Abstract
The efficient activation of p90rsk by MAP kinase requires their interaction through a docking site located at the C-terminal end of p90rsk. The MAP kinase p42mpk1 can associate with p90rsk in G2-arrested but not in mature Xenopus oocytes. In contrast, an N-terminally truncated p90rsk mutant named D2 constitutively interacts with p42mpk1. In this report we show that expression of D2 inhibits Xenopus oocyte maturation. The inhibition requires the p42mpk1 docking site. D2 expression uncouples the activation of p42mpk1 and p34cdc2/cyclin B in response to progesterone but does not prevent signaling through p90rsk. Instead, D2 interferes with a p42mpk1-triggered pathway, which regulates the phosphorylation and activation of Plx1, a potential activator of the Cdc25 phosphatase. This new pathway that links the activation of p42mpk1 and Plx1 during oocyte maturation is independent of p34cdc2/cyclin B activity but requires protein synthesis. Using D2, we also provide evidence that the sustained activation of p42mpk1 can trigger nuclear migration in oocytes. Our results indicate that D2 is a useful tool to study MAP kinase function(s) during oocyte maturation. Truncated substrates such as D2, which constitutively interact with MAP kinases, may also be helpful to study signal transduction by MAP kinases in other cellular processes.
INTRODUCTION
Xenopus oocytes are naturally arrested in prophase (late G2) of the first meiotic division and are induced to reinitiate meiosis and enter M-phase by progesterone stimulation. This process is called meiotic maturation and is associated with dramatic structural changes in the oocyte. During maturation the nucleus migrates to the cortex of the oocyte, and the nuclear membrane disappears (this is known as germinal vesicle breakdown [GVBD]) concomitant with the appearance of a white spot at the animal pole of the oocyte. The chromosomes also condense and a metaphase spindle assembles. The meiotic maturation is associated with the activation of the ubiquitous M-phase- or maturation-promoting factor (MPF) (Masui and Markert, 1971; Masui and Clarke, 1979), which is required for the initiation of mitosis and is also likely to be responsible for most of the structural changes associated with the progression through M-phase. MPF is a heterodimer of a B-type cyclin and the p34cdc2 protein kinase (reviewed by Nurse, 1990).
An essential requirement for progesterone-induced meiotic reinitiation is the translation of dormant maternal mRNAs stored in the oocyte. De novo synthesis of several proteins takes place during oocyte maturation (Sagata et al., 1988; Nebreda et al., 1995; Barkoff et al., 1998), one of them being the Mos protein kinase (Sagata et al., 1988, 1989; Freeman et al., 1990; Sheets et al., 1995). Mos is an efficient activator of the MAP kinase pathway (Nebreda et al., 1993a; Nebreda and Hunt, 1993; Posada et al., 1993; Shibuya and Ruderman, 1993), and several lines of evidence indicate that the activation of the Xenopus MAP kinase p42mpk1 (which is most similar to the mammalian ERK1/ERK2 MAP kinases) is required for meiotic reinitiation and p34cdc2/cyclin B activation (reviewed by Kosako et al., 1994a; Gotoh and Nishida, 1995). For example, injection of inhibitory anti-MAP kinase kinase antibodies (Kosako et al., 1994b, 1996) or a specific MAP kinase phosphatase (Gotoh et al., 1995) inhibits meiotic reinitiation. On the other hand, injection of constitutively active MAP kinase kinase or thio-phosphorylated p42mpk1 induces meiotic reinitiation in the absence of progesterone (Matsuda et al., 1992; Haccard et al., 1995; Huang et al., 1995). It has also been reported that MPF can trigger p42mpk1 activation, suggesting the existence of a positive feedback loop that links the activation of p42mpk1 and MPF (Gotoh et al., 1991; Matsuda et al., 1992). This may explain why the activation of p42mpk1 and MPF is coupled in progesterone-treated oocytes (Ferrell and Machleder, 1998). A positive feedback loop has also been described between p42mpk1 and the synthesis and/or stability of Mos (Matten et al., 1996; Roy et al., 1996; Howard et al., 1999).
G2-arrested oocytes contain a stockpile of p34cdc2/cyclin B complexes, which are inactivated by the phosphorylation of p34cdc2 on Thr-14 and Tyr-15 (Cyert and Kirschner, 1988; Gautier and Maller, 1991; Kobayashi et al., 1991). The kinase responsible for these inhibitory phosphorylations in oocytes is most likely to be Myt1, a membrane-associated member of the Wee1 protein kinase family (Atherton-Fessler et al., 1994; Kornbluth et al., 1994; Mueller et al., 1995; Palmer et al., 1998). The dephosphorylation of p34cdc2 at the onset of M-phase is triggered by the activation of the phosphatase Cdc25 (Dunphy and Kumagai, 1991; Gautier et al., 1991; Kumagai and Dunphy, 1991; Strausfeld et al., 1991). In G2-arrested oocytes, Cdc25 is maintained inactive by Chk1 phosphorylation and association with 14-3-3 proteins (Nakajo et al., 1999). The activation of the p34cdc2/cyclin B complexes in progesterone-treated oocytes may be brought about by Myt1 inhibition and/or Cdc25 activation. Myt1 and Cdc25 are both hyperphosphorylated in mature oocytes as in M-phase-arrested extracts, and this correlates with the inhibition of Myt1 and the activation of Cdc25 (Izumi et al., 1992; Kumagai and Dunphy, 1992; Mueller et al., 1995; Booher et al., 1997; Palmer et al., 1998). Both proteins can be phosphorylated by p34cdc2/cyclin B complexes in vitro (Hoffmann et al., 1993; Izumi and Maller, 1993; Strausfeld et al., 1994; Booher et al., 1997; Palmer et al., 1998), and in the case of Cdc25 this phosphorylation up-regulates its phosphatase activity (Hoffmann et al., 1993; Izumi and Maller, 1993; Strausfeld et al., 1994). The ability of MPF to regulate Cdc25 (and potentially Myt1) constitutes a positive feedback loop, the so-called MPF autoamplification process, by which a small amount of active p34cdc2/cyclin B would be able to trigger the activation of more p34cdc2/cyclin B from the stored pre-MPF complexes (Masui and Markert, 1971). However, p34cdc2/cyclin B is unlikely to be the triggering kinase that phosphorylates Myt1 and Cdc25 during oocyte maturation. We have recently found that the C-terminal regulatory domain of Myt1 can be efficiently phosphorylated by the p42mpk1-activated protein kinase p90rsk. Moreover, phosphorylation by p90rsk decreases the inhibitory activity of Myt1 on p34cdc2/cyclin B complexes in vitro, thereby providing a potential link between p42mpk1 and MPF activation at the onset of meiotic maturation (Palmer et al., 1998). On the other hand, p90rsk does not phosphorylate the N-terminal regulatory domain of Cdc25 (Palmer et al., 1998), but this is efficiently phosphorylated by the Polo-like kinase of Xenopus Plx1 (Kumagai and Dunphy, 1996). Plx1 phosphorylation can activate Cdc25 in vitro, suggesting that Plx1 may be one of the triggering kinases responsible for the phosphorylation and activation of Cdc25 at the onset of M-phase (Kumagai and Dunphy, 1996; Qian et al., 1998a). A protein kinase that can phosphorylate and activate Plx1 has been recently purified and cloned from Xenopus eggs and named xPlkk1 (Qian et al., 1998b).
p90rsk contains two protein kinase domains (Jones et al., 1988): the N-terminal domain (D1) is required for the phosphorylation of exogenous substrates, and the C-terminal domain (D2) is involved in p90rsk autophosphorylation (Bjørbaek et al., 1995; Leighton et al., 1995; Fischer and Blenis, 1996). Association between p90rsk family members and ERK MAP kinases has been detected in various cell types, including Xenopus oocytes, mammalian fibroblasts, and PC12 cells (Scimeca et al., 1992; Hsiao et al., 1994; Zhao et al., 1996). In G2-arrested Xenopus oocytes the unphosphorylated and inactive p90rsk is complexed with inactive p42mpk1, but the complex dissociates during oocyte maturation concomitant with the phosphorylation and activation of both protein kinases (Hsiao et al., 1994).
There is growing evidence that the efficient phosphorylation of some substrates requires specific sequences, which facilitate the kinase–substrate interaction and are distinct from the phosphoacceptor sites. These sequences, usually referred to as docking sites, were first described to be important for the phosphorylation of the transcription factor c-jun by JNK/MAP kinases (Kallunki et al., 1994). Docking sites have been later identified on Smads phosphorylated by transforming growth factor-β receptors (Chen et al., 1998; Lo et al., 1998), on some cdk2/cyclin A substrates (Adams et al., 1996; Schulman et al., 1998), and on the transcription factor Elk-1 (Yang et al., 1998a,b; Jacobs et al., 1999). The interaction between Elk-1 and activating ERK, JNK, and p38 MAP kinases appears to involve several distinct and overlapping interaction motifs, which have been suggested to work either synergistically or competitively (Jacobs et al., 1999). We and others have also recently characterized a novel MAP kinase docking site located at the C terminus of p90rsk, which is required for the efficient phosphorylation and activation of p90rsk both in vitro and in vivo (Gavin and Nebreda, 1999; Smith et al., 1999). This docking site is specific for ERK MAP kinases and shows no amino acid sequence similarity with other MAP kinase docking sites, suggesting that it may represent the prototype of a novel ERK MAP kinase interaction motif.
In this report we use an N-terminally truncated form of p90rsk named D2, which constitutively interacts with p42mpk1, to investigate the function(s) of p42mpk1 during oocyte maturation. Our results indicate that activation of p42mpk1 normally precedes the activation of pre-MPF in progesterone-treated oocytes. We also found that p42mpk1 can trigger an MPF-independent pathway that leads to the activation of Plx1 and that the activation of p42mpk1 in the absence of pre-MPF activation can trigger nuclear migration in Xenopus oocytes.
MATERIALS AND METHODS
cDNA Cloning and Site-directed Mutagenesis
Full-length Xenopus p90rsk α and the N-terminal (D1, amino acids 1–308) and C-terminal (D2, amino acids 309–733) domains alone were cloned in the FTX5 expression vector as described (Palmer et al., 1998). The mutants D2/KR (Lys-445 changed to Arg), D2Δ43 (stop codon following amino acid 690), and p90rsk 6xAla (phosphorylation sites at positions Ser-221, Thr-359, Ser-363, Ser-380, Thr-571, and Ser-730 changed to Ala) were prepared using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer’s instructions. To prepare D1+43Ct, the C-terminal 43 amino acids of p90rsk were amplified using a 5′ oligonucleotide that introduced an NcoI site at position 2069 of p90rsk (Lys-691 was changed to Met) and a 3′ oligonucleotide that introduced a BamHI site downstream of the stop codon. The PCR product was digested with NcoI and BamHI and subcloned into the corresponding sites of the plasmid FTX5. The resulting plasmid FTX5–43Ct was linearized with NcoI and ligated to the NcoI–NcoI D1 fragment from FXT5-D1.
FTX5-p42mpk1 was prepared by subcloning a PCR fragment containing the p42mpk1 coding sequence from pMalcRI-p42mpk1 (Rouse et al., 1994) into FTX5 previously digested with BamHI and XhoI. The mutant p42mpk1 AEF (Thr-187 and Tyr-189 changed to Ala and Phe, respectively) was prepared using the QuikChange site directed mutagenesis kit. FTX5-Mos was prepared by subcloning a 1.2-kb NcoI–XhoI DNA fragment containing the full-length Xenopus c-mos protooncogene from MLV-Mos (Nebreda et al., 1993a) into NcoI–XhoI digested FTX5.
