Abstract
We describe the isolation of fission yeast homologues of tubulin-folding cofactors B (Alp11) and E (Alp21), which are essential for cell viability and the maintenance of microtubules. Alp11B contains the glycine-rich motif (the CLIP-170 domain) involved in microtubular functions, whereas, unlike mammalian cofactor E, Alp21E does not. Both mammalian and yeast cofactor E, however, do contain leucine-rich repeats. Immunoprecipitation analysis shows that Alp11B interacts with both α-tubulin and Alp21E, but not with the cofactor D homologue Alp1, whereas Alp21E also interacts with Alp1D. The cellular amount of α-tubulin is decreased in both alp1 and alp11 mutants. Overproduction of Alp11B results in cell lethality and the disappearance of microtubules, which is rescued by co-overproduction of α-tubulin. Both full-length Alp11B and the C-terminal third containing the CLIP-170 domain localize in the cytoplasm, and this domain is required for efficient binding to α-tubulin. Deletion of alp11 is suppressed by multicopy plasmids containing either alp21+ or alp1+, whereas alp21 deletion is rescued by overexpression of alp1+ but not alp11+. Finally, the alp1 mutant is not complemented by either alp11+ or alp21+. The results suggest that cofactors operate in a linear pathway (Alp11B-Alp21E-Alp1D), each with distinct roles.
INTRODUCTION
Microtubules (MTs) are ubiquitous structures important for a wide variety of cellular processes, including motility, chromosome separation, protein and mRNA transport, and cell morphogenesis. They are composed of heterodimers of α- and β-tubulins, which are evolutionarily highly conserved. MTs undergo morphological alterations in cell cycle– and differentiation stage-specific manners; elucidation of the underlying molecular mechanisms is crucial to understanding how MTs regulate diverse cellular processes (Mitchison and Kirschner, 1986; Hyman and Karsenti, 1996). MTs are known to be regulated at several distinct levels in the cell. These include posttranscriptional autoregulation (Bachurski et al., 1994; Gonzalez-Garay and Cabral, 1996), treadmilling (Waterman-Storer and Salmon, 1997), assembly and disassembly through dynamic instability or severing reactions (Mitchison and Kirschner, 1984; Horio and Hotani, 1986; McNally and Vale, 1993; Belmont and Mitchison, 1996), and stabilization through a group of microtubule-associated proteins (MAPs) (Mandelkow and Mandelkow, 1995). MAPs are further classified into several categories depending on amino acid sequence homology and their biochemical characteristics. For example, classic MAPs consist of tau and MAP1, MAP2, and MAP4. MAPs in a broad sense include motor proteins such as kinesin and dynein (Hirokawa, 1998). Also, several proteins, including CLIP-170/restin and the p150Glued component of the dynactin complex, share a common motif of ∼50 amino acid residues, and some of these members have been shown to bind MTs (Gill et al., 1991; Bilbe et al., 1992; Pierre et al., 1992; Riehemann and Sorg, 1993).
In addition to these controls, the biogenesis of MTs is regulated by molecular chaperones, members of the GroEL- and Hsp60-related chaperonins (CCT/TriC/c-cpn) (Rommelaere et al., 1993; Kubota et al., 1994). Of particular interest is that, although newly synthesized actin and γ-tubulin are folded properly in vitro mainly by CCT/TriC/c-cpn (Gao et al., 1992; Melki et al., 1993; Geissler et al., 1998; Vainberg et al., 1998), folding reactions for α- and β-tubulin molecules are of far greater complexity. In addition to CCT/TriC/c-cpn, a group of proteins called cofactors are required for the production of assembly-competent α/β-tubulin heterodimers (Gao et al., 1993, 1994). Molecular cloning of these cofactors from vertebrates has revealed that cofactors comprise multiple proteins, cofactors A, B, C, D, and E (Llosa et al., 1996; Melki et al., 1996; Tian et al., 1996, 1997). Interestingly, both cofactors B and E contain the conserved motif that is found in several proteins, including CLIP-170 and the dynactin subunit mentioned above (Tian et al., 1996, 1997).
