Abstract
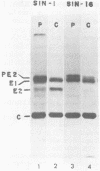
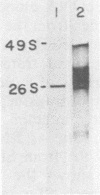
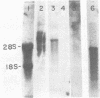
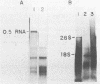
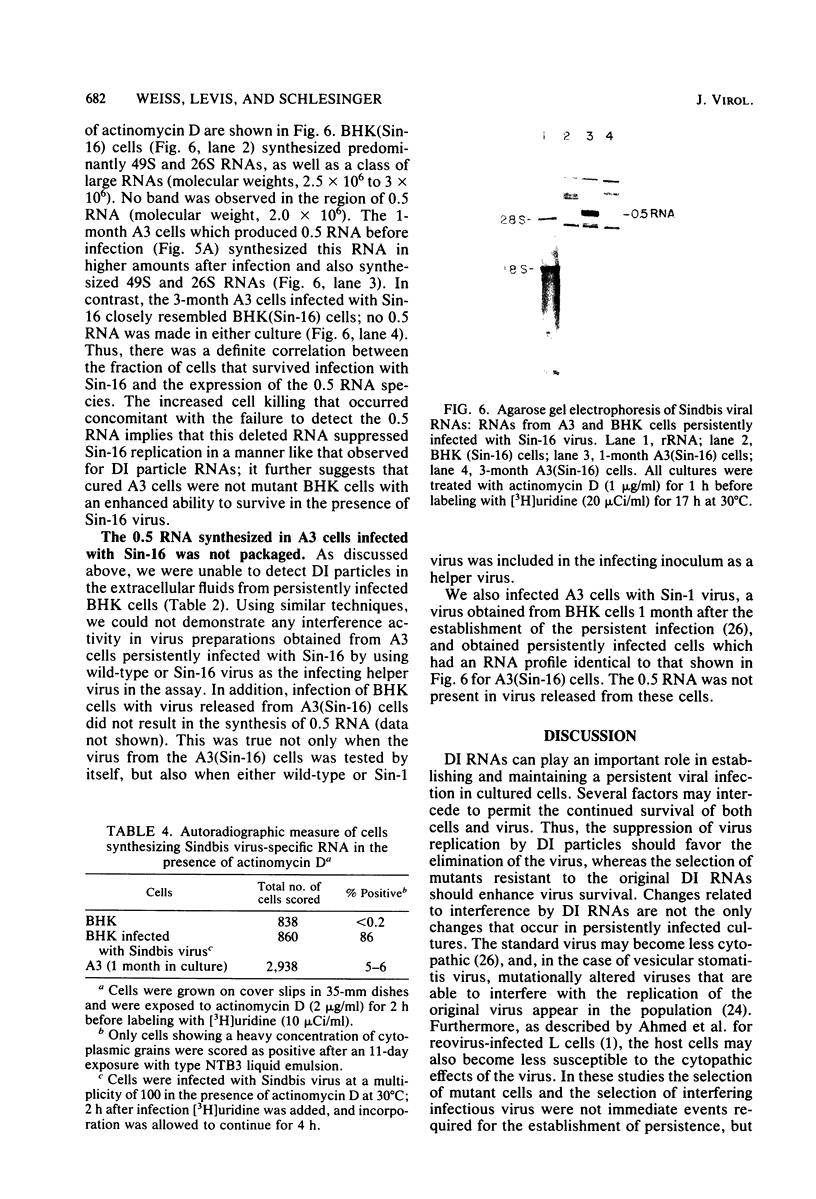
We analyzed a BHK cell line persistently infected with Sindbis virus for 16 months and a virus (Sin-16) cloned from these cells. Sin-16 virus was resistant to the defective interfering particles present in the original infection. We found that (i) cells infected with Sin-16 were impaired in the processing of a viral precursor glycoprotein, (ii) high-multiplicity passaging of Sin-16 gave rise to a variant that was able to generate and be inhibited by defective-interfering particles to which the original Sin-16 virus was resistant, and (iii) the persistently infected culture contained a heterogeneous mixture of defective Sindbis virus RNAs which were not packaged into extracellular particles. To determine whether these intracellular RNAs could interfere with the replication of Sin-16, we analyzed cells that were cloned from the persistently infected culture. One clone (A3) synthesized a single defective viral RNA which was lost with continued passaging in culture. Infection of A3 cells with Sin-16 showed that the presence of the defective RNA greatly enhanced cell survival and led to enrichment of this RNA. In contrast, cured cells were highly susceptible to killing by Sin-16, and survivors did not synthesize this RNA. Thus, A3 cells were not genetically altered in their response to Sin-16, but were protected from the cytopathic effects of infection by an RNA with the characteristics of a defective-interfering RNA.
