Abstract
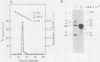
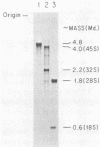
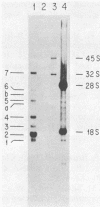
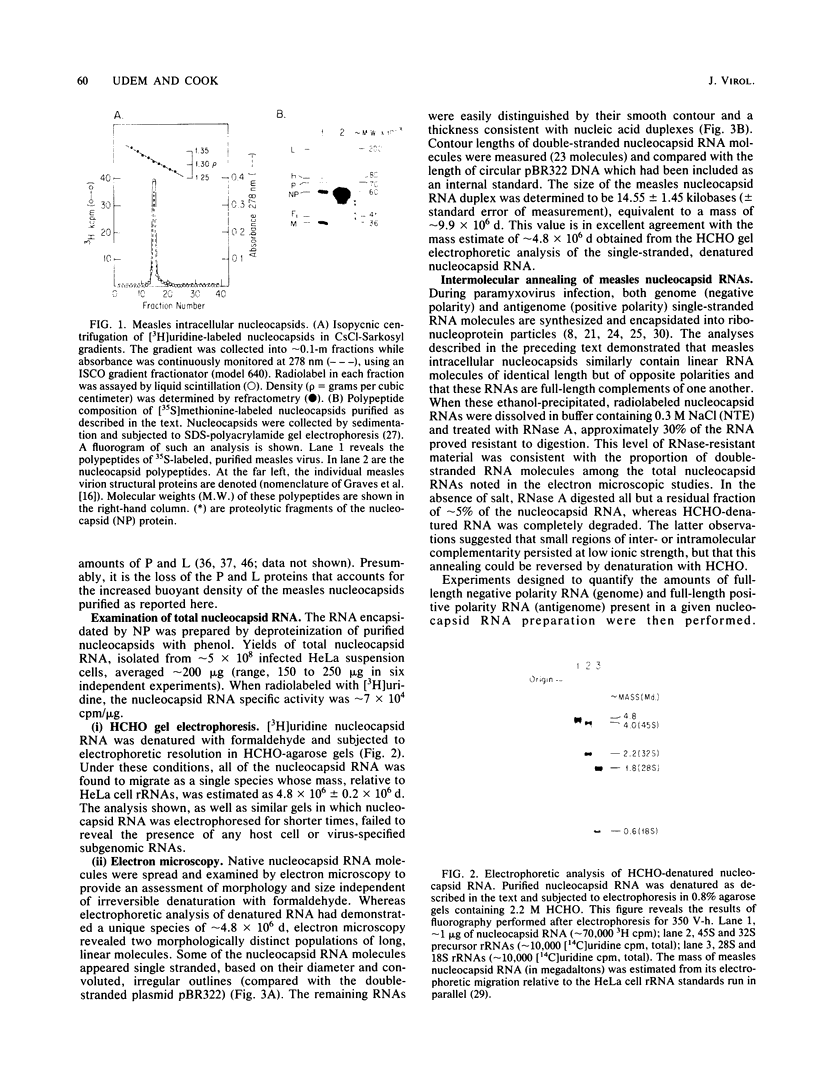
Protocols have been established for the preparation of large amounts of pure measles virus intracellular nucleocapsids. As a result, it has been possible to routinely achieve nucleocapsid RNA yields of approximately 200 micrograms (from approximately 5 X 10(8) infected cells). Electrophoretic analysis of this RNA under denaturing conditions revealed a single species whose mass was estimated at approximately 4.8 X 10(6) daltons. Electron microscopic assessment of nucleocapsid RNA contour lengths corroborated the electrophoretic size determination. Total nucleocapsid RNA was shown to contain both negative- and positive-stranded species distributed in a ratio of 2 to 3 genome polarity molecules for each antigenome RNA. Hybridization studies established that all of the virus-specified polyadenylated RNAs were encoded by the negative-stranded nucleocapsid RNA and, therefore, that this nucleocapsid RNA was the measles genome. Examination of the measles virus-specified, polyadenylated transcription products by HCHO-agarose gel electrophoresis revealed at least nine distinct RNA species (rather than the six predicted measles mRNAs). The significance of these observations is discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amesse L. S., Kingsbury D. W. Sendai virus gene sequences identified by oligonucleotide mapping. Virology. 1982 Apr 15;118(1):8–16. doi: 10.1016/0042-6822(82)90314-2. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczko K., Lazzarini R. A. Efficient propagation of measles virus in suspension cultures. J Virol. 1979 Sep;31(3):854–855. doi: 10.1128/jvi.31.3.854-855.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrer M. J., Bloom B. R., Udem S. Characterization of measles polypeptides by monoclonal antibodies. Virology. 1981 Jan 30;108(2):381–390. doi: 10.1016/0042-6822(81)90446-3. [DOI] [PubMed] [Google Scholar]
- Bishop J. M., Koch G. Infectious replicative intermediate of poliovirus: purification and characterization. Virology. 1969 Apr;37(4):521–534. doi: 10.1016/0042-6822(69)90270-0. [DOI] [PubMed] [Google Scholar]
- Blair C. D., Robinson W. S. Replication of Sendai virus. II. Steps in virus assembly. J Virol. 1970 May;5(5):639–650. doi: 10.1128/jvi.5.5.639-650.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman L. H., Rabin B., Schlessinger D. Multiple ribosomal RNA cleavage pathways in mammalian cells. Nucleic Acids Res. 1981 Oct 10;9(19):4951–4966. doi: 10.1093/nar/9.19.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Hightower L. E., Ball L. A. Transcriptional map for Newcastle disease virus. J Virol. 1980 Sep;35(3):682–693. doi: 10.1128/jvi.35.3.682-693.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Wertz G. W., Ball L. A., Hightower L. E. Coding assignments of the five smaller mRNAs of Newcastle disease virus. J Virol. 1982 Sep;43(3):1024–1031. doi: 10.1128/jvi.43.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Wertz G. W. cDNA cloning and transcriptional mapping of nine polyadenylylated RNAs encoded by the genome of human respiratory syncytial virus. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3208–3212. doi: 10.1073/pnas.80.11.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Choppin P. W. Isolation and properties of the helical nucleocapsid of the parainfluenza virus SV5. Proc Natl Acad Sci U S A. 1967 Apr;57(4):949–956. doi: 10.1073/pnas.57.