According to biochemical analysis performed in mammalian systems with tubulins expressed in vitro with purified chaperonins and cofactors, the pathways leading to correctly folded α/β-tubulin heterodimers are as follows. Newly translated free tubulins first enter a 900-kDa toroidal complex consisting of several chaperonin subunits (Lewis et al., 1992; Yaffe et al., 1992). After release from the chaperonin complex, α- and β-tubulins are captured by cofactors B and A, respectively, which subsequently are replaced by cofactors E and D. Then, the two pathways (α-tubulin-cofactor E and β-tubulin-cofactor D) converge, forming a complex consisting of all four molecules. Finally, cofactor C joins and, upon GTP hydrolysis, assembly-competent α/β-tubulin heterodimers are released. Cofactors D and E are indispensable and play parallel roles in the folding reactions of β- and α-tubulin, respectively (Lewis et al., 1997).
Cofactors are also evolutionarily conserved from humans to yeasts. In budding yeast, homologues of all the cofactors except cofactor C are found in the genome, and mutants and corresponding genes were independently isolated in genetic screens to identify genes involved in MT function (Hoyt et al., 1990, 1997; Stearns et al., 1990; Ursic and Culbertson, 1991; Archer et al., 1995; Tian et al., 1997). These analyses have shown that, surprisingly, none of the cofactor homologues are essential for cell viability in budding yeast; MTs appear to be capable of assembling in the absence of these cofactors, although modest defects in MT function are observed in mutants defective in these homologues, such as hypersensitivity to MT-destabilizing drugs and cold-sensitive growth. This raises the question as to the function of these cofactors in MT biogenesis: do cofactors play a crucial role in MT function in vivo? Analysis in fission yeast, however, has given a quite different view. The cofactor D homologue–encoding alp1+ gene is essential, and temperature-sensitive alp1 mutants show a variety of phenotypes attributed to defects in MT function, such as abnormal bent or branched morphology, displacement of the nucleus and septum, and mitotic delay. More importantly, MT structures become highly fragile and no intact MTs are observed at the restrictive temperature, demonstrating that cofactors are required for MT function in fission yeast (Hirata et al., 1998).
alp1 was originally identified as one of 15 loci (alp1 to alp15), temperature-sensitive mutations of which result in growth polarity defects, including curved, bent, or branched cell shape at the restrictive temperature. Previous analysis has shown that four loci (alp1+, alp2+, alp11+, and alp12+) are responsible for the maintenance of MT structures (Radcliffe et al., 1998). As described above, alp1+ encodes a homologue of cofactor D and alp2+ and alp12+ are allelic to atb2+ and nda3+, encoding α2-tubulin and β-tubulin, respectively.
In the current study, we have isolated and characterized the fourth gene, alp11+, which turns out to encode a homologue of cofactor B. Furthermore, we have isolated a homologue of cofactor E (alp21+) as a multicopy suppressor of the temperature-sensitive alp11 mutant. Genetic analysis indicates that, consistent with findings for alp1+, both alp11+ and alp21+ are essential for MT function and cell viability. In agreement with the result obtained from mammalian in vitro systems, we show that Alp21E functions downstream of Alp11B. However, contrary to the notion that cofactors D and E act in a parallel manner, our analysis suggests that in fission yeast Alp1D acts downstream of Alp21E. Structure-function relationships of these cofactors are discussed in terms of evolutionary conservation in the biogenesis and regulation of MTs.
MATERIALS AND METHODS
Strains, Media, and Genetic Methods
The strains used in this study are listed in Table 1. YPD (2% dextrose, 2% polypeptone, and 1% yeast extract) and YE5S (Moreno et al., 1991) were used as rich media. The standard methods were followed as described (Moreno et al., 1991).
Table 1.
