [DOI] [PubMed] [Google Scholar]
- Paoletti A, Moudjou M, Paintrand M, Salisbury JL, Bornens M. Most of centrin in animal cells is not centrosome-associated and centrosomal centrin is confined to the distal lumen of centrioles. J Cell Sci. 1996;109:3089–3102. doi: 10.1242/jcs.109.13.3089. [DOI] [PubMed] [Google Scholar]
- Perreault SD, Naish SJ, Zirken BR. The timing of hamster sperm nuclear decondensation and male pronucleus formation is related to sperm nuclear disulfide bond content. Biol Reprod. 1987;36:239–244. doi: 10.1095/biolreprod36.1.239. [DOI] [PubMed] [Google Scholar]
- Perreault SD, Wolf RA, Zirken BR. The role of disulfide bond reduction during mammalian sperm nuclear decondensation in vivo. Dev Biol. 1984;101:160–167. doi: 10.1016/0012-1606(84)90126-x. [DOI] [PubMed] [Google Scholar]
- Pinto-Correia C, Poccia DL, Chang T, Robl JM. Dephosphorylation of sperm midpiece antigens initiates aster formation in rabbit oocytes. Proc Natl Acad Sci USA. 1994;91:7894–7898. doi: 10.1073/pnas.91.17.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodman TC, Pruslin FH, Hoffmann HP, Allfrey VG. Turnover of basic chromosomal proteins in fertilized eggs: a cytoimmunochemical study of events in vivo. J Cell Biol. 1981;90:351–361. doi: 10.1083/jcb.90.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MD, Biggins S, Satterwhite LL. Unravelling the tangled web at the microtubule-organizing center. Curr Opin Cell Biol. 1993;5:105–115. doi: 10.1016/s0955-0674(05)80015-8. [DOI] [PubMed] [Google Scholar]
- Salisbury JL. Centrin, centrosomes, and mitotic spindle poles. Curr Opin Cell Biol. 1995;7:39–45. doi: 10.1016/0955-0674(95)80043-3. [DOI] [PubMed] [Google Scholar]
- Sanders MA, Salisbury JL. Centrin-mediated microtubule severing during flagellar excision in Chlamydomonas reinhardtii. J Cell Biol. 1989;108:1751–1760. doi: 10.1083/jcb.108.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MA, Salisbury JL. Centrin plays an essential role in microtubule severing during flagellar excision in Chlamydomonas reinhardtii. J Cell Biol. 1994;124:795–805. doi: 10.1083/jcb.124.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatten G. Centrosome inheritance: the reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev Biol. 1994;165:299–335. doi: 10.1006/dbio.1994.1256. [DOI] [PubMed] [Google Scholar]
- Schatten G, Simerly C, Schatten H. Maternal inheritance of centrosomes in mammals? Studies on parthenogenesis and polyspermy in mice. Proc Natl Acad Sci USA. 1991;88:6785–6789. doi: 10.1073/pnas.88.15.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatten H, Schatten G, Mazia D, Balczon R, Simerly C. Behavior of centrosomes during fertilization and cell division in mouse oocytes and in sea urchin eggs. Proc Natl Acad Sci USA. 1986;83:105–109. doi: 10.1073/pnas.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M, Pryzwansky KB, Euteneuer U. Centrosome splitting in neutrophils: an unusual phenomenon related to cell activation and motility. Cell. 1982;31:705–717. doi: 10.1016/0092-8674(82)90325-7. [DOI] [PubMed] [Google Scholar]
- Shalgi R, Seligman J, Kosower NS. Dynamics of the thiol status of rat spermatozoa during maturation: analysis with the fluorescent labeling agent monobromobimane. Biol Reprod. 1989;40:1037–1045. doi: 10.1095/biolreprod40.5.1037. [DOI] [PubMed] [Google Scholar]
- Simerly C, Schatten G. Techniques for localization of specific molecules in oocytes and embryos. Methods Enzymol. 1993;225:516–552. doi: 10.1016/0076-6879(93)25035-z. [DOI] [PubMed] [Google Scholar]