Xp9 was amplified from a Xenopus oocyte cDNA library using oligonucleotides that created EcoRI sites both upstream of the first ATG and downstream of the stop codon. A PCR product with the same sequence as the cDNA described by Patra and Dunphy (1996) was cloned into EcoRI-digested FTX5.
Oocyte Maturation and Expression of myc-tagged Proteins
In vitro–transcribed mRNAs were obtained from FTX5 constructs linearized with either XbaI (p90rsk, D1, D2, Mos, and Xp9) or XmnI (p42mpk1) using the MEGAscript kit (Ambion, Austin, TX) according to the manufacturer’s instructions. The mRNAs were resuspended in 25 μl of diethylpyrocarbonate-treated water and further diluted 1:5 in diethylpyrocarbonate-treated water before microinjection.
Fully grown oocytes were sorted either manually or after collagenase B treatment (Boehringer Mannheim, Indianapolis, IN; 0.5 mg/ml, 30–60 min) and left at 18°C in mBarth for at least 2 h before injection. Meiotic maturation was induced by incubation with 5 μg/ml progesterone (Sigma, St. Louis, MO) or by injection with 50 nl of either malE-mos protein (40 ng, Nebreda and Hunt, 1993) or Mos mRNA. Maturation was scored by the appearance of a white spot at the animal pole of the oocyte, and GVBD was confirmed after either fixation in 5% trichloroacetic acid or by boiling for 90 s in PBS.
The oocytes were lysed in 10 μl per oocyte histone H1 kinase (H1K) buffer (80 mM β-glycerophosphate, 20 mM EGTA, 15 mM MgCl2, 2 μg/ml leupeptin, 2 μg/ml aprotinin, 2.5 mM benzamidine, 1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride, and 1 mM DTT, pH 7.4) and centrifuged at 14,000 rpm (Eppendorf centrifuge), and the supernatant (oocyte lysate) was stored at −70°C. To prepare concentrated extracts, oocytes were lysed in 1–2 μl per oocyte H1K buffer or lysis buffer (80 mM β-glycerophosphate, pH 7.4, 10 mM EDTA, 1 mM sodium orthovanadate, 2 mM PMSF, 2 μM microcystin, 10 mM p-nitrophenylphosphate, 100 μg/ml leupeptin, and 100 μg/ml aprotinin) and clarified by centrifugation at 14,000 rpm for 90 s. The clear cytoplasm was removed from between the overlying lipid layer and the yolk protein and was further clarified by a second centrifugation at 14,000 rpm for 10 min. Concentrated oocyte extracts were stored in aliquots at −70°C.
Immunoblotting and Immunoprecipitation
Immunoblotting was performed as previously described (Palmer et al., 1998) using the following antibodies: the monoclonal antibody 3E1 (provided by J. Gannon and T. Hunt, Imperial Cancer Research Fund, South Mimms, United Kingdom) for p34cdc2 (Nebreda et al., 1995); the rabbit antiserum 3297.1 for p42mpk1 (Palmer et al., 1998); a mixture of purified anti-Rsk1 and anti-Rsk2 goat antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) for p90rsk; a rabbit anti-Pl×1 antiserum (provided by A. Tavares, C. Avides, and D. Glover, University of Dundee, Dundee, United Kingdom) for Plx1; and the 9E10 monoclonal antibody for myc-tagged proteins.
For the immunoprecipitation of myc-tagged proteins, 10 μl of anti-myc agarose conjugate (Santa Cruz; sc-40 AC) was incubated with 70 μl of oocyte lysate for 2 h at 4°C. The beads were then washed three times in immunoprecipitation buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 0.5% NP-40, 5 mM EGTA, 5 mM EDTA, 20 mM NaF, 100 μM NaVO4, 1 mM PMSF, 2.5 mM benzamidine, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 2 μM microcystin) and once in S6 kinase buffer (see below) and immediately used for an in vitro kinase assay. Coimmunoprecipitation experiments were performed essentially as described above, except that 40–70 μl of concentrated oocyte extracts were used.
For the immunoprecipitation of p42mpk1, we used an anti-ERK2 antibody conjugated to agarose (Santa Cruz). As a control, we used purified rabbit immunoglobulin G (IgG; Sigma) covalently bound to protein A beads using dimethylpimelimidate. Concentrated oocyte extracts (50–100 μl) were precleared with 20 μl of protein A beads for 30 min at 4°C and incubated for 2 h at 4°C with the bead-bound antibody. The beads were then washed three times in either H1K buffer supplemented with 2 μg/ml microcystin, 4 mM p-nitrophenylphosphate, 1 mM PMSF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin or lysis buffer and then used for immunoblotting.
For the immunoprecipitation of Plx1, 7 μl of oocyte lysate were diluted to 200 μl in buffer B (50 mM Tris, pH 8.0, 0.5% NP-40, 120 mM NaCl, 20 mM EDTA, and 1 mM DTT) and mixed with 5 μl of anti-Plx1 antiserum and 20 μl of protein A-Sepharose beads. After 2 h, the beads were washed twice in 10 mM Tris, pH 7.0, 0.1% NP-40, 1 M NaCl, and 1 mM DTT, twice in buffer B, and twice in kinase buffer (20 mM HEPES, pH 7.2, 2 mM DTT, 10 mM MgCl2, 0.1 mM EGTA, and 0.01% Brij 35).
The immunoprecipitation of endogenous p90rsk was performed as described by Palmer et al. (1998) but using a mixture of both anti-Rsk1 and anti-Rsk2 antibodies (Santa Cruz).
In Vitro Kinase Assays
Myc-tagged and endogenous p90rsk immunoprecipitates were assayed for 40 min at room temperature in a final volume of 15 μl of S6 kinase buffer (50 mM 3-morpholinepropanesulfonic acid, pH 7.2, 1 mM DTT, 10 mM MgCl2, 20 mM p-nitrophenylphosphate, 0.1% Triton X-100, 1 mM NaVO3, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM PMSF or 4-(2-aminoethyl)-benzenesulfonyl fluoride) containing 100 μM ATP, 5 μCi of [γ-32P]ATP (3000 Ci/mmol) and 0.4 μg of GST-Myt1 Ct (Palmer et al., 1998).
Plx1 immunoprecipitates were incubated for 30 min at 30°C in 15 μl of kinase buffer containing 67 μM ATP, 5 μCi [γ-32P]ATP, and 1 μg/μl dephosphorylated casein (Sigma).
Phosphorylation reactions were analyzed by SDS-PAGE followed by Coomassie staining and autoradiography.
Gel Filtration
Concentrated oocyte extracts were further clarified by centrifugation at 50,000 rpm in a Beckman Instruments (Palo Alto, CA) TLA 100 rotor at 4°C for 1 h. Samples (100 μl) from 100 oocytes were chromatographed on a Superose 12 column (Pharmacia, Piscataway, NJ; flow rate 0.4 ml/min) equilibrated with lysis buffer containing 80 mM β-glycerophosphate, pH 7.4, 10 mM EDTA, 1 mM sodium orthovanadate, 0.1 mM PMSF, 0.2 μM microcystin, 0.5 mM p-nitrophenylphosphate, 1 μg/ml leupeptin, and 1 μg/ml aprotinin. Fractions of 100 μl were collected and analyzed by immunoblotting. The molecular mass standards were aldolase (158 kDa), BSA (67 kDa), ovalbumin (43 kDa), chymotrypsinogen A (25 kDa), and ribonuclease (13.7 kDa).
RESULTS
A N-Terminally Truncated Form of p90rsk Constitutively Interacts with p42mpk1
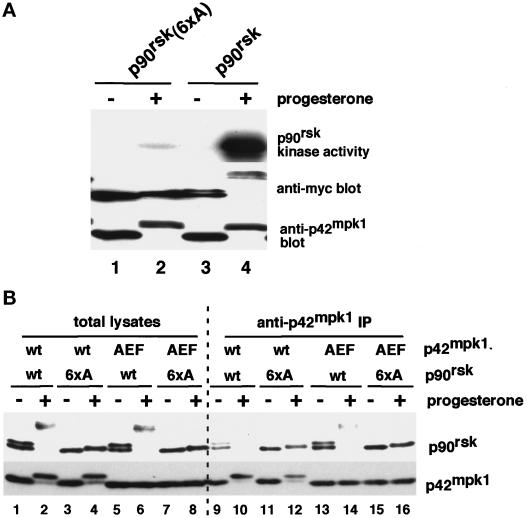
In G2-arrested Xenopus oocytes, p90rsk and p42mpk1 are associated in a complex that dissociates upon activation of both kinases (Hsiao et al., 1994), suggesting that the interaction may be regulated by the phosphorylation state of either p90rsk or p42mpk1 or both. To investigate this possibility, we prepared a p90rsk mutant with the amino acids that are known to be phosphorylated upon activation (Dalby et al., 1998), being replaced by nonphosphorylatable Ala residues (p90rsk/6xA). As expected, this mutated p90rsk was neither hyperphosphorylated nor activated when expressed in progesterone-treated oocytes (Figure 1A, compare lanes 2 and 4 in the upper and middle panels). We also prepared a p42mpk1 mutant in which the sequence TEY in the activation loop was changed to AEF to prevent phosphorylation. The wild-type and mutant forms of both p90rsk and p42mpk1 were coexpressed in oocytes, which were either left untreated or treated with progesterone to induce the activation of the p42mpk1 pathway and meiotic reinitiation (Figure 1B). The association between p90rsk and p42mpk1 was determined by anti-p42mpk1 immunoprecipitation followed by immunoblotting using an anti-p90rsk antibody (Figure 1B). The wild-type p42mpk1 associated with the wild-type p90rsk only in G2-arrested oocytes (Figure 1B, lanes 9 and 10). Similarly, p42mpk1/AEF interacted much more efficiently with the unphosphorylated than with the phosphorylated p90rsk (Figure 1B, lanes 13 and 14), suggesting that the interaction between p42mpk1 and p90rsk might be regulated by the phosphorylation of p90rsk. Consistent with this possibility, the nonphosphorylatable mutant p90rsk/6xA was able to interact with p42mpk1 both in prophase- and progesterone-treated oocytes (Figure 1B, lanes 11, 12, 15, and 16), indicating that the phosphorylation of p90rsk is responsible for the dissociation of the p42mpk1/p90rsk complex.
Figure 1.
Mutation to alanine of six phosphorylatable residues in p90rsk impairs its activation by progesterone and its ability to dissociate from p42mpk1. (A) Lysates were prepared from untreated or progesterone-treated oocytes expressing either myc-tagged p90rsk or p90rsk (6xA) and were analyzed by immunoblotting using anti-myc and anti-p42mpk1, as indicated. Anti-myc immunoprecipitates were prepared from the same oocyte lysates, and their associated kinase activity was assayed using GST-Myt1 as an in vitro substrate. (B) Lysates were prepared from either untreated or progesterone-treated oocytes expressing the indicated p90rsk and p42mpk1 proteins and were immunoprecipitated with anti-p42mpk1 antibodies. The total lysates (lanes 1–8) and the anti-p42mpk1 immunoprecipitates (lanes 9–16) were analyzed by immunoblotting with anti-p90rsk and anti-myc antibodies.