Strain list
| Strain | Genotype | Derivation |
|---|---|---|
| HM123 | h−leu1 | Our stock |
| DH2-8D | h−leu1alp1-1315 | Hirata et al. (1998) |
| DH924 | h−leu1alp11-924 | This study |
| ME1 | h−/h+leu1/leu1ura4/ura4his7/his7 ade6-210/ade6-216alp1∷ura4+/+ | Hirata et al. (1998) |
| PR6 | h−leu1ura4alp11-924 | This study |
| PR7 | h−/h+leu1/leu1ura4/ura4his7/his7 | This study |
| ade6-M210/ade6-M216alp11∷ura4+/+ | ||
| PR8 | h−/h+leu1/leu1ura4/ura4his7/his7 | This study |
| ade6-M210/ade6-M216alp21∷ura4+/+ | ||
| PR9a | h−leu1ura4his7ade6alp11∷ura4+ | This study |
| PR10 | h−leu1ura4his7ade6alp21∷ura4+ containing pAL100 (alp1+) | This study |
| PR11 | h−leu1alp11+-GFP-ura4+ | This study |
| PR12 | h−leu1alp21+-GFP-ura4+ | This study |
| PR13 | h−/h+leu1/leu1ura4/ura4his7/his7 | This study |
| ade6-M210/ade6-M216alp111–163∷ura4+/+ | ||
| PR14 | h−leu1ura4his7ade6alp111–163∷ura4+ | This study |
| PR15 | h−leu1ura4his7ade6alp1+-HA-kan | This study |
| PR16 | h−leu1ura4his7ade6alp21+-GFP-ura4+alp1+-HA-kan | This study |
| PR17 | h−leu1ura4his7ade6alp111–163∷ura4+alp21+-GFP-ura4+ | This study |
| PR18 | h−leu1alp21+-GFP-ura4+pop3+-HA-kan | This study |
This strain was spontaneously obtained from tetrads derived from PR7, which could be propagated, possibly ascribable to the occurrence of some suppressor mutation.
Cloning of the alp11+ and alp21+ Genes
A Schizosaccharomyces pombe genomic library was used for the isolation of genes that complemented the temperature-sensitive alp11-924 mutant (PR6). Two of 20,000 colonies were capable of growing at 36°C. Plasmid DNAs were recovered from Escherichia coli, and restriction mapping indicated that two different plasmids (pCR1 and pCR11; the corresponding genes were designated alp11+ and alp21+, respectively) were isolated. Allelism between a gene on pCR1 and alp11+ was determined by tetrad analysis between the viable Δalp11 disruptant (PR9; see below) and the alp11-924 strain (PR6). Ts+ (wild-type) recombinants did not appear among 13 tetrads.
Nucleic Acids Preparation and Manipulation
Standard molecular biology techniques were followed as described (Sambrook et al., 1989). Enzymes were used as recommended by the suppliers (New England Biolabs, Beverly, MA). Nucleotide sequence data reported in this paper are in the DDBJ/EMBL/GenBank databases under accession numbers AB008750 (alp11+) and AB008749 (alp21+).
Gene Disruption
The alp11+ and alp21+ genes were deleted with the use of PCR-generated fragments (Bähler et al., 1998). Dissection of asci from heterozygous diploid cells (PR7 and PR8, respectively) showed that both genes were essential for cell viability, as two viable and two nonviable spores were obtained from 20 tetrads dissected and viable colonies were Ura− in both cases. Extended incubation (5 d at 29°C) of tetrads derived from PR7 led to the appearance of tiny colonies (Ura+) at a frequency of around 50%.
Chromosomal Deletion of alp11 CLIP-170 Sequence
Oligonucleotides (100 bases long) were designed to create an alp11-truncation mutant with the use of a PCR-generated fragment (Bähler et al., 1998) in which a stop codon was introduced at amino acid 164 in one of the chromosomal alp11+ genes in a diploid (PR13; Table 1).
Overexpression and Epitope Tagging
The entire ORFs of the alp11+ and alp21+ genes were cloned by PCR into pREP1 under control of the nmt1 promoter (Maundrell, 1990): oligonucleotides PO1 and PO2 (Table 2) were used for alp11+, yielding pREP-alp11+, and PO3 and PO4 were used for alp21+, yielding pREP-alp21+. Both were functional, as they complemented the temperature-sensitive alp11 mutant. Truncated alp11 genes carried on pREP1 encoding amino acids 1–163 or 131–234 were made by PCR with the use of the following oligonucleotides: PO1 and PO5 or PO2 and PO6 (Table 2), designated pREP-alp111–163 and pREP-alp11131–234, respectively.
