Abstract
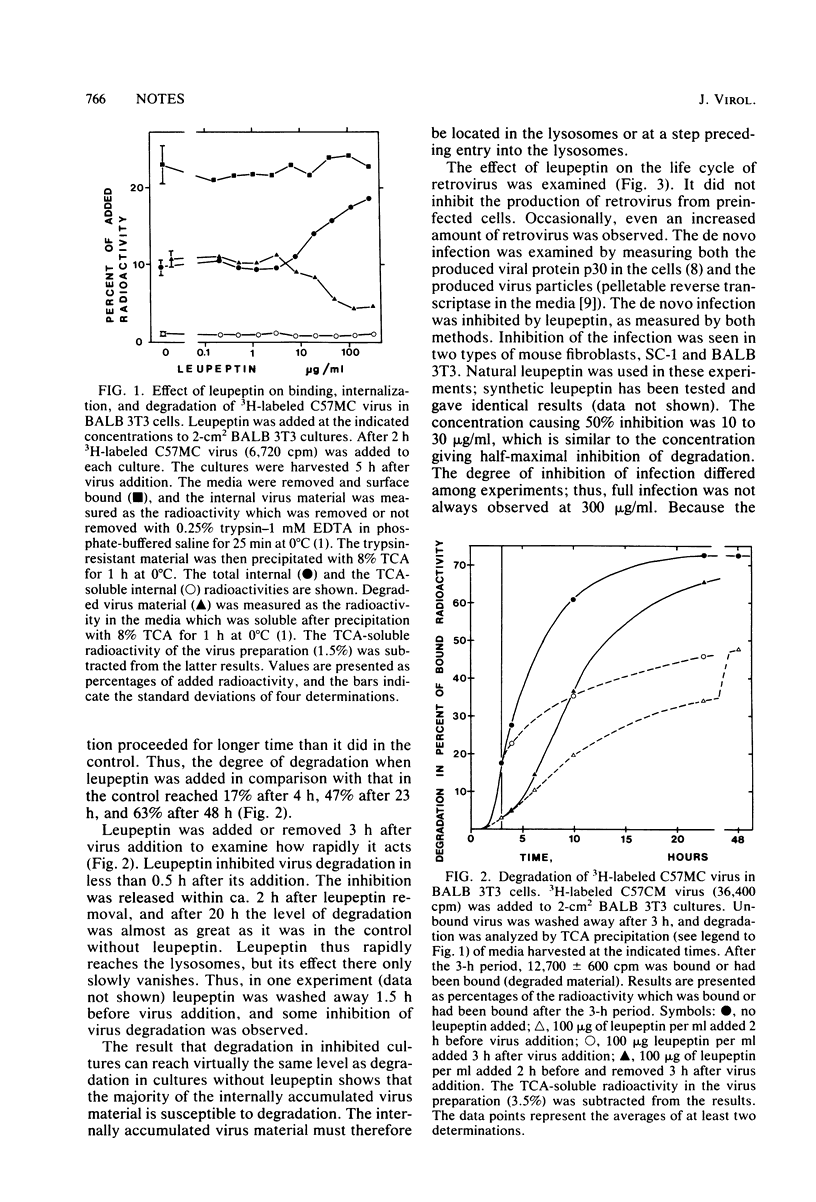
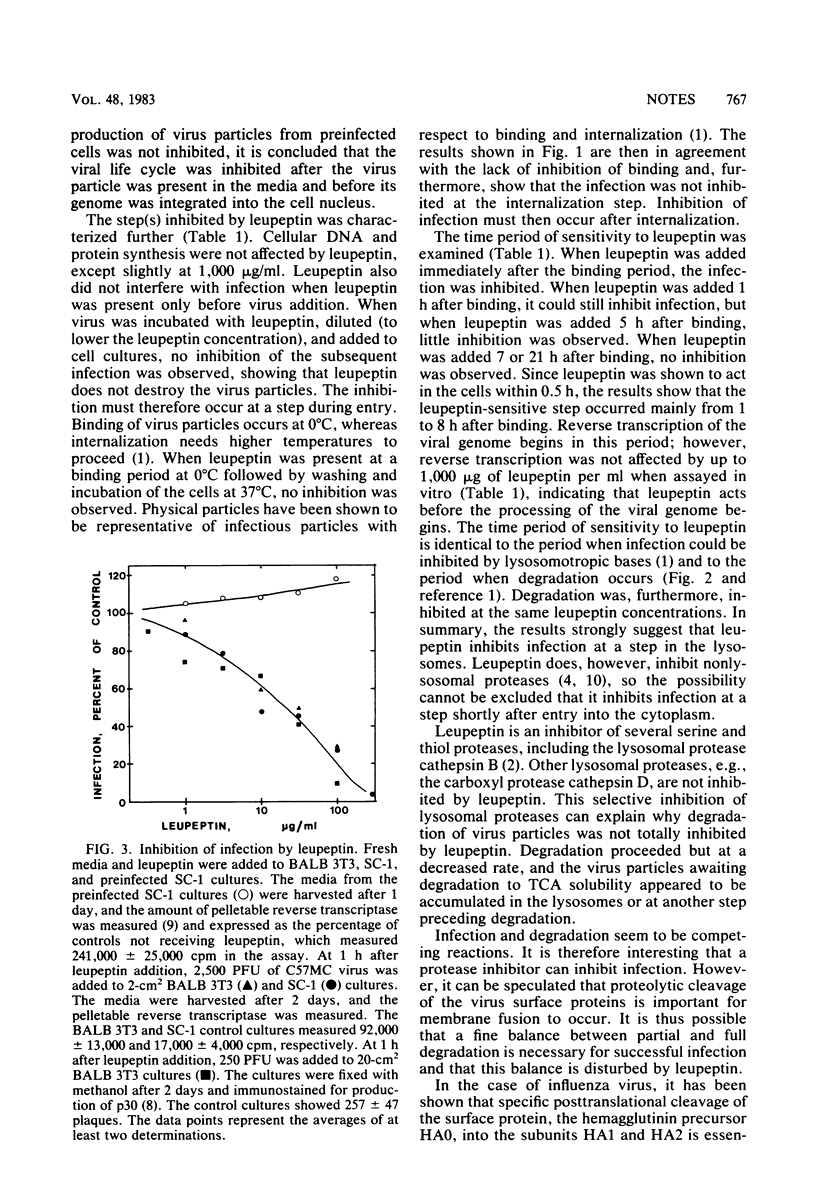
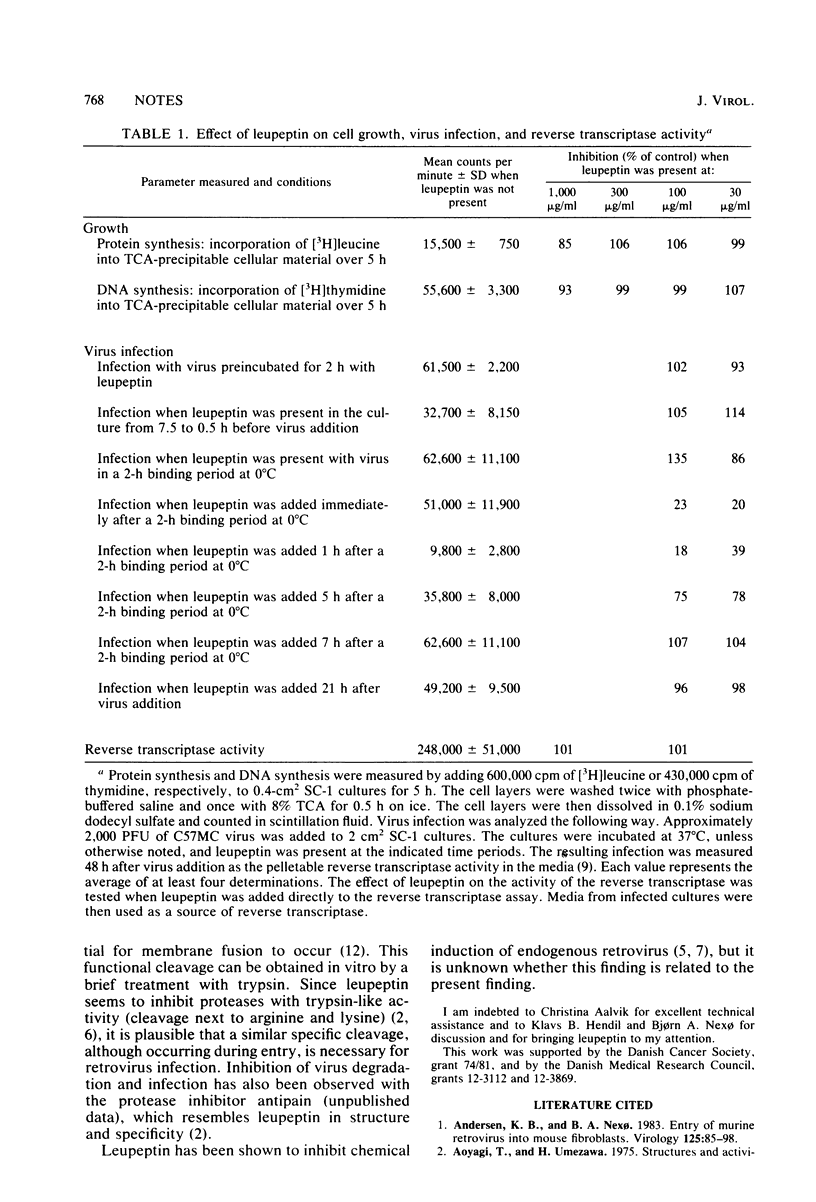
The protease inhibitor leupeptin was shown to inhibit retrovirus infection in mouse fibroblasts at a step shortly after internalization of the virus particles. The inhibited step most likely was the passage of virus particles through the lysosomes or other acid vesicles. Leupeptin was also shown to inhibit degradation of virus particles in the lysosomes. The results are discussed with respect to the involvement of proteases in the infectious route.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K. B., Nexø B. A. Entry of murine retrovirus into mouse fibroblasts. Virology. 1983 Feb;125(1):85–98. doi: 10.1016/0042-6822(83)90065-x. [DOI] [PubMed] [Google Scholar]
- Grinde B., Seglen P. O. Differential effects of proteinase inhibitors and amines on the lysosomal and non-lysosomal pathways of protein degradation in isolated rat hepatocytes. Biochim Biophys Acta. 1980 Sep 17;632(1):73–86. doi: 10.1016/0304-4165(80)90250-0. [DOI] [PubMed] [Google Scholar]