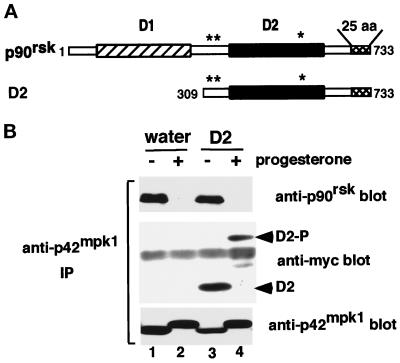
We have previously shown that a truncated form of p90rsk named D2, which lacks the N-terminal kinase domain (Figure 2A), was able to bind to p42mpk1. This interaction was mediated by a p42mpk1 docking site, which was located at the C-terminal 25 amino acids of p90rsk (Gavin and Nebreda, 1999). Moreover, both nonphosphorylated and phosphorylated GST-D2 were able to bind p42mpk1 with similar affinity in pulldown experiments (Gavin and Nebreda, 1999). To further characterize the ability of this truncated p90rsk to interact with p42mpk1, myc-tagged D2 was expressed in Xenopus oocytes, which were then either treated with progesterone or left untreated. The association between p42mpk1 and either p90rsk or D2 was determined by anti-p42mpk1 immunoprecipitation followed by immunoblotting using either anti-p90rsk or anti-myc antibodies (Figure 2B). As expected, p90rsk was found to coimmunoprecipitate with p42mpk1 in control oocytes (Figure 2B, lanes 1 and 3) but not in progesterone-treated oocytes, in which p90rsk is phosphorylated (Figure 2B, lanes 2 and 4). In contrast, D2 associated with p42mpk1 both in control and in progesterone-treated oocytes (Figure 2B, lanes 3 and 4, anti-myc blot). Moreover, the electrophoretic mobility of D2 was significantly reduced upon progesterone treatment (Figure 2B, lanes 3 and 4), suggesting that it became hyperphosphorylated. This is consistent with previous reports showing that the major phosphorylation sites for p42mpk1 map in the C-terminal half of p90rsk (Dalby et al., 1998).
Figure 2.
The C-terminal D2 domain of p90rsk constitutively interacts with endogenous p42mpk1 in oocytes. (A) Schematic representation of full-length p90rsk indicating the two kinase domains, D1 and D2, the p42mpk1 docking site located in the last 25 amino acids (25 aa) and the three p42mpk1 phosphorylation sites (asterisks). The N-terminally truncated p90rsk protein D2 is also shown. (B) Oocytes injected with water (lanes 1 and 2) or expressing myc-tagged D2 (lanes 3 and 4) were induced to mature by progesterone or not as indicated. Anti-p42mpk1 immunoprecipitates prepared from the oocyte lysates were analyzed by immunoblotting with anti-p90rsk, anti-myc and anti-p42mpk1 antibodies. The unphosphorylated and hyperphosphorylated D2 are indicated as D2 and D2-P, respectively.
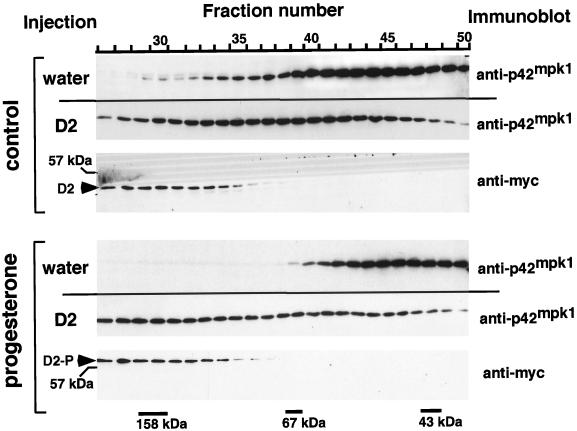
We further tested the ability of D2 to interact with p42mpk1 by gel filtration chromatography. Oocyte lysates were chromatographed on a Superose 12 column, and the fractions were analyzed by immunoblotting (Figure 3). In lysates prepared from G2-arrested oocytes, p42mpk1 exhibited a broad size distribution, which, as described by others (Hsiao et al., 1994), may correspond to two overlapping peaks of ∼40 and 110 kDa (Figure 3, control/water). After progesterone treatment, the high-molecular-weight peak of p42mpk1 disappeared (Figure 3, progesterone/water), consistent with p42mpk1 being mainly present in its monomeric form (Hsiao et al., 1994). Interestingly, in lysates prepared from D2-expressing oocytes, a significant amount of p42mpk1 was shifted toward a higher-molecular-weight form, which coeluted with D2 (Figure 3, control/D2). Moreover, the pattern of elution of p42mpk1 (and D2) was not changed by progesterone treatment of the oocytes, despite the hyperphosphorylation of D2 (Figure 3, progesterone/D2). These results confirm that D2 interacts with p42mpk1 in the oocytes and that, in contrast to full-length p90rsk, the association between D2 and p42mpk1 does not appear to be regulated by the phosphorylation state of D2.
Figure 3.
D2 expressed in oocytes comigrates with endogenous p42mpk1 in a high-molecular-weight complex upon gel filtration chromatography. Oocytes injected with water or expressing myc-tagged D2 were induced to mature by progesterone or not as indicated. The oocyte lysates were separated by gel filtration chromatography on Superose 12, and the fractions were analyzed by immunoblot using anti-myc and anti-p42mpk1 antibodies. The elution of the molecular weight markers is indicated at the bottom. The unphosphorylated and hyperphosphorylated D2 are indicated as D2 and D2-P, respectively.
Overexpression of the p42mpk1 Docking Site Negatively Regulates Oocyte Maturation
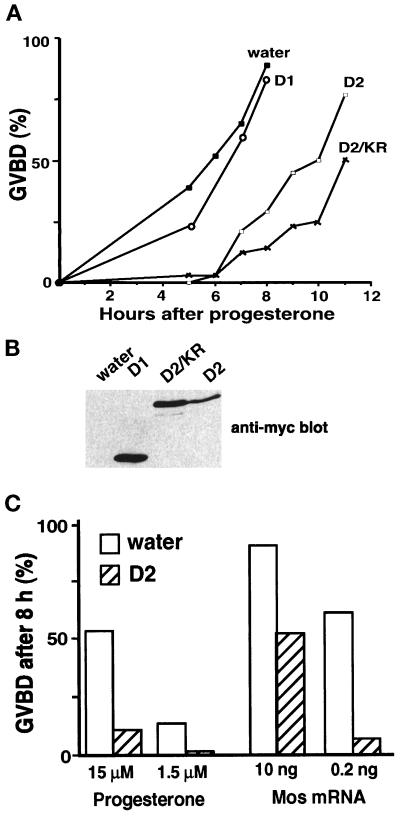
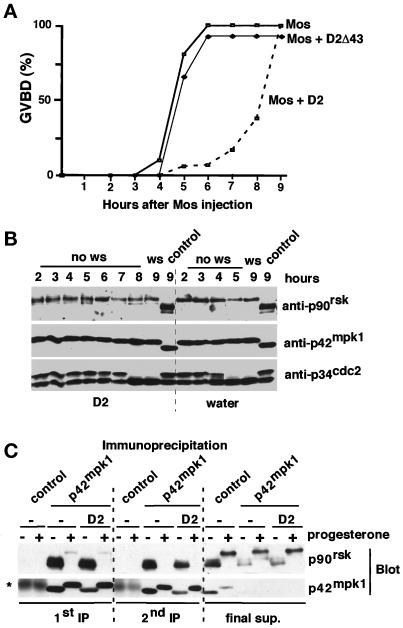
The overexpression of the p42mpk1 docking site located at the C terminus of p90rsk interferes with the phosphorylation of p90rsk by p42mpk1 in reticulocyte lysates (Gavin and Nebreda, 1999). We wanted to investigate whether D2, which contains the p42mpk1 docking site and constitutively interacted with p42mpk1, could also inhibit signaling by p42mpk1 in oocytes. We observed that expression of D2 in oocytes significantly delayed the kinetics of progesterone-induced maturation (Figure 4A). As a control, expression of similar levels of the D1 catalytic domain alone (Figure 4B) did not affect meiotic reinitiation (Figure 4A). Moreover, a catalytically inactive mutant of D2 (D2/KR) was as efficient as D2 in delaying oocyte maturation (Figure 4A), indicating that the autophosphorylation activity of D2 (Fischer and Blenis, 1996; our unpublished data) was not required for the inhibition of oocyte maturation. The inhibitory effect of D2 was also observed when oocyte maturation was induced by the injection of either Mos mRNA or bacterially produced Mos protein (Figure 4C; also see Figure 7A).
Figure 4.
Expression of D2 inhibits oocyte maturation. (A) Groups of 25–30 oocytes were injected with water or mRNAs encoding the indicated proteins and after overnight incubation were treated with progesterone and scored for GVBD. D2 was found to inhibit or delay progesterone-induced maturation in 27 of 34 experiments. (B) Lysates were prepared from the same oocytes as in A after overnight incubation and analyzed by immunoblotting using anti-myc antibodies. (C) Groups of 25–30 oocytes were injected with water or D2 mRNA, incubated overnight, and then either injected with Mos mRNA or incubated with progesterone. GVBD was scored 8 h later.
Figure 7.
Expression of D2 does not affect the Mos-induced activation of p42mpk1 and p90rsk. (A) Groups of 25–30 oocytes were injected with water or mRNAs encoding either D2 or D2Δ43 and after overnight incubation were injected with malE-mos protein and scored for GVBD. (B) Lysates were prepared from the same oocytes as in A and analyzed by immunoblotting with anti-p90rsk, anti-p42mpk1, and anti-p34cdc2 antibodies. (C) Groups of 100 oocytes were injected with either water or D2 mRNA, incubated overnight, and then treated or not treated with progesterone for 12 h. Oocyte lysates were subjected to two sequential rounds of immunoprecipitation using either anti-p42mpk1 or control rabbit IgG. The immunoprecipitates and the final supernatant (after the second round of immunoprecipitation) were analyzed by immunoblotting with anti-p90rsk and anti-p42mpk1 antibodies. The asterisk indicates a nonspecific band that probably corresponds to control IgG leaking from the beads.
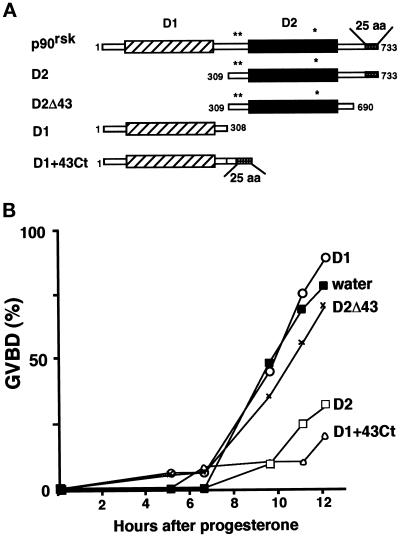
To investigate whether the inhibitory effect of D2 required the p42mpk1 docking site, we used a truncated form of D2, which lacks the last 43 amino acids (Figure 5A, D2Δ43) and does not interact with p42mpk1 (Gavin and Nebreda, 1999). We also used a chimera in which the last 43 amino acids of p90rsk have been added to the C terminus of D1 (Figure 5A, D1+43Ct) resulting in a fusion protein that can interact with p42mpk1 in oocytes (Gavin and Nebreda, 1999). Oocytes were injected with either water or the mRNAs encoding these proteins and then were treated with progesterone. We found that truncation of the C-terminal 43 amino acids abrogated the inhibitory effect of D2 (Figure 5B, D2Δ43). Furthermore, in contrast to D1 alone, which had no effect on progesterone-induced maturation, D1+43Ct also delayed the kinetics of maturation to the same extent as D2 (Figure 5B). These results indicate that overexpression of the p42mpk1 docking site is able to inhibit the meiotic reinitiation in oocytes.