Full text
PDF

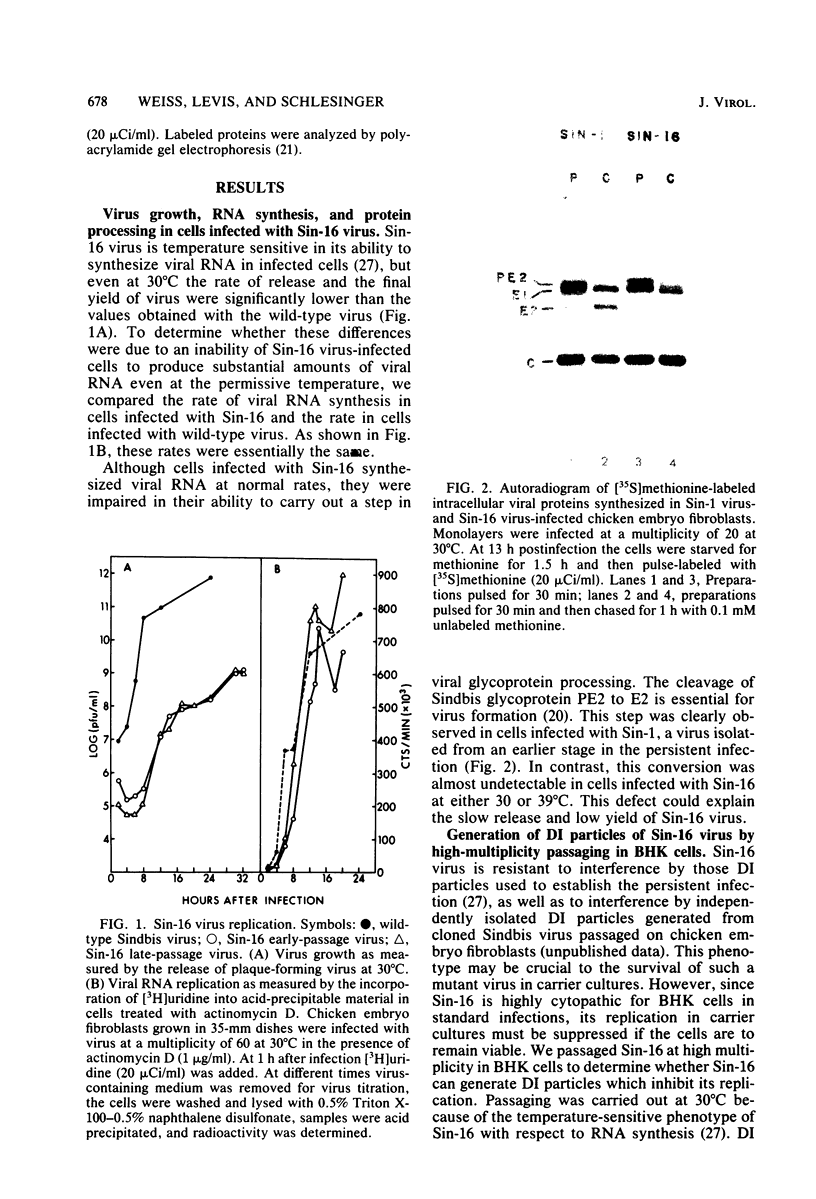

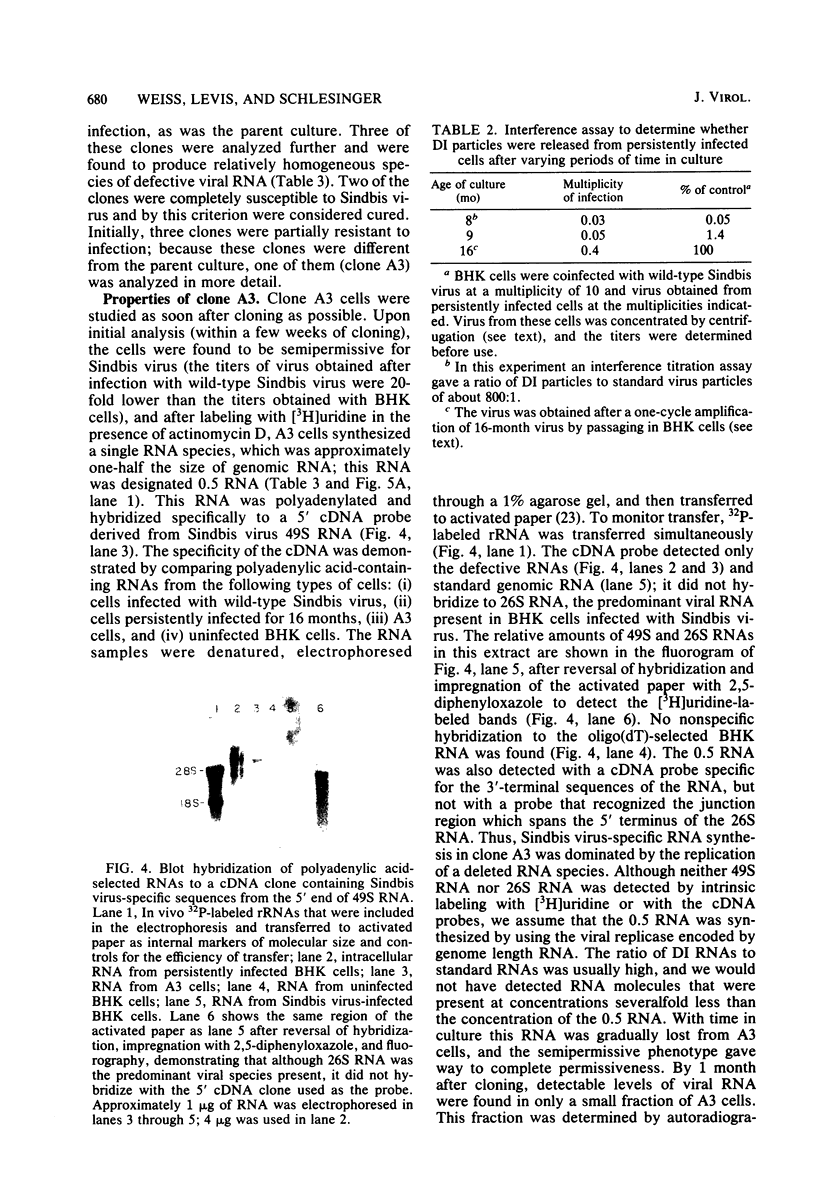
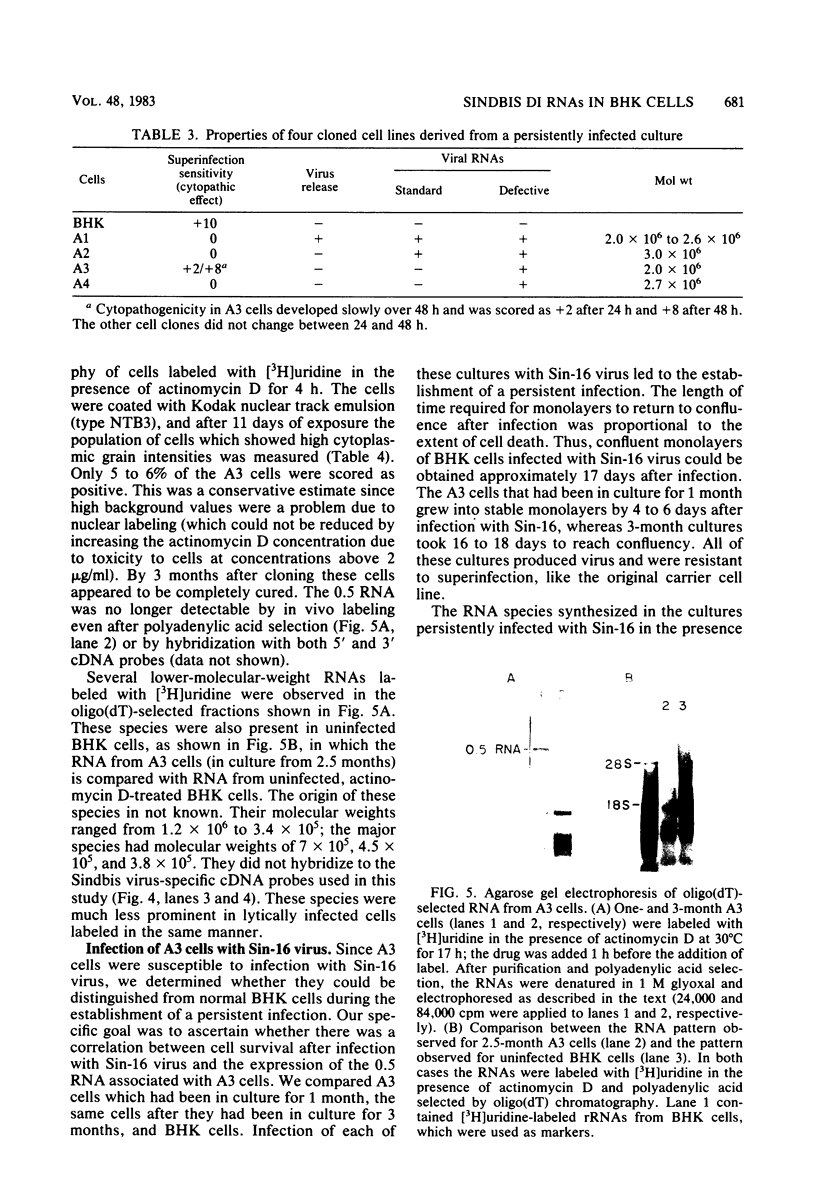


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Canning W. M., Kauffman R. S., Sharpe A. H., Hallum J. V., Fields B. N. Role of the host cell in persistent viral infection: coevolution of L cells and reovoirus during persistent infection. Cell. 1981 Aug;25(2):325–332. doi: 10.1016/0092-8674(81)90050-7. [DOI] [PubMed] [Google Scholar]
- Carmichael G. G., McMaster G. K. The analysis of nucleic acids in gels using glyoxal and acridine orange. Methods Enzymol. 1980;65(1):380–391. doi: 10.1016/s0076-6879(80)65049-6. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Wallis J. Colony hybridization. Methods Enzymol. 1979;68:379–389. doi: 10.1016/0076-6879(79)68027-8. [DOI] [PubMed] [Google Scholar]
- Guild G. M., Stollar V. Defective interfering particles of Sindbis virus. III. Intracellular viral RNA species in chick embryo cell cultures. Virology. 1975 Sep;67(1):24–41. doi: 10.1016/0042-6822(75)90400-6. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P. Persistent noncytocidal vesicular stomatitis virus infections mediated by defective T particles that suppress virion transcriptase. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2956–2960. doi: 10.1073/pnas.71.8.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P., Welsh R. M., Oldstone M. B., Kohne D., Lazzarini R., Scolnick E. Long-term persistent vesicular stomatitis virus and rabies virus infection of cells in vitro. J Gen Virol. 