Abstract
Immunochemical research into the antigenic structure of a given disease agent presupposes the availability of undegraded antigen. Some types of immunochemical studies of plasmodia can be carried out with the intracellular parasites in situ in the host cell (for example, studies using the fluorescent antibody technique). In other techniques (such as double diffusion in gel, disc electrophoresis) the presence of host cell contaminants is undesirable, and these require to be reduced to a minimum.
A method is described for harvesting mammalian (rodent, simian, and human) plasmodia. Plasmodia in the product are significantly concentrated as compared with the original samples. This point is particularly important in harvesting human plasmodia, in which parasitaemias tend to be very low. Significant reduction of red- and white-cell contaminants is achieved. Antigens in the cell-free plasmodial products obtained are apparently in their native state, and give replicable results in studies of double diffusion in gel, immunoelectrophoresis and disc electrophoresis, passive haemagglutination and vaccination.
Full text
PDF



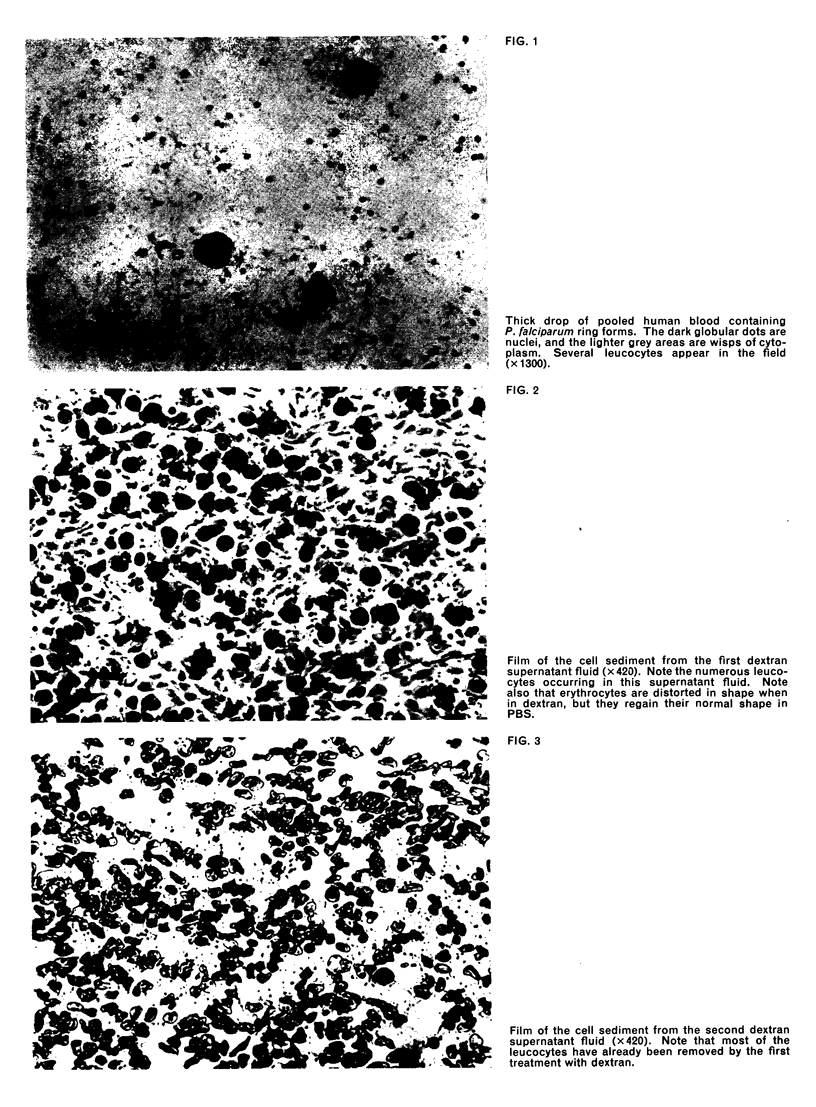



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray R. S. The Stein and Desowitz haemagglutination test for the detection of antibodies to Plasmodium berghei and Plasmodium vinckei. Ann Soc Belges Med Trop Parasitol Mycol. 1965;45(4):397–402. [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- McGregor I. A., Hall P. J., Williams K., Hardy C. L. Demonstration of circulating antibodies to Plasmodium falciparum by gel-diffusion techniques. Nature. 1966 Jun 25;210(5043):1384–1386. doi: 10.1038/2101384a0. [DOI] [PubMed] [Google Scholar]
- NELKEN D., GILBOA-GARBER N., GUREVITCH J. A method for the simultaneous separation of human thrombocytes and leucocytes. J Clin Pathol. 1960 May;13:266–266. doi: 10.1136/jcp.13.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPIRAD, ZUCKERMAN A. Antigenic structure of Plasmodium vinckei. Science. 1962 Aug 17;137(3529):536–537. doi: 10.1126/science.137.3529.536. [DOI] [PubMed] [Google Scholar]
- Sodeman W. A., Jr, Meuwissen H. E. Disk electrophoresis of Plasmodium berghei. J Parasitol. 1966 Feb;52(1):23–25. [PubMed] [Google Scholar]
- TRAGER W. Studies on the extracellular cultivation of an intracellular parasite (avian malaria). I. Development of the organisms in erythrocyte extracts, and the favoring effect of adenosinetriphosphate. J Exp Med. 1950 Oct 1;92(4):349–366. doi: 10.1084/jem.92.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdaneta-Morales S., Tengler de McLure M. The endocommensal ciliates of Venezuelan sea urchins. J Protozool. 1966 Feb;13(1):5–8. doi: 10.1111/j.1550-7408.1966.tb01859.x. [DOI] [PubMed] [Google Scholar]