Figure 5.
Expression of the p42mpk1 docking site is necessary and sufficient to inhibit oocyte maturation. (A) Schematic representation of the recombinant proteins expressed in oocytes. (B) Groups of 25–30 oocytes were injected with water or mRNAs encoding the indicated proteins and after overnight incubation were treated with progesterone and scored for GVBD.
D2 Uncouples the Activation of p42mpk1 and MPF in Response to Progesterone
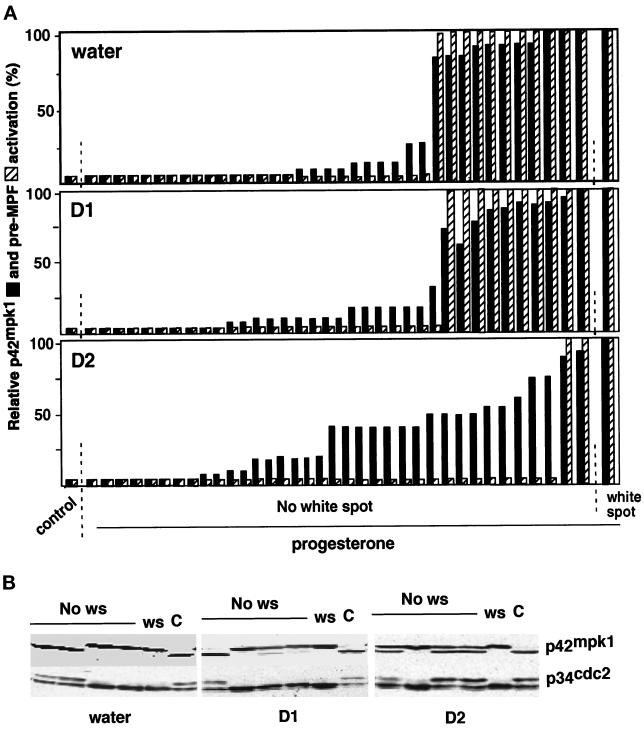
The observation that D2 can also interfere with oocyte maturation induced by Mos (Figures 4C) suggested that it might inhibit downstream of p42mpk1. To test this possibility, oocytes were injected with either water or the D1 or D2 mRNAs and then treated with progesterone. When the oocytes started to mature (as determined by the appearance of a white spot), single oocytes were taken and analyzed by immunoblotting with anti-p42mpk1 and anti-p34cdc2 antibodies. The activation of p42mpk1 was scored based on the upward mobility shift of the p42mpk1 protein in SDS-PAGE, which correlates with its phosphorylation and the activation of pre-MPF for the disappearance of the slow-migrating p34cdc2 band, which corresponds to tyrosine-phosphorylated and cyclin B-bound p34cdc2 (Figure 6). We found that in every progesterone-treated oocyte that had a white spot there was activation of both p42mpk1 and pre-MPF, independently of whether they had been injected with water or the D1 or the D2 mRNAs (Figure 6, A, white spot, and B, ws, show a representative example). When progesterone-treated oocytes without a white spot were analyzed, we found two categories of oocytes from each injection with either water or the D1 mRNA (Figure 6, A, No white spot, and B, No ws): oocytes with neither p42mpk1 nor pre-MPF activation, which looked like G2-arrested oocytes (Figure 6, A, control, and B and C) or oocytes with both kinases in an active state as in oocytes with a white spot (these oocytes were probably about to undergo maturation when they were collected). This confirmed previous work showing that the activation of p42mpk1 is always coupled to the activation of preformed p34cdc2/cyclin B complexes (pre-MPF) in response to progesterone stimulation (Ferrell and Machleder, 1998). Interestingly, when we analyzed single progesterone-treated oocytes expressing D2, we found that about half of the oocytes without a white spot fell into a third category in which p42mpk1 was partially activated (>40% of the p42mpk1 protein showed reduced electrophoretic mobility), but p34cdc2 was tyrosine phosphorylated, and thus preMPF was still inactive (Figure 6, A and B, D2).
Figure 6.
Expression of D2 uncouples the activation of p42mpk1 and pre-MPF in response to progesterone. (A) Groups of 30 oocytes were injected with water or mRNAs encoding either D1 or D2 and after overnight incubation were treated with progesterone. Every hour, starting 3 h after the addition of progesterone, two or three progesterone-treated oocytes without a white spot were transferred to dry ice. Untreated oocytes (control) and progesterone-treated oocytes that had a white spot were also taken for comparison. Oocyte lysates were prepared from single oocytes and analyzed by immunoblotting with anti-p42mpk1 and anti-p34cdc2 antibodies. Each bar represents a single oocyte. A total of 30 oocytes were analyzed in three independent experiments. The activation of p42mpk1 was scored by the phosphorylation of the protein, which causes its upward mobility shift in the blots. The activation of pre-MPF was scored for the disappearance in the blots of the slow migrating p34cdc2 form, which corresponds to Tyr-phosphorylated cyclin B-bound p34cdc2. (B) Representative examples of the p42mpk1 and p34cdc2 immunoblots from single oocytes described in A.
The uncoupling between the activation of p42mpk1 and MPF in response to progesterone suggested that D2 interferes with p42mpk1 signaling in oocytes. These results also indicate that p42mpk1 activation normally precedes the activation of pre-MPF during progesterone-induced oocyte maturation. The partial activation of p42mpk1 is probably a consequence of the lack of an MPF-activated positive feedback loop, which has been proposed to be required for full p42mpk1 activation in response to progesterone (Gotoh et al., 1991; Matsuda et al., 1992). Consistent with this possibility, we observed that D2 expression in Mos-injected oocytes delayed GVBD (Figure 7A) and the activation of pre-MPF (Figure 7B, lower panel) but did not interfere with the activation of p42mpk1 (Figure 7B, middle panel). This suggested that D2 does not directly prevent p42mpk1 activation and is more likely to act downstream of p42mpk1, uncoupling the activation of p34cdc2/cyclin B from the activation of p42mpk1.
D2 Does Not Prevent p90rsk Activation
We have recently shown that after activation by p42mpk1, p90rsk associates with and phosphorylates the p34cdc2 inhibitory kinase Myt1 (Palmer et al., 1998). Thus, one possibility is that D2 uncouples p42mpk1 from p34cdc2/cyclin B activation by interfering with p42mpk1 signaling through p90rsk.
We observed, however, that overexpression of D2 inhibits neither the phosphorylation nor the activation of p90rsk when p42mpk1 was activated by Mos injection (Figure 7B, upper panel; see Figure 9D). Furthermore, we could detect approximately the same amount of p90rsk in the anti-p42mpk1 immunoprecipitates prepared from either control or D2-expressing oocytes (Figure 2B), suggesting that D2 did not disrupt the endogenous p42mpk1/p90rsk complexes in G2-arrested oocytes. These results were confirmed when two consecutive rounds of anti-p42mpk1 immunoprecipitation were performed (Figure 7C).
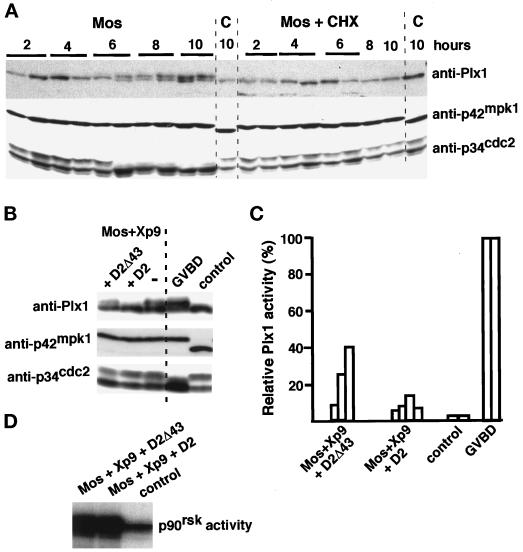
Figure 9.
Activation of Plx1 by Mos requires protein synthesis and is inhibited by expression of D2. (A) Groups of 30 oocytes that had been either preincubated for 45 min or not with cycloheximide (CHX, 50 μg/ml) were injected with malE-mos protein. At the indicated times oocytes were individually transferred to dry ice. Uninjected oocytes (C) were also taken for comparison. Oocyte lysates were prepared from single oocytes and analyzed by immunoblotting with anti-Plx1, anti-p42mpk1, and anti-p34cdc2 antibodies. (B) Groups of 25 oocytes were injected first with mRNAs encoding either D2 or D2Δ43 and 2 h later with Xp9 mRNA. After overnight incubation the oocytes were injected again with malE-mos protein and incubated for 12 h. Lysates prepared from three oocytes were analyzed by immunoblotting with anti-Plx1, anti-p42mpk1, and anti-p34cdc2 antibodies. Uninjected oocytes (control) and Mos-matured oocytes (GVBD) were also analyzed in parallel. (C) Groups of 35 oocytes were injected first with mRNAs encoding either D2 or D2Δ43 and 2 h later with Xp9 mRNA. After overnight incubation the oocytes were injected again with malE-mos protein, and 4 h later oocytes that did not have a normal white spot were individually taken every hour and stored on dry ice. Lysates were prepared from single oocytes and immunoprecipitated with anti-Plx1 antibodies to assay their associated kinase activity using casein as an in vitro substrate. Each bar represents single oocytes that were uninjected (control), malE-mos injected and matured (GVBD), or injected with malE-mos plus Xp9 and either D2 or D2Δ43. The Plx1 activity taken as 100% in mature oocytes (GVBD) was usually 30- to 40-fold higher than in control oocytes. (D) Groups of 25 oocytes were injected first with mRNAs encoding Xp9 and either D2 or D2Δ43 and later with malE-mos protein as in A. After 12 h, lysates were prepared from three oocytes, immunoprecipitated with anti-p90rsk antibodies, and assayed for kinase activity using GST-Myt1 Ct as an in vitro substrate. Uninjected oocytes (control) were also analyzed in parallel.