Table 2.
Oligonucleotides used in this study
| Name | Sequence (5′–3′) |
|---|---|
| PO1 | TCGACATATGAATGAGATAACTCT |
| PO2 | TCGAAGATCTTCAAAGACCTTCTAAAA |
| PO3 | TCGACATATGCATATATCCACTGGA |
| PO4 | TCGAGGATCCTTAGACGTTTCCACTTT |
| PO5 | TATGGGATCCTTACTCTCCTGCTGCACAGC |
| PO6 | TCGACATATGGACTTTGAAGCAAGTAAAG |
| PO7 | TATGCTGCAGTAATCATATATCTTAAG |
| PO8 | TATGAGATCTATTAACATGGAACCTTG |
| PO9 | TCGACATATGATTAACAATTTGAA |
| PO10 | TATGCATATGAGAGAGATCATTTCC |
| PO11 | TATGGGATCCTTAGTACTCTTCTTC |
C-terminal tagging of the chromosomal alp11+, alp21+, and alp1+ genes with GFP and the hemagglutinin (HA) peptide was performed with the use of a PCR-generated fragment (PR11, PR12, and PR15; Table 1) (Bähler et al., 1998). The entire ORF of alp11+ was also subcloned into pREP42GFP (Craven et al., 1998), yielding pREP42-GFP-alp11+. The tagged gene was functional, as pREP42-GFP-alp11+ rescued the temperature-sensitive alp11-924 mutant. A truncated alp11 gene, which encodes the CLIP-170 domain, was inserted into pREP42GFP, yielding pREP42-GFP-alp11131–124. The entire ORF of atb2+ was also subcloned into pREP2 with the use of oligonucleotides PO10 and PO11 (Table 2), yielding pREP2-atb2+. pREP2-nda3+ was described previously (Hirata et al., 1998).
Immunochemical Assays
Rabbit polyclonal anti-Alp11 and anti-Alp21 antibodies were prepared as follows. To express Alp11 protein, a 700-base pair fragment (corresponding to the entire ORF; PO1 and PO2 were used for PCR) was inserted into pET10c (Invitrogen, Carlsbad, CA). In the case of Alp21, a 740-base pair fragment (corresponding to amino acid residues 269–512; PO4 and PO9 were used for PCR) (Table 2) was used. Immunoblotting was performed with crude sera or affinity-purified antibodies. Affinity-purified rabbit polyclonal anti-Alp1 antibody (Hirata et al., 1998), mouse monoclonal anti-α-tubulin antibody (TAT-1; provided by Dr. Keith Gull), mouse monoclonal anti-β-tubulin antibody (KMX-1; provided by Dr. Keith Gull), mouse monoclonal anti-Cdc2 antibody (provided by Dr. Hiroyuki Yamano), mouse monoclonal anti-GFP antibody (used for immunoblotting; 8362-1, Clontech, Palo Alto, CA), and rabbit polyclonal anti-GFP antibody (used for immunoprecipitation; provided by Dr. Kenneth Sawin) were also used. Horseradish peroxidase–conjugated goat anti-rabbit immunoglobulin G, goat anti-mouse immunoglobulin G (Bio-Rad Laboratories, Richmond, CA), and a chemiluminescence system (ECL, Amersham, Arlington Heights, IL) were used to detect bound antibody. Fission yeast whole cell extracts were prepared with glass beads to disrupt cells as described by Kominami et al. (1998). For immunoprecipitation, PME buffer (0.1 M piperazine-N,N′-bis[2-ethanesulfonic acid], 2 mM EGTA, 1 mM MgCl2, pH 6.9), plus a cocktail of inhibitors as described (Vega et al., 1998), was used.
Gel Filtration Chromatography
Gel filtration chromatography was performed on a Superose-6 column by fast-performance liquid chromatography (Pharmacia Biotech, Piscataway, NJ) in buffer A (20 mM Tris-HCl, pH 7.5, 20% glycerol, 0.1 mM EDTA, 1 mM mercaptoethanol, 5 mM ATP, plus a cocktail of inhibitors). The column was equilibrated with 2 column volumes of buffer A containing 100 mM NaCl. To determine molecular mass, a parallel column was run with standards consisting of dextran (2000 kDa), thyroglobulin (669 kDa), and α-amylase (232 kDa). Fractions (50 μl each) were separated by SDS-PAGE on 10% gels, and fractionated proteins were detected with individual antibodies.