- Simerly C, et al. The paternal inheritance of the centrosome, the cell’s microtubule-organizing center, in humans, and the implications for infertility. Nat Med. 1995;1:47–52. doi: 10.1038/nm0195-47. [DOI] [PubMed] [Google Scholar]
- Stearns T. The form and the substance. Nat Med. 1995;1:19–20. doi: 10.1038/nm0195-19. [DOI] [PubMed] [Google Scholar]
- Stearns T, Evans L, Kirschner M. γ-Tubulin is a highly conserved component of the centrosome. Cell. 1991;65:825–836. doi: 10.1016/0092-8674(91)90390-k. [DOI] [PubMed] [Google Scholar]
- Stearns T, Kirschner M. In vitro reconstitution of centrosome assembly and function: the central role of γ-tubulin. Cell. 1994;76:623–637. doi: 10.1016/0092-8674(94)90503-7. [DOI] [PubMed] [Google Scholar]
- Stearns T, Winey M. The cell center at 100. Cell. 1997;91:303–309. doi: 10.1016/s0092-8674(00)80414-6. [DOI] [PubMed] [Google Scholar]
- Steffen-Zoran S, Simerly CR, Schatten G. Microtubule organization in mouse oocytes after microinjection of sea urchin sperm heads, midpieces and centrosomal complexes. Biol Res. 1993;26:453–464. [Google Scholar]
- Sutovsky P, Fléchon JE, Fléchon B, Motlík J, Peynot N, Chesné P, Heyman Y. Dynamic changes of gap junctions and cytoskeleton during in vitro culture of cattle oocyte cumulus complexes. Biol Reprod. 1993;49:1277–1287. doi: 10.1095/biolreprod49.6.1277. [DOI] [PubMed] [Google Scholar]
- Vandré DD, Davis FM, Rao PN, Borisy GG. Distribution of cytoskeletal proteins sharing a conserved phosphorylated epitope. Eur J Cell Biol. 1986;41:72–81. [PubMed] [Google Scholar]
- Verde F, Labbé JC, Dorée M, Karsenti E. Regulation of microtubule dynamics by cdc2 protein kinase in cell-free extracts of Xenopus eggs. Nature. 1990;343:233–238. doi: 10.1038/343233a0. [DOI] [PubMed] [Google Scholar]
- Verlhac M-H, Kubiak JZ, Clarke HJ, Maro B. Microtubule and chromatin behavior follow MAP kinase activity but not MPF activity during meiosis in mouse oocytes. Development. 1994;120:1017–1025. doi: 10.1242/dev.120.4.1017. [DOI] [PubMed] [Google Scholar]
- Ward WS, Coffey DS. DNA packaging and organization in mammalian spermatozoa: comparison with somatic cells. Biol Reprod. 1991;44:569–574. doi: 10.1095/biolreprod44.4.569. [DOI] [PubMed] [Google Scholar]
- Whitaker M, Swann K. Lighting the fuse at fertilization. Development. 1993;117:1–12. [Google Scholar]
- Xu Y-S, Overton WR, Marmar JL, Leonard JC, McCoy JP, Jr, Butler GH, Li H. Complete replication of human sperm genome in egg extracts from Xenopus laevis. Biol Reprod. 1998;58:641–647. doi: 10.1095/biolreprod58.3.641. [DOI] [PubMed] [Google Scholar]
- Zamboni L, Stefanini M. The fine structure of the neck of mammalian spermatozoa. Anat Rec. 1971;169:155–172. doi: 10.1002/ar.1091690203. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Jung MK, Oakley BR. γ-Tubulin is present in Drosophila melanogaster and Homo sapiens and is associated with the centrosome. Cell. 1991;65:817–823. doi: 10.1016/0092-8674(91)90389-g. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Wong ML, Alberts B, Mitchison T. A γ-tubulin ring complex purified from the unfertilized egg of Xenopus laevis can nucleate microtubule assembly in vitro. Nature. 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman W, Sparks CA, Doxsey SJ. Amorphous no longer: the centrosome comes into focus. Curr Opin Cell Biol. 1999;11:122–128. doi: 10.1016/s0955-0674(99)80015-5. [DOI] [PubMed] [Google Scholar]