A Possible Role for p42mpk1 Upstream of Plx1
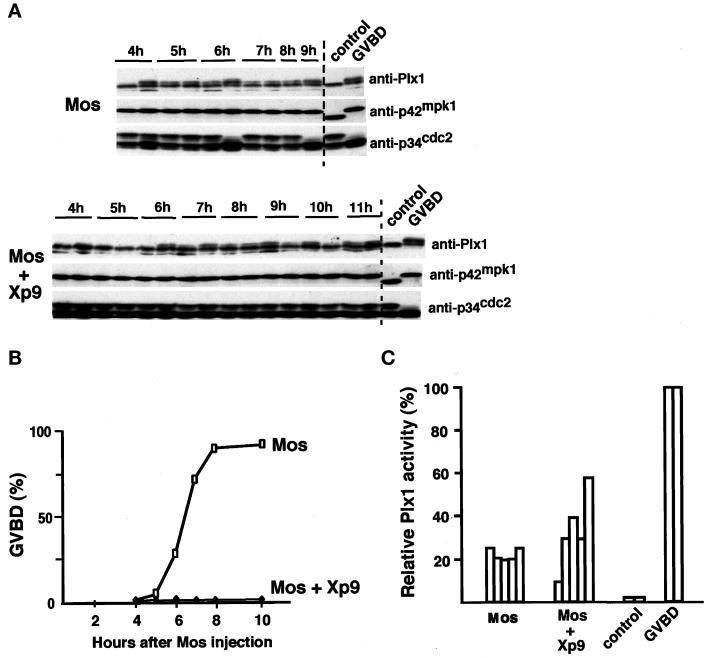
Because D2 did not interfere with p42mpk1 signaling through p90rsk, we investigated alternative pathways that could potentially be regulated by p42mpk1. One obvious candidate was the Plx1/Cdc25 pathway (Kumagai and Dunphy, 1996; Abrieu et al., 1998; Qian et al., 1998a). To investigate the possible role of p42mpk1 upstream of Plx1, oocytes were injected with Mos to activate p42mpk1. We then analyzed by immunoblotting the phosphorylation state of p42mpk1, p34cdc2, and Plx1 in single oocytes. As expected, the appearance of the white spot always correlated with the activation of pre-MPF (tyrosine dephosphorylation of p34cdc2) and with the phosphorylation of both p42mpk1 and Plx1 (Figure 8A, GVBD). When Mos-injected oocytes not showing a white spot were analyzed, we observed some Plx1 phosphorylation occurring before any detectable tyrosine dephosphorylation of p34cdc2 (Figure 8A, Mos). This result was observed in three experiments of five; in the other two experiments Plx1 phosphorylation was coupled to the activation of preMPF (see example in Figure 9A). To confirm that Plx1 could be phosphorylated independently of pre-MPF activation, we injected the small p34cdc2-binding protein Xp9, which can interfere with p34cdc2/cyclin B activation and delay M-phase entry in Xenopus egg extracts (Patra and Dunphy, 1996). We found that expression of Xp9 inhibited Mos-induced (Figure 8B) as well as progesterone-induced (our unpublished results) oocyte maturation, as measured by the absence of GVBD. These Xp9-injected oocytes, however, had a less pigmented area, which resembled an irregular white spot (see below and Figure 10A), although the nucleus was still detected upon dissection of fixed oocytes. The activation of p42mpk1 by Mos was not affected in Xp9-expressing oocytes, whereas p34cdc2/cyclin B activation was either blocked or severely delayed depending on the batch of oocytes (Figure 8A). However, Plx1 phosphorylation was still observed in the absence of pre-MPF activation in oocytes coinjected with Mos and Xp9 (Figure 8A).
Figure 8.
Mos induces partial phosphorylation and activation of Plx1 independently of pre-MPF activation. (A) Groups of 35 oocytes were injected with either Xp9 mRNA or water and after overnight incubation were injected again with malE-mos protein. At the indicated times injected oocytes that did not have a normal white spot were transferred to dry ice. Uninjected oocytes (control) and malE-mos matured oocytes (GVBD) were also taken for comparison. Lysates were prepared from single oocytes, and half of this lysate was analyzed by immunoblotting with anti-Plx1, anti-p42mpk1, and anti-p34cdc2 antibodies. (B) Kinetics of maturation of the same oocytes as in A. (C) Lysates corresponding to half an oocyte (the second half of the lysates prepared from single oocytes that in A were used for immunblotting) were immunoprecipitated with anti-Plx1 antibodies, and their associated kinase activity was assayed using casein as an in vitro substrate. Each bar represents single oocytes that were uninjected (control), malE-mos injected and matured (GVBD), or injected with malE-mos either alone or plus Xp9 but had no GVBD (these corresponded to the oocytes that in A showed partial Plx1 phosphorylation but had no preMPF activation). The Plx1 activity taken as 100% in mature oocytes (GVBD) was usually 30- to 40-fold higher than in control oocytes.
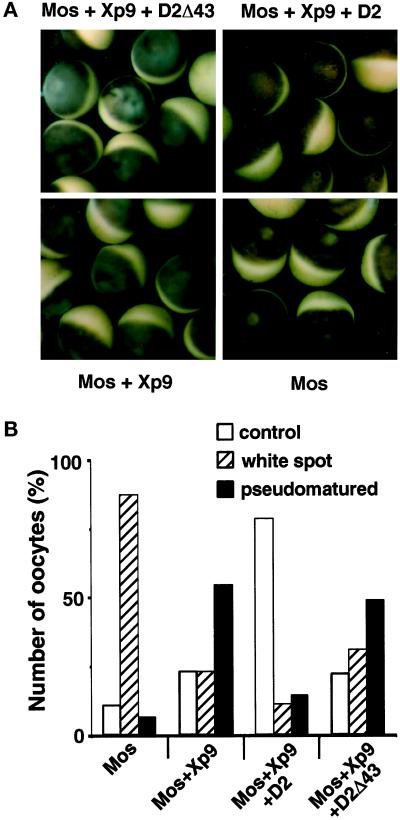
Figure 10.
The sustained activation of p42mpk1 in the absence of pre-MPF activation produces morphological changes in the oocytes. (A) Oocytes were injected with Xp9 mRNA alone or together with either D2 or D2Δ43 mRNAs, incubated overnight, and then injected again with malE-mos protein. Pictures were taken 10 h after malE-mos injection. (B) Oocytes were injected as in A and 8–24 h later were fixed by boiling for 90 s in PBS. After dissection, the oocytes were monitored for the presence of the nucleus (control), GVBD (white spot), or nuclear migration (pseudomatured). The results represent four independent experiments with ∼40 oocytes being dissected for each treatment.
The early Plx1 phosphorylation in Mos-injected oocytes was associated with a partial increase in the kinase activity of Plx1 immunoprecipitates (usually 20–50% of the activity measured in GVBD oocytes) (Figure 8C), in agreement with previous reports showing that the phosphorylation of Plx1 correlates with its activation (Qian et al., 1998a; Karaiskou et al., 1998). These results suggested that the activation of p42mpk1 could lead to partial Plx1 activation. However, full Plx1 activation apparently required the activation of MPF, which is consistent with the existence of a positive feedback loop linking p34cdc2/cyclin B and Plx1 (Abrieu et al., 1998; Karaiskou et al., 1998). Interestingly, although p42mpk1 can be activated as early as 1 h after Mos injection, the phosphorylation and activation of Plx1 was detected only 3–5 h later (Figure 8A). This suggested that the link between p42mpk1 and Plx1 was not direct. To determine whether Plx1 activation by p42mpk1 might require protein synthesis, oocytes were preincubated with cycloheximide before injection of the recombinant Mos protein. We observed that the phosphorylation of Plx1 induced by Mos injection was completely inhibited in the presence of cycloheximide (Figure 9A). The same results were obtained when Xp9 was overexpressed before Mos injection to uncouple Plx1 phosphorylation from the activation of pre-MPF. Altogether these results suggested that p42mpk1 can induce partial phosphorylation and activation of Plx1 via a pathway that requires protein synthesis but is independent of MPF activity. We then investigated whether D2 expression could interfere with the p42mpk1-dependent phosphorylation of Plx1. For this experiment, we coexpressed in oocytes Xp9 with either D2 or D2Δ43 and then injected Mos protein to activate p42mpk1. We observed that expression of D2 but not D2Δ43 inhibited Plx1 phosphorylation in Mos/Xp9-injected oocytes, whereas p90rsk activation was not significantly affected (Figure 9, B and D). This also correlated with a reduction in the extent of Plx1 activation in D2 versus D2Δ43-expressing oocytes (Figure 9C). The results suggested that D2 interfered with this pathway and further supports that the early Plx1 phosphorylation observed after Mos injection is mediated by p42mpk1.
We also found that in oocytes coinjected with Mos and Xp9 the animal pole tends to become either mottled or opaque, suggesting that sustained p42mpk1 activation in the absence of MPF activity may have some unexpected effects. These oocytes also had a less pigmented area, which resembled a kind of irregular white spot (Figure 10A, Mos+Xp9). However when the oocytes were fixed and dissected, ∼50% of them still contained a nucleus, which was usually located closer to the cortex than in uninjected oocytes and sometimes was also flatter in shape (we refer to these oocytes as pseudomatured; Figure 10B). In the same experiment, the majority of Mos-injected oocytes showed a normal white spot, and the nucleus was not detected inside (Figure 10, A and B, Mos). This suggested that some of the morphological changes associated with meiotic maturation, such as the germinal vesicle migration, can occur independently of pre-MPF activation. A role for p42mpk1 in this process was confirmed by the observation that when D2 was expressed together with Mos and Xp9, the pseudomaturation was blocked, and both the oocytes and their nucleus had the appearance of normal uninjected oocytes (Figure 10, A and B, Mos+Xp9+D2). In contrast, the expression of D2Δ43 did not block oocyte pseudomaturation (Figure 10, A and B, Mos+Xp9+D2Δ43), consistent with the inability of this truncated form to associate with p42mpk1. These results suggest that nuclear migration may be regulated by the activation of p42mpk1; however, GVBD appears to require MPF activation.
DISCUSSION
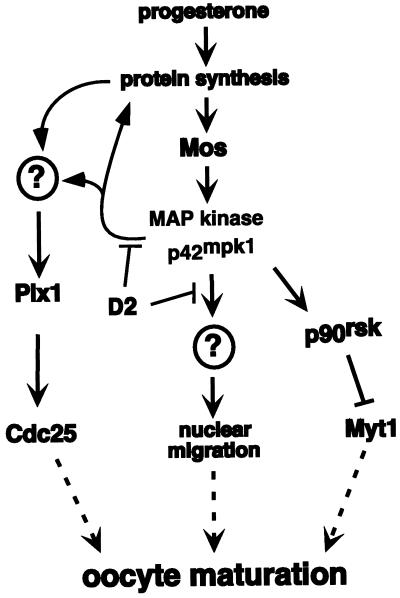
In this report we show that expression of D2, an N-terminally truncated p90rsk that contains a MAP kinase docking site, interferes with Xenopus oocyte maturation. D2 inhibition is mediated by the p42mpk1 docking site and uncouples the activation of p42mpk1 and p34cdc2/cyclin B in response to progesterone. The uncoupling does not correlate with the inhibition of p90rsk, suggesting that p42mpk1 can target pre-MPF activation through alternative pathways. Consistent with this hypothesis, we found that in Mos-injected oocytes the activation of p42mpk1 is followed by the partial phosphorylation and activation of Plx1 via a pathway that requires protein synthesis. Plx1 activation triggered by p42mpk1 was independent of pre-MPF activation and was decreased by D2 expression in the oocytes. We also provide evidence that D2 interferes with nuclear migration triggered by the sustained activation of p42mpk1 in the absence of MPF activity (Figure 11).
Figure 11.