Indirect Immunofluorescence Microscopy
Cells were fixed with methanol, and primary antibodies (anti-Alp11, TAT-1, or anti-GFP) were applied, followed by Cy3-conjugated goat anti-rabbit immunoglobulin G (Sigma) or fluorescein-linked sheep anti-mouse immunoglobulin G (Amersham).
Cross-Suppression Experiments
Diploids heterozygous for alp1+ (ME1), alp11+ (PR7), or alp21+ (PR8) were transformed with multicopy plasmids containing alp1+ (pAL100 and pREP-alp1+; Hirata et al., 1998), alp11+ (pREP-alp11+), or alp21+ (pREP-alp21+). Leu+ transformants were allowed to sporulate, and free spores were directly plated on minimal plates supplemented with adenine and histidine with or without uracil and thiamine. If Ura+Leu+ haploid colonies were obtained in which plasmids (Leu+ prototrophy) were mitotically stable, this plasmid was assigned to be capable of suppressing deletion of the gene of interest. Δalp11 (PR9) and temperature-sensitive alp1 mutants (DH2-8D) were also used.
RESULTS
The alp11-924 Mutation Results in Loss of Microtubule Structures and Growth Polarity Defects
The alp11-924 mutant was isolated in a screen for genes involved in growth polarity control (Hirata et al., 1998). Mutant cells divided once at the restrictive temperature and showed bent or branched morphology (26 and 5%, respectively, after 8 h at 36°C). Wild-type cells do not show this type of abnormal morphology. Mitotic delay was also evident, as condensed chromosomes were observed to a high extent (30%, in contrast to the only 1–2% of exponentially growing wild-type cells that show condensed chromosomes). The percentage of septated cells increased from 20% (at 26°C) to 31% (6 h at 36°C), of which 62% had septa displaced from the center of the cell. The position of the nucleus was also often (24%) displaced from the medial region, and 3% of cells were anucleate, attributed to asymmetrical cell division.
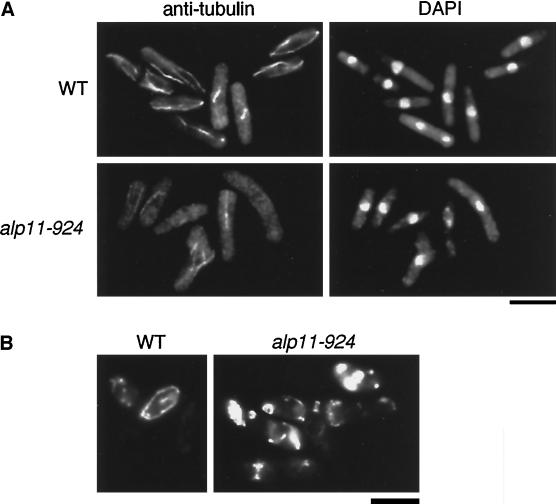
These phenotypes are very similar to those of temperature-sensitive alp1, alp2/atb2, and alp12/nda3 mutants, which are defective in MT function (Hirata et al., 1998; Radcliffe et al., 1998). To examine MT structures in the temperature-sensitive alp11-924 mutant, MTs were stained with anti-α-tubulin antibody. As shown in Figure 1A, MTs became fragile in this mutant. No intact MTs were observed; instead, either none, short, or misoriented MTs clustered around the nucleus remained. Also, mitochondria, the localization of which is dependent on and often coincident with cytoplasmic MTs in fission yeast (Yaffe et al., 1996), became aggregated just as in tubulin mutants (Figure 1B). In line with the defects in MTs, alp11-924 cells were hypersensitive to MT-depolymerizing drugs such as thiabendazole, even at the permissive temperature (26°C; our unpublished results). These results showed that alp11+ is required for the maintenance or establishment of MTs.
Figure 1.