- Hellman K. B., Brewer P. P., Twardzik D. R., Hellman A. Protease inhibitors modify induction of endogenous type C oncornavirus. Proc Soc Exp Biol Med. 1981 Jan;166(1):28–34. doi: 10.3181/00379727-166-41019. [DOI] [PubMed] [Google Scholar]
- Ikezawa H., Aoyagi T., Takeuchi T., Umezawa H. Effect of protease inhibitors of actinomycetes on lysosomal peptide-hydrolases from swine liver. J Antibiot (Tokyo) 1971 Jul;24(7):488–490. doi: 10.7164/antibiotics.24.488. [DOI] [PubMed] [Google Scholar]
- Long C. W., Bruszewski J. A., Christensen W. L., Suk W. A. Effects of protease inhibitors on chemical induction of type C virus. Cancer Res. 1979 Aug;39(8):2995–2999. [PubMed] [Google Scholar]
- Nexo B. A. A plaque assay for murine leukemia virus using enzyme-coupled antibodies. Virology. 1977 Apr;77(2):849–852. doi: 10.1016/0042-6822(77)90504-9. [DOI] [PubMed] [Google Scholar]
- Pedersen F. S., Crowther R. L., Tenney D. Y., Reimold A. M., Haseltine W. A. Novel leukaemogenic retroviruses isolated from cell line derived from spontaneous AKR tumour. Nature. 1981 Jul 9;292(5819):167–170. doi: 10.1038/292167a0. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Grinde B., Solheim A. E. Inhibition of the lysosomal pathway of protein degradation in isolated rat hepatocytes by ammonia, methylamine, chloroquine and leupeptin. Eur J Biochem. 1979 Apr 2;95(2):215–225. doi: 10.1111/j.1432-1033.1979.tb12956.x. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Guntaka R. V., Fan W. J., Heasley S., Bishop J. M. Synthesis of viral DNA in the cytoplasm of duck embryo fibroblasts and in enucleated cells after infection by avian sarcoma virus. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3874–3878. doi: 10.1073/pnas.71.10.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Helenius A., Gething M. J. Haemagglutinin of influenza virus expressed from a cloned gene promotes membrane fusion. Nature. 1982 Dec 16;300(5893):658–659. doi: 10.1038/300658a0. [DOI] [PubMed] [Google Scholar]
- White J., Kartenbeck J., Helenius A. Fusion of Semliki forest virus with the plasma membrane can be induced by low pH. J Cell Biol. 1980 Oct;87(1):264–272. doi: 10.1083/jcb.87.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]