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkind P. R., Cross R. K., Lamb R. A., Merz D. C., Choppin P. W. In vitro synthesis of structural and nonstructural proteins of Sendai and SV5 viruses. Virology. 1980 Jan 15;100(1):22–33. doi: 10.1016/0042-6822(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Glazier K., Raghow R., Kingsbury D. W. Regulation of Sendai virus transcription: evidence for a single promoter in vivo. J Virol. 1977 Mar;21(3):863–871. doi: 10.1128/jvi.21.3.863-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorecki M., Rozenblatt S. Cloning of DNA complementary to the measles virus mRNA encoding nucleocapsid protein. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3686–3690. doi: 10.1073/pnas.77.6.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves M. C., Silver S. M., Choppin P. W. Measles virus polypeptides synthesis in infected cells. Virology. 1978 May 1;86(1):254–263. doi: 10.1016/0042-6822(78)90025-9. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Kiessling W., ter Meulen V. Membrane proteins of subacute sclerosing panencephalitis and measles viruses. Nature. 1978 Mar 30;272(5652):460–462. doi: 10.1038/272460a0. [DOI] [PubMed] [Google Scholar]
- Herman R. C., Schubert M., Keene J. D., Lazzarini R. A. Polycistronic vesicular stomatitis virus RNA transcripts. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4662–4665. doi: 10.1073/pnas.77.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. T., Wertz G. W. The genome of respiratory syncytial virus is a negative-stranded RNA that codes for at least seven mRNA species. J Virol. 1982 Jul;43(1):150–157. doi: 10.1128/jvi.43.1.150-157.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G., Udem S., Rager-Zisman B., Bloom B. R. Isolation of a heterogeneous population of temperature-sensitive mutants of measles virus from persistently infected human lymphoblastoid cell lines. J Exp Med. 1978 Jun 1;147(6):1637–1652. doi: 10.1084/jem.147.6.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakofsky D., Boy de la Tour E., Bruschi A. Self-annealing of Sendai virus RNA. J Virol. 1974 Jul;14(1):33–39. doi: 10.1128/jvi.14.1.33-39.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakofsky D., Boy de la Tour E., Delius H. Molecular weight determination of Sendai and Newcastle disease virus RNA. J Virol. 1974 Feb;13(2):261–268. doi: 10.1128/jvi.13.2.261-268.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakofsky D., Bruschi A. Antigenomes in Sendai virions and Sendai virus-infected cells. Virology. 1975 Jul;66(1):185–191. doi: 10.1016/0042-6822(75)90189-0. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D. Isolation and characterization of Sendai virus DI-RNAs. Cell. 1976 Aug;8(4):547–555. doi: 10.1016/0092-8674(76)90223-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Determination by peptide mapping of the unique polypeptides in Sendai virions and infected cells. Virology. 1978 Feb;84(2):469–478. doi: 10.1016/0042-6822(78)90263-5. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- McCarthy M., Lazzarini R. A. Intracellular nucleocapsid RNA of mumps virus. J Gen Virol. 1982 Jan;58(Pt 1):205–209. doi: 10.1099/0022-1317-58-1-205. [DOI] [PubMed] [Google Scholar]
- Moore P. M., Hayes E. C., Miller S. E., Wright L. L., Machamer C. E., Zweerink H. J. Measles virus nucleocapsids: large-scale purification and use in radioimmunoassays. Infect Immun. 1978 Jun;20(3):842–846. doi: 10.1128/iai.20.3.842-846.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle W. E., Choppin P. W. A comparison of the polypeptides of four measles virus strains. Virology. 1977 May 15;78(2):463–474. doi: 10.1016/0042-6822(77)90123-4. [DOI] [PubMed] [Google Scholar]