Defective phenotypes of temperature-sensitive alp11-924 mutants. (A) Microtubule structures. Wild-type (WT; HM123) and alp11-924 (DH924) cells were incubated at 36°C for 6 h and processed for immunofluorescence microscopy. Anti-tubulin staining (left, TAT-1) and nuclear staining (right, DAPI) are shown. Bar, 10 μm. (B) Distribution of mitochondria. Wild-type and alp11-924 cells were stained with 2-(4-dimethyaminostyryl)-1-methlpyridinium iodide, as described previously (Yaffe et al., 1996). Bar, 10 μm.
alp11+ and a Multicopy Suppressor alp21+ Encode Homologues of Tubulin-folding Cofactors B and E, Respectively
A fission yeast genomic library was used to isolate genes that complemented the temperature-sensitive alp11-924 mutation. Two different plasmids (pCR1 and pCR11) that contained nonoverlapping inserts were isolated. Genetic linkage analysis (see MATERIALS AND METHODS) indicated that the gene in pCR1 is alp11+ and the gene in pCR11 is a multicopy suppressor (designated alp21+). Nucleotide sequencing showed that alp11+ and alp21+ encode fission yeast homologues of cofactors B and E, respectively (Tian et al., 1996, 1997). Amino acid sequence identity is 35% between human cofactor B and Alp11 and 27% between cofactor E and Alp21.
Comparison of the amino acid sequences of fission yeast and vertebrate cofactors B and E illuminated some interesting features of structural conservation and divergence through evolution. As in cofactor B from other organisms, Alp11B contains a glycine-rich region of around 50 amino acid residues that shows a high degree of homology to a motif found in a group of proteins related to MT-dependent processes. These include CLIP-170/restin, dynactin, kinesin-73, vertebrate cofactor E, and fission yeast Ssm4 (Figure 2, A and B; hereafter this domain will be called the CLIP-170 domain) (Gill et al., 1991; Bilbe et al., 1992; Pierre et al., 1992; Tian et al., 1996; Li et al., 1997; Yamashita et al., 1997). Unlike vertebrate cofactor E and also the budding yeast homologue Pac2 (Hoyt et al., 1997), Alp21E does not contain the analogous domain in the N-terminal region (Figure 2A). However, we did find that both vertebrate cofactor E and the yeast homologues contain leucine-rich repeating (LRR) motifs in their central regions. The repeating motif consists of the consensus LX2LX2LX1–2LX1–3NX1–3L (where X is any amino acid), and 10 repeats are found in Alp21 and vertebrate cofactor E (Figure 2, A and C). LRRs are believed to mediate protein-protein interactions (Kobe and Deisenhofer, 1994).
Figure 2.
Structural motifs found in cofactors B and E. (A) Overall structural organization of various proteins containing the glycine-rich tubulin-interacting (CLIP-170) domain and/or leucine-rich repeats (LRRs). The CLIP-170 domain and LRR units are shown in closed and small open boxes, respectively. Coiled-coil regions are shown with hatched boxes. (B) Sequence alignment of the CLIP-170 domain. Invariant glycines are indicated with asterisks. (C) Sequence alignment of LRRs in Alp21. Consensus amino acid residues are boxed, and a repeating unit is shown below.