A summary of possible p42mpk1 functions during Xenopus oocyte maturation. Progesterone stimulates the translation of maternal mRNAs and triggers the synthesis of the protein kinase Mos, which activates p42mpk1 via MAP kinase kinase. p42mpk1 activates p90rsk, which in turn down-regulates the p34cdc2 inhibitory kinase Myt1. Our results indicate that D2 interferes with p42mpk1 signaling in oocytes but does not affect the activation of p42mpk1 and p90rsk. Instead, D2 interferes with the phosphorylation and activation of Plx1. This pathway, which may lead to Cdc25 up-regulation, is triggered by p42mpk1 independently of pre-MPF activation but requires protein synthesis. Expression of D2 also inhibits the nuclear migration and pseudomaturation, which appears to be triggered by the sustained activation of p42mpk1 in the absence of MPF activation.
A Regulated Interaction between p42mpk1 and p90rsk Is Essential for Signal Transduction by p42mpk1
The efficient activation of p90rsk requires binding to the Xenopus ERK MAPK p42mpk1 through a specific docking site located in the last 25 amino acids of p90rsk. In G2-arrested oocytes, this interaction is regulated, and the p90rsk/p42mpk1 complex dissociates during maturation (Hsiao et al., 1994). We found that in contrast to wild-type p90rsk, a mutant with Ala substitutions in the six residues that are known to be phosphorylated upon p90rsk activation (p90rsk/6xA) was able to interact with both the inactive and the active p42mpk1. This suggests that the phosphorylation state of p90rsk regulates the interaction between the full-length p90rsk and p42mpk1. However, D2, which lacks the N-terminal kinase domain but is efficiently phosphorylated by p42mpk1 (Fischer and Blenis, 1996; Dalby et al., 1998; Gavin and Nebreda, 1999), can still constitutively interact with p42mpk1. This suggests that upon p90rsk phosphorylation, the D1 kinase domain is somehow required for the dissociation of the p90rsk/p42mpk1 complex. D1 has been reported to phosphorylate Ser-730 of p90rsk, which is located in the p42mpk1 docking site (Dalby et al., 1998), but this is unlikely to prevent the binding to p42mpk1, because Ser-730 is apparently phosphorylated both in active and inactive p90rsk (Dalby et al., 1998). An alternative possibility is that the phosphorylation of p90rsk induces some conformational change, so that D1 will then sterically prevent p42mpk1 from accessing its docking site in p90rsk. Finally, it is also possible that the D1 kinase domain is required for the interaction of phosphorylated p90rsk with other proteins, which in turn might prevent p42mpk1 binding. Interestingly, the C-terminal regulatory domain of Myt1 can specifically bind to the phosphorylated form of p90rsk and this association requires the full-length p90rsk (Palmer et al., 1998). It is thus possible that p90rsk interaction with Myt1 prevents p42mpk1 binding.
The observation that a truncated form of p90rsk, which constitutively binds to p42mpk1 can inhibit signaling by p42mpk1, suggests an important role for the regulated interaction between p42mpk1 and p90rsk. The association between these two kinases in G2-arrested oocytes, in which p42mpk1 is inactive, would ensure the preferential phosphorylation of the bound p90rsk. However, upon progesterone stimulation, dissociation of the complex is probably necessary for the accessibility of p42mpk1 and p90rsk to other substrates. Expression of peptides based on the sequence of an ERK MAP kinase docking site from Elk-1 has been also reported to inhibit Xenopus oocyte maturation (Jacobs et al., 1999). Thus, truncated substrates and docking site peptides may be helpful inhibitory tools to study signal transduction by MAP kinases. They might also allow the differential inhibition of specific MAP kinase pathways, because some docking sites appear to target specific MAP kinase family members. Furthermore, these tools might preferentially inhibit particular subsets of substrates, because their ability to compete with endogenous substrates may depend on the relative affinities of the different docking sites for the MAP kinases.
The Inhibitory Effect of D2 Requires Binding to p42mpk1
The inhibitory effect of D2 on meiotic reinitiation does not depend on its kinase activity, suggesting that D2 inhibits by interaction with endogenous proteins and not catalytically. Fragments of monomeric enzymes can sometimes work in a dominant negative way by interfering with the folding of the wild-type endogenous protein (Michaels et al., 1996). However, it appears unlikely that D2 works by targeting p90rsk in a similar way. We have not detected interactions between D2 and either p90rsk or the D1 or D2 kinase domain alone in a yeast two-hybrid system (our unpublished results). Furthermore, we showed that D2 associates with endogenous p42mpk1 in the oocytes (which is recruited to higher-molecular-weight complexes), whereas the elution pattern of p90rsk in gel filtration was unchanged upon D2 expression (our unpublished results). These results together with the observation that D2 inhibition is mediated by the p42mpk1 docking site suggest that the inhibitory effect of D2 is brought about by binding to p42mpk1.
A New Pathway That Links the Activation of p42mpk1 and p34cdc2/Cyclin B during Oocyte Maturation
We have taken advantage of the inhibitory effect of D2 to investigate the function of p42mpk1 during Xenopus oocyte maturation. We found that D2 expression uncouples the activation of p42mpk1 and p34cdc2/cyclin B. This confirms previous results showing that the interference with p42mpk1 activation prevents meiotic reinitiation and p34cdc2/cyclin B activation (Kosako et al., 1994b, 1996; Gotoh et al., 1995). However, in contrast to previous studies in which the activation of p42mpk1 was also prevented, we use a tool that does not block p42mpk1 activation but inhibits downstream of p42mpk1 and prevents the activation of p34cdc2/cyclin B. Thus, our work indicates that p42mpk1 activation normally precedes the activation of pre-MPF during progesterone-induced meiotic maturation.
We found that D2 did not disrupt the preformed p90rsk/p42mpk1 complexes present in G2-arrested oocytes. This observation is surprising because we have not detected significant differences in the affinities of either D2 or p90rsk for p42mpk1. One possible explanation is that in G2-arrested oocytes the interaction between p42mpk1 and p90rsk might not be dynamic. In support of this possibility, when either p42mpk1 or full-length p90rsk is overexpressed, it does not bind to the endogenous partners (our unpublished results); however, the overexpressed p42mpk1 and p90rsk are able to interact with each other (Figure 1B). These observations suggest that there may be mechanisms to stablilise the preformed p90rsk/p42mpk1 complexes in oocytes. Whether this is due to the binding of additional proteins that stabilize the complex or to the localization of the complexes in particular subcellular compartments where they are not accessible to the exogenous, overexpressed proteins remains to be investigated. Consistent with the observation that D2 did not disrupt the preformed p90rsk/p42mpk1 complex, D2 did not delay the kinetics of phosphorylation and activation of p90rsk by p42mpk1, suggesting that D2 does not interfere with the p90rsk/Myt1 pathway. Thus, p42mpk1 might target pre-MPF activation through alternative pathways. In G2-arrested oocytes only part of the p42mpk1 is found associated with p90rsk, and our gel-filtration experiments suggest that D2 interacts with the p42mpk1 subpopulation, which is not associated with p90rsk. This p42mpk1 subpopulation is likely to target substrates other than p90rsk, which might also participate in the regulation of p34cdc2/cyclin B activity (and hence the activation of pre-MPF) during oocyte maturation. We cannot rule out, however, that upon progesterone stimulation D2 might also interact with the active p42mpk1 released from the p42mpk1/p90rsk complex.
The Cdc25 activating kinase Plx1 can be phosphorylated and activated by the protein kinase xPlkk1, which itself is also regulated by phosphorylation by one or more protein kinases (Qian et al., 1998b). Several reports indicate that Plx1 activation may be triggered by p34cdc2/cyclin B as part of the MPF autoamplification loop (Abrieu et al., 1998; Karaiskou et al., 1998). Here we provide evidence for a p42mpk1-triggered pathway, which also regulates Plx1 phosphorylation and activation. We observed that in Mos-injected oocytes, the activation of p42mpk1 is followed by partial phosphorylation of Plx1 before any detectable pre-MPF activation. In some batches of oocytes, however, the phosphorylation of Plx1 induced by Mos injection coincides with pre-MPF activation. One interpretation is that Plx1 phosphorylation normally precedes the activation of pre-MPF, although in some oocytes the two events may take place too close in time to be separated experimentally. To rule out an implication of p34cdc2/cyclin B in the phosphorylation of Plx1 induced by Mos, we coexpressed the Xenopus Suc1/Cks protein Xp9 together with Mos. Xp9 is a p34cdc2-binding protein that appears to be important for targeting p34cdc2/cyclin complexes to some substrates (Kusubata et al., 1992; Patra and Dunphy, 1998). For example, Xp9 can enhance the phosphorylation of the Cdc27 component of the anaphase-promoting complex by p34cdc2/cyclin B and affect cyclin B stability (Patra and Dunphy, 1998). When overexpressed in Xenopus egg extracts, Xp9-like proteins can delay entry into mitosis by interfering with p34cdc2 tyrosine dephosphorylation (Dunphy and Newport, 1989; Patra and Dunphy, 1996). Consistent with this, we found that injection of Xp9 into oocytes prevented Mos-induced meiotic reinitiation by interfering with the activation of p34cdc2/cyclin B. In these oocytes, however, ectopic Mos was still able to activate p42mpk1, and we could confirm that Plx1 phosphorylation and activation occurred independently of pre-MPF activation. Importantly, the phosphorylation and partial activation of Plx1 induced by Mos was inhibited by the expression of D2, indicating that it involves p42mpk1. A link between p42mpk1 and Plx1 has not been found in extracts prepared from G2-arrested oocytes (Karaiskou et al., 1998). In these extracts, however, the activation of p42mpk1 and p34cdc2/cyclin B appear to be independent events (Huang and Ferrell, 1996) probably attributable to the impaired ability of the oocyte extracts to translate mRNAs (our unpublished results). In agreement with this, we show that the p42mpk1-dependent phosphorylation of Plx1 is abolished by cycloheximide and thus requires protein synthesis. This observation together with the 3- to 5-h delay observed between the activation of p42mpk1 and Plx1 argue against a direct link between p42mpk1 and either Plx1 or xPlkk1 and rather suggest that p42mpk1 triggers the accumulation of a protein or proteins that are required for the activation of Plx1. Whether this link between p42mpk1 and Plx1 activation is related to the triggering pathway that leads to Plx1 activation at the onset of M-phase remains to be determined. In any case, the delayed kinetics observed between p42mpk1 and Plx1 activation suggest that Plx1 activation is probably not an early p42mpk1-dependent step in maturation and may be the basis of a positive feedback loop ensuring the coordinated activation of both p42mpk1 and MPF.
During oocyte maturation p42mpk1 can participate in p34cdc2/cyclin B activation via several pathways (Figure 11). One pathway may involve phosphorylation and inhibition of Myt1 by p90rsk (Palmer et al., 1998). Accumulation of Mos is also stimulated by p42mpk1 through a positive feedback loop (Matten et al., 1996; Roy et al., 1996), and it has recently been shown that p42mpk1 can up-regulate cytoplasmic polyadenylation of the Mos mRNA (Howard et al., 1999). However, the initial progesterone-induced synthesis of Mos is probably independent of p42mpk1, because it occurs before any detectable p42mpk1 activation. Our results suggest a new connection between p42mpk1 and a protein or proteins that are required for the phosphorylation and partial activation of Plx1. p42mpk1 could stimulate synthesis of these proteins and/or post-translationally regulate their activity, for example, at the level of protein stability. As in the case of Mos, synthesis of these proteins might be also initiated during progesterone-induced maturation before the activation of p42mpk1. This suggests the possibility that the susceptibility of the oocytes to inhibition by D2 may depend on the initial rate of protein synthesis induced by progesterone.