1976 Nov;33(2):193–211. doi: 10.1099/0022-1317-33-2-193. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Horodyski F. M., Holland J. J. Viruses isolated from cells persistently infected with vesicular stomatitis virus show altered interactions with defective interfering particles. J Virol. 1980 Nov;36(2):627–631. doi: 10.1128/jvi.36.2.627-631.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S., Dutko F. J., Pfau C. J. Determinants of spontaneous recovery and persistance in MDCK cells infected with lymphocytic choriomeningitis virus. J Gen Virol. 1979 Jul;44(1):113–122. doi: 10.1099/0022-1317-44-1-113. [DOI] [PubMed] [Google Scholar]
- Kawai A., Matsumoto S. Interfering and noninterfering defective particles generated by a rabies small plaque variant virus. Virology. 1977 Jan;76(1):60–71. doi: 10.1016/0042-6822(77)90282-3. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I., Bruton C. J., Weiss B., Schlesinger S. Defective interfering passages of Sindbis virus: nature of the defective virion RNA. J Virol. 1976 Sep;19(3):1034–1043. doi: 10.1128/jvi.19.3.1034-1043.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe S. S., Schlesinger S. RNAs from two independently isolated defective interfering particles of Sindbis virus contain a cellular tRNA sequence at their 5' ends. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3279–3283. doi: 10.1073/pnas.80.11.