Gene Disruption of the alp11+ and alp21+ Genes
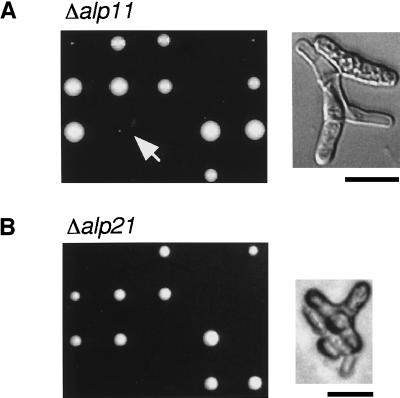
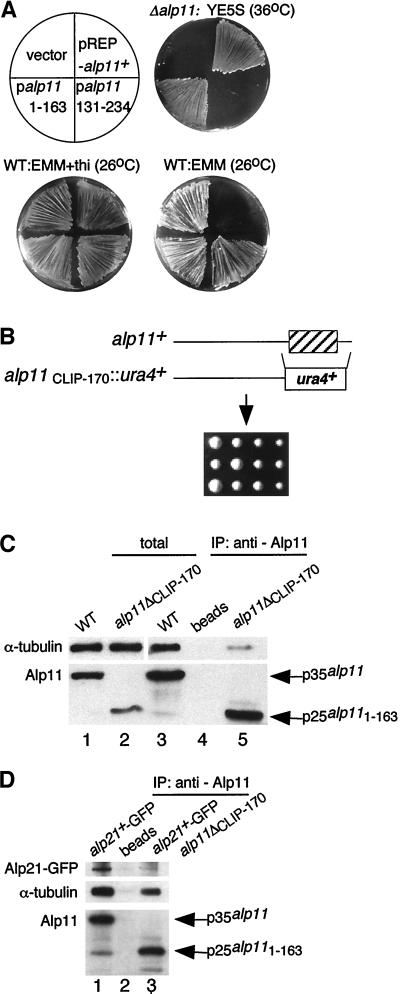
Gene disruption experiments showed that both genes were essential for cell viability (Figure 3, A and B, left). Microscopic observation of inviable spores indicated that in both cases spores were capable of germinating; they divided one or two times and arrested, with abnormally bent or branched cell morphology (Figure 3B, right). We also noticed that after longer incubation (5 d at 29°C) ∼50% of alp11-deleted spores formed tiny Ura+ (Δalp11) colonies (Figure 3A, left, arrow), some of which managed to survive upon subculture. Cells from these colonies were defective in cell morphology (Figure 3A, right) and showed temperature-sensitive and also cold-sensitive growth. We have not genetically analyzed these viable strains further but suspect that a suppressor mutation(s) may have arisen that rescues Δalp11. This result showed that, like cofactor D–encoding alp1+ (Hirata et al., 1998), cofactors B– and E–encoding alp11+ and alp21+ genes are essential for cell viability.
Figure 3.
Gene disruption of alp11+ and alp21+ genes. Tetrad analysis of diploids heterozygous for alp11 (A; PR7) or alp21 (B; PR8) is shown. Note that tiny colonies grow from some tetrads of PR7 (arrow). Cell morphology of an alp11-deleted haploid cell (A) and an alp21-deleted cell (B) is shown. Bars, 10 μm.
Physical Interaction among Cofactors and α-Tubulin
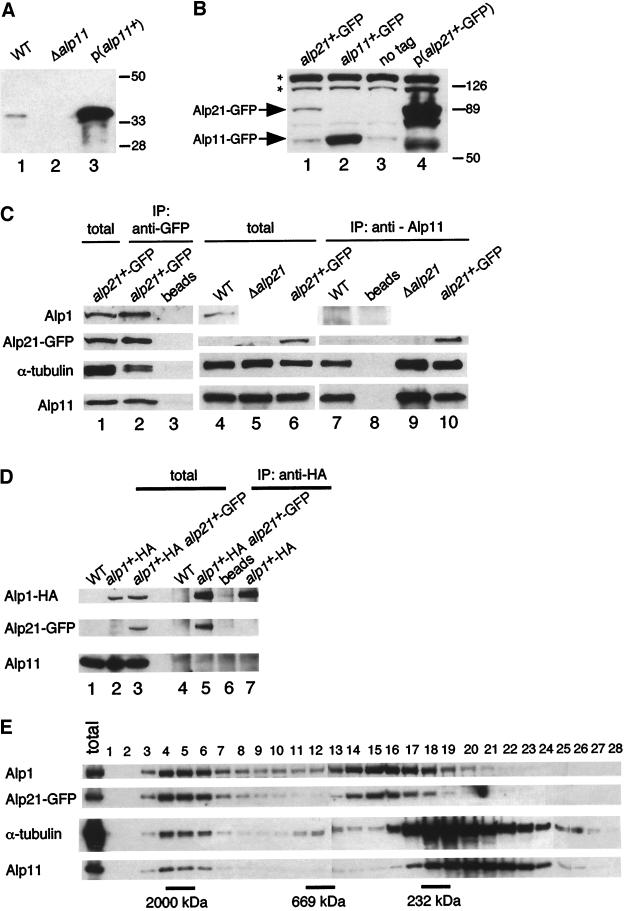
Polyclonal antibodies against Alp11B and Alp21E were produced. Immunoblotting with the use of anti-Alp11 antibody against total cell extracts prepared from wild type identified a single band of 35 kDa (p35alp11; Figure 4A, lane 1), which was missing in Δalp11 and highly enhanced in alp11+-overexpressing cells (Figure 4A, lanes 2 and 3). In the case of anti-Alp21 antibody, sera were not specific enough to identify the alp21+ gene product derived from a single chromosomal gene. Therefore, the chromosomal alp21+ gene (and also alp11+) was tagged with GFP at the C terminus. Also the HA epitope was used to tag the chromosomal alp1+ gene (see MATERIALS AND METHODS). Tagging did not interfere with Alp11B, Alp21E, or Alp1D function, as haploid strains containing integrated GFP or HA behaved like wild-type cells. Immunoblotting with anti-GFP antibody showed a specific band of around 90 kDa in an alp21+-GFP strain (Figure 4B, lane 1). This band was missing in the untagged and alp11+-GFP strains (Figure 4B, lanes 2 and 3) and highly increased in a strain carrying alp21+-GFP on a multicopy plasmid (Figure 4B, lane 4). Alp11B-GFP was detected as a 60-kDa band (Figure 4B, lane 2). It should be noted that Alp11B appears to exist in a greater amount than Alp21E; the difference between the levels of Alp11B-GFP and Alp21E-GFP detected with the same anti-GFP antibody was at least 10-fold (Figure 4B; compare lanes 1 and 2).