A Possible Role for p42mpk1 in Nuclear Migration
MPF is a key regulator of M-phase progression during the cell cycle and is likely to be responsible for most of the structural changes associated with M-phase. ERK MAP kinases and p34cdc2/cyclin B, however, have some overlapping substrate specificity. For example, both can phosphorylate nuclear lamins at the sites that are involved in lamin disassembly before nuclear envelope breakdown (Peter et al., 1992). In mouse oocytes and embryos ERK MAP kinases have also been implicated in some M-phase events (Gavin et al., 1994; Moos et al., 1996; Verlhac et al., 1996). However, a possible role for p42mpk1 in controlling M-phase structural changes in Xenopus oocytes has been difficult to address, because p42mpk1 is usually activated at the same time as MPF during meiotic maturation. Here we show that activation of p42mpk1 by Mos in the absence of p34cdc2/cyclin B activation may induce some of the morphological changes associated with meiotic maturation such as nuclear migration to the cortex of the oocyte. This is likely to be triggered by p42mpk1 activation, because it can be inhibited by the overexpression of D2, but not of a truncated version of D2, which lacks the p42mpk1 docking site. However, the sustained activation of p42mpk1 in the absence of MPF activation appears to lead to an abnormal maturation as indicated by the morphological appearance of the oocytes. Similar effects (including germinal vesicle migration without breakdown) have also been described when maturation is induced by the injection of oncogenic Ras in the presence of either drugs that increase cAMP levels (Birchmeier et al., 1985) or protein synthesis inhibitors (Nebreda et al., 1993b). Our results also show that activation of p42mpk1 alone is not enough to trigger GVBD in Xenopus oocytes, indicating that nuclear envelope breakdown during M-phase is likely to require the activation of MPF.
In summary, we have found that overexpression of the p42mpk1 docking site located at the C-terminal end of p90rsk can inhibit Xenopus oocyte maturation, and we have used this as a tool to elucidate the role(s) of p42mpk1 in the process. Our main conclusions are that activation of p42mpk1 normally precedes the activation of pre-MPF and that there is an MPF-independent pathway that links the activation of p42mpk1 and the Cdc25-activating kinase Plx1 during oocyte maturation. We also show that p42mpk1 may have a role in nuclear migration during Xenopus oocyte maturation. Finally, our results indicate that the regulated interaction with substrates is essential for signal transduction by MAP kinases.
ACKNOWLEDGMENTS
We are grateful to A. Tavares, C. Avides, and D. Glover for the anti-Plx1 antiserum, to A. Björnson for preparation of FTX5-Mos, and to D. Barila, K. Dorey, A. Palmer, and G. Superti-Furga for critically reading the manuscript. A.-C.G. was supported by the Swiss National Science Foundation and the European Molecular Biology Laboratory.
Abbreviations used:
- GVBD
germinal vesicle breakdown
- H1K
histone H1 kinase
- IgG
immunoglobulin G
- MPF
M-phase- or maturation-promoting factor
REFERENCES
- Abrieu A, Brassac T, Galas S, Fisher D, Labbe JC, Doree M. The polo-like kinase plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J Cell Sci. 1998;111:1751–1757. doi: 10.1242/jcs.111.12.1751. [DOI] [PubMed] [Google Scholar]
- Adams PD, Sellers WR, Sharma SK, Wu AD, Nalin CM, Kaeling WG. Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol Cell Biol. 1996;16:6623–6633. doi: 10.1128/mcb.16.12.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton-Fessler S, Liu F, Gabrielli B, Lee MS, Peng CY, Piwnica-Worms H. Cell cycle regulation of the p34cdc2 inhibitory kinases. Mol Biol Cell. 1994;5:989–1001. doi: 10.1091/mbc.5.9.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkoff A, Ballantyne S, Wickens M. Meiotic maturation in Xenopus requires polyadenylation of multiple mRNAs. EMBO J. 1998;17:3168–3175. doi: 10.1093/emboj/17.11.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier C, Broek D, Wigler M. RAS proteins can induce meiosis in Xenopus oocytes. Cell. 1985;43:615–621. doi: 10.1016/0092-8674(85)90233-8. [DOI] [PubMed] [Google Scholar]
- Bjørbaek C, Zhao Y, Moller DE. Divergent functional roles for p90rsk kinase domains. J Biol Chem. 1995;270:18848–18852. doi: 10.1074/jbc.270.32.18848. [DOI] [PubMed] [Google Scholar]
- Booher RN, Holman PS, Fattaey A. Human Myt1 is a cell cycle-regulated kinase that inhibits cdc2 but not cdk2 activity. J Biol Chem. 1997;272:22300–22306. doi: 10.1074/jbc.272.35.22300. [DOI] [PubMed] [Google Scholar]
- Chen YG, Hata A, Lo RS, Wotton D, Shi Y, Pavletich N, Massague J. Determinants of specificity in TGF-beta signal transduction. Genes & Dev. 1998;12:2144–2152. doi: 10.1101/gad.12.14.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert MS, Kirschner MW. Regulation of MPF activity in vitro. Cell. 1988;53:185–195. doi: 10.1016/0092-8674(88)90380-7. [DOI] [PubMed] [Google Scholar]
- Dalby KN, Morrice N, Caudwell FB, Avruch J, Cohen P. Identification of regulatory phosphorylation sites in mitogen-activated protein kinase (MAPK)-activated protein kinase-1a/p90rsk that are inducible by MAPK. J Biol Chem. 1998;273:1496–1505. doi: 10.1074/jbc.273.3.1496. [DOI] [PubMed] [Google Scholar]
- Dunphy W, Newport J. Fission yeast p13 blocks mitotic activation and tyrosine dephosphorylation of the Xenopus cdc2 protein kinase. Cell. 1989;58:181–191. doi: 10.1016/0092-8674(89)90414-5. [DOI] [PubMed] [Google Scholar]
- Dunphy WG, Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991;67:189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- Ferrell JE, Machleder EM. The biochemical basis of an all-or-none cell fate switch in Xenopus oocytes. Science. 1998;280:895–898. doi: 10.1126/science.280.5365.895. [DOI] [PubMed] [Google Scholar]
- Fischer TL, Blenis J. Evidence for two catalytically active kinase domains in pp90rsk. Mol Cell Biol. 1996;16:1212–1219. doi: 10.1128/mcb.16.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman RS, Kanki JP, Ballantyne SM, Pickham KM, Donoghue DJ. Effects of the v-mos oncogene on Xenopus development: meiotic induction in oocytes and mitotic arrest in cleaving embryos. J Cell Biol. 1990;111:533–541. doi: 10.1083/jcb.111.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J, Maller JL. Cyclin B in Xenopus oocytes: implications for the mechanism of preMPF activation. EMBO J. 1991;10:177–182. doi: 10.1002/j.1460-2075.1991.tb07934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Gavin AC, Cavadore JC, Schorderet-Slatkine S. Histone H1 kinase activity, germinal vesicle breakdown and M-phase entry in mouse oocytes. J Cell Sci. 1994;107:275–283. doi: 10.1242/jcs.107.1.275. [DOI] [PubMed] [Google Scholar]
- Gavin AC, Nebreda AR. A MAP kinase docking site is required for phosphorylation and activation of p90rsk/MAPKAP kinase-1. Curr Biol. 1999;9:281–284. doi: 10.1016/s0960-9822(99)80120-1. [DOI] [PubMed] [Google Scholar]
- Gotoh Y, Masuyama N, Dell K, Shirakabe K, Nishida E. Initiation of Xenopus oocyte maturation by activation of the mitogen-activated protein kinase cascade. J Biol Chem. 1995;270:25898–25904. doi: 10.1074/jbc.270.43.25898. [DOI] [PubMed] [Google Scholar]
- Gotoh Y, Moriyama K, Matsuda S, Okumura E, Kishimoto T, Kawasaki H, Suzuki K, Yahara I, Sakai H, Nishida E. Xenopus M-phase MAP kinase: isolation of its cDNA and activation by MPF. EMBO J. 1991;10:2661–2668. doi: 10.1002/j.1460-2075.1991.tb07809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y, Nishida E. Activation mechanism and function of the MAP kinase cascade. Mol Reprod Dev. 1995;42:486–492. doi: 10.1002/mrd.1080420417. [DOI] [PubMed] [Google Scholar]
- Haccard O, Lewellyn A, Hartley RS, Erikson E, Maller JL. Induction of Xenopus oocyte meiotic maturation by MAP kinase. Dev Biol. 1995;168:677–682. doi: 10.1006/dbio.1995.1112. [DOI] [PubMed] [Google Scholar]
- Hoffmann I, Clarke PR, Marcote MJ, Karsenti E, Draetta G. Phosphorylation and activation of human cdc25c by cdc2:cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J. 1993;12:53–63. doi: 10.1002/j.1460-2075.1993.tb05631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard EL, Charlesworth A, Welk J, MacNicol AM. The mitogen-activated protein kinase signaling pathway stimulates mos mRNA cytoplasmic polyadenylation during Xenopus oocyte maturation. Mol Cell Biol. 1999;19:1990–1999. doi: 10.1128/mcb.19.3.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao KM, Chou SY, Shih SJ, Ferrell JE. Evidence that inactive p42 mitogen-activated protein kinase and inactive Rsk exist as a heterodimer in vivo. Proc Natl Acad Sci USA. 1994;91:5480–5484. doi: 10.1073/pnas.91.12.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CF, Ferrell JE. Dependence of mos-induced Cdc2 activation on MAP kinase function in a cell-free system. EMBO J. 1996;15:2169–2173. [PMC free article] [PubMed] [Google Scholar]
- Huang W, Kessler DS, Erikson RL. Biochemical and biological analysis of Mek1 phosphorylation site mutants. Mol Biol Cell. 1995;6:237–245. doi: 10.1091/mbc.6.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Maller JL. Elimination of cdc2 phosphorylation sites in the cdc25 phosphatase blocks initiation of M-phase. Mol Biol Cell. 1993;4:1337–1350. doi: 10.1091/mbc.4.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Walker DH, Maller JL. Periodic changes in phosphorylation of the Xenopus cdc25 phosphatase regulate its activity. Mol Biol Cell. 1992;3:927–939. doi: 10.1091/mbc.3.8.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs D, Glossip D, Xing H, Muslin AJ, Kornfeld K. Multiple docking sites on substrate proteins form a modular system that mediates recognition by ERK MAP kinase. Genes & Dev. 1999;13:163–175. [PMC free article] [PubMed] [Google Scholar]
- Jones SW, Erikson E, Blenis J, Maller JL, Erikson RL. A Xenopus ribosomal protein S6 kinase has two apparent kinase domains that are each similar to distinct protein kinases. Proc Natl Acad Sci USA. 1988;85:3377–3381. doi: 10.1073/pnas.85.10.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallunki T, Su B, Tsigelny I, Sluss HK, Dérijard B, Moore G, Davis R, Karin M. JNK2 contains a specificity-determining region responsible for efficient c-Jun binding and phosphorylation. Genes & Dev. 1994;8:2996–3007. doi: 10.1101/gad.8.24.2996. [DOI] [PubMed] [Google Scholar]