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Buchmeier M. J. Restricted expression of viral glycoprotein in cells of persistently infected mice. Nature. 1982 Nov 25;300(5890):360–362. doi: 10.1038/300360a0. [DOI] [PubMed] [Google Scholar]
- Ou J. H., Rice C. M., Dalgarno L., Strauss E. G., Strauss J. H. Sequence studies of several alphavirus genomic RNAs in the region containing the start of the subgenomic RNA. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5235–5239. doi: 10.1073/pnas.79.17.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. M., Strauss J. H. Synthesis, cleavage and sequence analysis of DNA complementary to the 26 S messenger RNA of Sindbis virus. J Mol Biol. 1981 Aug 15;150(3):315–340. doi: 10.1016/0022-2836(81)90550-7. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roux L., Holland J. J. Role of defective interfering particles of Sendai virus in persistent infections. Virology. 1979 Feb;93(1):91–103. doi: 10.1016/0042-6822(79)90278-2. [DOI] [PubMed] [Google Scholar]
- Roux L., Waldvogel F. A. Instability of the viral M protein in BHK-21 cells persistently infected with Sendai virus. Cell. 1982 Feb;28(2):293–302. doi: 10.1016/0092-8674(82)90347-6. [DOI] [PubMed] [Google Scholar]
- Rowlands K., Grabau E., Spindler K., Jones C., Semler B., Holland J. Virus protein changes and RNA termini alterations evolving during persistent infection. Cell. 1980 Apr;19(4):871–880. doi: 10.1016/0092-8674(80)90078-1. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S. Large-molecular-weight precursors of sindbis virus proteins. J Virol. 1973 Jun;11(6):1013–1016. doi: 10.1128/jvi.11.6.1013-1016.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Relation of fatty acid attachment to the translation and maturation of vesicular stomatitis and Sindbis virus membrane glycoproteins. J Biol Chem. 1980 Apr 25;255(8):3334–3339. [PubMed] [Google Scholar]
- Seed B. Diazotizable arylamine cellulose papers for the coupling and hybridization of nucleic acids. Nucleic Acids Res. 1982 Mar 11;10(5):1799–1810. doi: 10.1093/nar/10.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler K. R., Holland J. J. A mutant standard virus isolated from vesicular stomatitis virus persistent infection interferes specifically with wild-type virus replication. J Gen Virol. 1982 Oct;62(Pt 2):363–367. doi: 10.1099/0022-1317-62-2-363. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weiss B., Rosenthal R., Schlesinger S. Establishment and maintenance of persistent infection by Sindbis virus in BHK cells. J Virol. 1980 Jan;33(1):463–474. doi: 10.1128/jvi.33.1.463-474.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Schlesinger S. Defective interfering particles of Sindbis virus do not interfere with the homologous virus obtained from persistently infected BHK cells but do interfere with Semliki Forest virus. J Virol. 1981 Feb;37(2):840–844. doi: 10.1128/jvi.37.2.840-844.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]