- Nakai T., Shand F. L., Howatson A. F. Development of measles virus in vitro. Virology. 1969 May;38(1):50–67. doi: 10.1016/0042-6822(69)90127-5. [DOI] [PubMed] [Google Scholar]
- Portner A. Synthesis of message and genome RNAs in vitro by Sendai virus-infected cell nucleocapsids. J Gen Virol. 1982 May;60(Pt 1):67–75. doi: 10.1099/0022-1317-60-1-67. [DOI] [PubMed] [Google Scholar]
- Robbins S. J., Bussell R. H., Rapp F. Isolation and partial characterization of two forms of cytoplasmic nucleocapsids from measles virus-infected cells. J Gen Virol. 1980 Apr;47(2):301–310. doi: 10.1099/0022-1317-47-2-301. [DOI] [PubMed] [Google Scholar]
- Robbins S. J., Bussell R. H. Structural phosphoproteins associated with purified measles virions and cytoplasmic nucleocapsids. Intervirology. 1979;12(2):96–102. doi: 10.1159/000149074. [DOI] [PubMed] [Google Scholar]
- Roux L., Holland J. J. Role of defective interfering particles of Sendai virus in persistent infections. Virology. 1979 Feb;93(1):91–103. doi: 10.1016/0042-6822(79)90278-2. [DOI] [PubMed] [Google Scholar]
- Roux L., Kolakofsky D. Isolation of RNA transcripts from the entire Sendai viral genome. J Virol. 1975 Dec;16(6):1426–1434. doi: 10.1128/jvi.16.6.1426-1434.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozek C. E., Davidson N. Drosophila has one myosin heavy-chain gene with three developmentally regulated transcripts. Cell. 1983 Jan;32(1):23–34. doi: 10.1016/0092-8674(83)90493-2. [DOI] [PubMed] [Google Scholar]
- Rozenblatt S., Gesang C., Lavie V., Neumann F. S. Cloning and characterization of DNA complementary to the measles virus mRNA encoding hemagglutinin and matrix protein. J Virol. 1982 Jun;42(3):790–797. doi: 10.1128/jvi.42.3.790-797.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluederberg A. Measles virus RNA. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1012–1015. doi: 10.1016/0006-291x(71)90004-0. [DOI] [PubMed] [Google Scholar]
- Soeiro R., Birnboim H. C., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. II. Base composition and cellular localization of a heterogeneous RNA fraction. J Mol Biol. 1966 Aug;19(2):362–372. doi: 10.1016/s0022-2836(66)80010-4. [DOI] [PubMed] [Google Scholar]
- Sprague J., Eron L. J., Seifried A. S., Milstien J. B. Cell-free synthesis of measles virus proteins. J Virol. 1979 Nov;32(2):688–691. doi: 10.1128/jvi.32.2.688-691.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruance S. L., Ashton B. N., Smith C. B. Preparation and characterization of high-specific activity radiolabeled 50 S measles virus RNA. J Virol Methods. 1980;1(4):223–228. doi: 10.1016/0166-0934(80)90062-2. [DOI] [PubMed] [Google Scholar]
- Stallcup K. C., Wechsler S. L., Fields B. N. Purification of measles virus and characterization of subviral components. J Virol. 1979 Apr;30(1):166–176. doi: 10.1128/jvi.30.1.166-176.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D. L., Norrby E. Structural polypeptides of measles virus. J Gen Virol. 1978 May;39(2):219–229. doi: 10.1099/0022-1317-39-2-219. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Soeiro R. Nascent ribosomes from HeLa cells. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1984–1990. doi: 10.1073/pnas.58.5.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler S. L., Fields B. N. Intracellular synthesis of measles virus-specified polypeptides. J Virol. 1978 Jan;25(1):285–297. doi: 10.1128/jvi.25.1.285-297.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]