- Karaiskou A, Cayla X, Haccard O, Jessus C, Ozon R. MPF amplification in Xenopus oocyte extracts depends on a two-step activation of cdc25 phosphatase. Exp Cell Res. 1998;244:491–500. doi: 10.1006/excr.1998.4220. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Minshull J, Ford C, Golsteyn R, Poon R, Hunt T. On the synthesis and destruction of A- and B-type cyclins during oogenesis and meiotic maturation in Xenopus laevis. J Cell Biol. 1991;114:755–765. doi: 10.1083/jcb.114.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth S, Sebastian B, Hunter T, Newport J. Membrane localization of the kinase which phosphorylates p34cdc2 on threonine 14. Mol Biol Cell. 1994;5:273–282. doi: 10.1091/mbc.5.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosako H, Akamatsu Y, Tsurushita N, Lee KK, Gotoh Y, Nishida E. Isolation and characterization of neutralizing single-chain antibodies against Xenopus mitogen-activated protein kinase kinase from phage display libraries. Biochemistry. 1996;35:13212–13221. doi: 10.1021/bi960956f. [DOI] [PubMed] [Google Scholar]
- Kosako H, Gotoh Y, Nishida E. Regulation and function of the MAP kinase cascade in Xenopus oocytes. J Cell Sci suppl. 1994a;18:115–119. doi: 10.1242/jcs.1994.supplement_18.17. [DOI] [PubMed] [Google Scholar]
- Kosako H, Gotoh Y, Nishida E. Requirement for the MAP kinase kinase/MAP kinase cascade in Xenopus oocyte maturation. EMBO J. 1994b;13:2131–2138. doi: 10.1002/j.1460-2075.1994.tb06489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. The cdc25 protein controls tyrosine dephosphorylation of the cdc2 protein in a cell-free system. Cell. 1991;64:903–914. doi: 10.1016/0092-8674(91)90315-p. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992;70:139–151. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- Kusubata M, et al. p13suc1 suppresses the catalytic function of p34cdc2 kinase for intermediate filament proteins, in vitro. J Biol Chem. 1992;267:20937–20942. [PubMed] [Google Scholar]
- Leighton IA, Dalby KN, Caudwell FB, Cohen PT, Cohen P. Comparison of the specificities of p70 S6 kinase and MAPKAP kinase-1 identifies a relatively specific substrate for p70 S6 kinase: the N-terminal kinase domain of MAPKAP kinase-1 is essential for peptide phosphorylation. FEBS Lett. 1995;375:289–293. doi: 10.1016/0014-5793(95)01170-j. [DOI] [PubMed] [Google Scholar]
- Lo RS, Chen YG, Shi Y, Pavletich NP, Massague J. The L3 loop: a structural motif determining specific interactions between SMAD proteins and TGF-beta receptors. EMBO J. 1998;17:996–1005. doi: 10.1093/emboj/17.4.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui Y, Clarke HJ. Oocyte maturation. Int Rev Cytol. 1979;57:185–282. doi: 10.1016/s0074-7696(08)61464-3. [DOI] [PubMed] [Google Scholar]
- Masui Y, Markert CL. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971;177:129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Kosako H, Takenaka K, Moriyama K, Sakai H, Akiyama T, Gotoh Y, Nishida E. Xenopus MAP kinase activator: identification and function as a key intermediate in the phosphorylation cascade. EMBO J. 1992;11:973–982. doi: 10.1002/j.1460-2075.1992.tb05136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matten WT, Copeland TD, Ahn NG, Vande Woude GF. Positive feedback between MAP kinase and Mos during Xenopus oocyte maturation. Dev Biol. 1996;179:485–492. doi: 10.1006/dbio.1996.0277. [DOI] [PubMed] [Google Scholar]
- Michaels J-EA, Schimmel P, Shiba K, Miller WT. Dominant negative inhibition by fragments of a monomeric enzyme. Proc Natl Acad Sci USA. 1996;93:14452–14455. doi: 10.1073/pnas.93.25.14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos J, Xu Z, Schultz RM, Kopf GS. Regulation of nuclear envelope assembly/disassembly by MAP kinase. Dev Biol. 1996;175:358–361. doi: 10.1006/dbio.1996.0121. [DOI] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Kumagai A, Dunphy WG. Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science. 1995;270:86–90. doi: 10.1126/science.270.5233.86. [DOI] [PubMed] [Google Scholar]
- Nakajo N, Oe T, Uto K, Sagata N. Involvement of Chk1 in prophase I arrest of Xenopus oocytes. Dev Biol. 1999;207:432–444. doi: 10.1006/dbio.1998.9178. [DOI] [PubMed] [Google Scholar]
- Nebreda AR, Gannon JV, Hunt T. Newly synthesized protein(s) must associate with p34cdc2 to activate MAP kinase and MPF during progesterone-induced maturation of Xenopus oocytes. EMBO J. 1995;14:5597–5607. doi: 10.1002/j.1460-2075.1995.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebreda AR, Hill C, Gomez N, Cohen P, Hunt T. The protein kinase Mos activates MAP kinase kinase in vitro and stimulates the MAP kinase pathway in mammalian somatic cells in vivo. FEBS Lett. 1993a;333:183–187. doi: 10.1016/0014-5793(93)80401-f. [DOI] [PubMed] [Google Scholar]
- Nebreda AR, Hunt T. The c-mos protooncogene protein kinase turns on and maintains the activity of MAP kinase but not MPF, in cell-free extracts of Xenopus oocytes and eggs. EMBO J. 1993;12:1979–1986. doi: 10.1002/j.1460-2075.1993.tb05847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebreda AR, Porras A, Santos E. p21ras-induced meiotic maturation of Xenopus oocytes in the absence of protein synthesis: MPF activation is preceded by activation of MAP and S6 kinases. Oncogene. 1993b;8:467–477. [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating cell cycle timing of M-phase. Nature. 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Palmer A, Gavin A-C, Nebreda AR. A link between MAP kinase and p34cdc2/cyclin B during oocyte maturation: p90rsk phosphorylates and inactivates the p34cdc2 inhibitory kinase Myt 1. EMBO J. 1998;17:5037–5047. doi: 10.1093/emboj/17.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra D, Dunphy WG. Xe-p9, a Xenopus Suc1/Cks homolog, has multiple essential roles in cell cycle control. Genes & Dev. 1996;10:1503–1515. doi: 10.1101/gad.10.12.1503. [DOI] [PubMed] [Google Scholar]
- Patra D, Dunphy WG. Xe-p9, a Xenopus Suc1/Cks protein, is essential for the Cdc2-dependent phosphorylation of the anaphase-promoting complex at mitosis. Genes & Dev. 1998;12:2549–2559. doi: 10.1101/gad.12.16.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M, Sanghera JS, Pelech SL, Nigg EA. Mitogen-activated protein kinases phosphorylate nuclear lamins and display sequence specificity overlapping that of mitotic protein kinase p34cdc2. Eur J Biochem. 1992;205:287–294. doi: 10.1111/j.1432-1033.1992.tb16779.x. [DOI] [PubMed] [Google Scholar]
- Posada J, Yew N, Ahn N, VandeWoude GF, Cooper JA. Mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro. Mol Cell Biol. 1993;13:2546–2553. doi: 10.1128/mcb.13.4.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian YW, Erikson E, Li C, Maller JL. Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis. Mol Cell Biol. 1998a;18:4262–4271. doi: 10.1128/mcb.18.7.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian YW, Erikson E, Maller JL. Purification and cloning of a protein kinase that phosphorylates and activates the polo-like kinase Plx1. Science. 1998b;282:1701–1704. doi: 10.1126/science.282.5394.1701. [DOI] [PubMed] [Google Scholar]
- Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Roy LM, Haccard O, Izumi T, Lattes BG, Lewellyn AL, Maller JL. Mos proto-oncogene function during oocyte maturation in Xenopus. Oncogene. 1996;12:2203–2211. [PubMed] [Google Scholar]
- Sagata N, Daar I, Oskarsson M, Showalter SD, Vande Woude GF. The product of the mos proto-oncogene as a candidate “initiator”for oocyte maturation. Science. 1989;245:643–646. doi: 10.1126/science.2474853. [DOI] [PubMed] [Google Scholar]
- Sagata N, Oskarsson M, Copeland T, Brumbaugh J, Vande Woude GF. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988;335:519–526. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Schulman BA, Lindstrom DL, Harlow E. Substrate recruitment to cyclin-dependent kinase 2 by a multipurpose docking site on cyclin A. Proc Natl Acad Sci USA. 1998;95:10453–10458. doi: 10.1073/pnas.95.18.10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimeca JC, Nguyen TT, Filloux C, Van Obberghen E. Nerve growth factor-induced phosphorylation cascade in PC12 pheochromocytoma cells. Association of S6 kinase II with the microtubule-associated protein kinase, ERK1. J Biol Chem. 1992;267:17369–17374. [PubMed] [Google Scholar]
- Sheets MD, Wu M, Wickens M. Polyadenylation of c-mos mRNA as a control point in Xenopus meiotic maturation. Nature. 1995;274:511–516. doi: 10.1038/374511a0. [DOI] [PubMed] [Google Scholar]
- Shibuya EK, Ruderman JV. Mos induces the in vitro activation of mitogen-activated protein kinases in lysates of frog oocytes and mammalian somatic cells. Mol Biol Cell. 1993;4:781–790. doi: 10.1091/mbc.4.8.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Poteet-Smith CE, Malarkey K, Sturgill TW. Identification of an extracellular signal-regulated kinase (ERK) docking site in ribosomal S6 kinase, a sequence critical for activation by ERK in vivo. J Biol Chem. 1999;274:2893–2898. doi: 10.1074/jbc.274.5.2893. [DOI] [PubMed] [Google Scholar]
- Strausfeld U, Fernandez A, Capony JP, Girard F, Lautredou N, Derancourt J, Labbé JC, Lamb NJ. Activation of p34cdc2 protein kinase by microinjection of human cdc25C into mammalian cells. Requirement for prior phosphorylation of cdc25C by p34cdc2 on sites phosphorylated at mitosis. J Biol Chem. 1994;269:5989–6000. [PubMed] [Google Scholar]
- Strausfeld U, Labbé JC, Fesquet D, Cavadore JC, Picard A, Sadhu K, Russell P, Dorée M. Dephosphorylation and activation of a p34cdc2/cyclin B complex in vitro by human CDC25 protein. Nature. 1991;351:242–245. doi: 10.1038/351242a0. [DOI] [PubMed] [Google Scholar]
- Verlhac MH, Kubiak JZ, Weber M, Geraud G, Colledge WH, Evans MJ, Maro B. Mos is required for MAP kinase activation and is involved in microtubule organization during meiotic maturation in the mouse. Development. 1996;122:815–822. doi: 10.1242/dev.122.3.815. [DOI] [PubMed] [Google Scholar]
- Yang S-H, Whitmarsh AJ, Davis RJ, Sharrocks AD. Differential targeting of MAP kinases to the ETS-domain transcription factor Elk-1. EMBO J. 1998a;17:1740–1749. doi: 10.1093/emboj/17.6.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Yates PR, Whitmarsh AJ, Davis RJ, Sharrocks AD. The Elk-1 EST-domain transcription factor contains a Mitogen-activated protein kinase targeting motif. Mol Cell Biol. 1998b;18:710–720. doi: 10.1128/mcb.18.2.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Bjørbæk C, Moller DE. Regulation and interaction of pp90rsk isoforms with mitogen-activated protein kinases. J Biol Chem. 1996;271:29773–29779. doi: 10.1074/jbc.271.47.29773. [DOI] [PubMed] [Google Scholar]