Figure 4.
Interaction and gel filtration analysis of cofactors and tubulins. (A) Identification of the alp11+ gene product. Immunoblotting was performed with anti-Alp11 antibody against cell extracts prepared from wild type (lane 1), Δalp11 (lane 2; PR9), or wild type carrying multicopy alp11+ (lane 3). The positions of size markers (kilodaltons) are shown on the right. (B) Identification of the alp21+-GFP gene product. Immunoblotting was performed with anti-GFP antibody against cell extracts prepared from wild type (lane 3), strains containing integrated alp11+-GFP (lane 2; PR11) or alp21+-GFP (lane 1; PR12), or the strain carrying multicopy alp21+-GFP (lane 4). Asterisks show nonspecific bands. (C and D) Interaction among cofactors and α-tubulin. Cell extracts were prepared from wild type (C, lanes 4 and 7, and D, lanes 1 and 4), a strain containing integrated alp21+-GFP (C, lanes 1, 2, 3, 6, 8, and 10; PR12), Δalp21 containing pDB-alp1+ (C, lanes 5 and 9; PR10), a strain containing integrated alp1+-HA (D, lanes 2 and 7; PR15), or a strain containing alp1+-HA and alp21+-GFP (D, lanes 3, 5, and 6; PR16), and immunoprecipitation (shown as IP) was performed with anti-GFP antibody (C, lane 2), anti-Alp11 antibody (C, lanes 7, 9, and 10), anti-HA antibody (D, lanes 4, 5, and 7), or mock treatment (C, lanes 3 and 8, and D, lane 6). Precipitated proteins were detected with anti-Alp1, anti-GFP, anti-α-tubulin, anti-Alp11, and anti-HA antibodies. Total cell extracts (corresponding to 1/30th the amount used for immunoprecipitation) were also run (C, lanes 1, 4, 5, and 6, and D, lanes 1, 2, and 3). (E) Gel filtration chromatography. Soluble extracts from a strain containing integrated alp21+-GFP (PR18) were separated through a Superose-6 column, and fractions were analyzed by immunoblotting with the antibodies shown. Total extract (10 μg) was run on the far left panel. The positions of size markers (232, 669, and 2000 kDa) are also shown.
To explore in vivo physical interactions between cofactors and tubulins, immunoprecipitation experiments were performed. Using the integrated alp21+-GFP strain and anti-GFP antibody, we found that Alp21E interacts with Alp1D, Alp11B, and α-tubulin (Figure 4C, lane 2). Reciprocal immunoprecipitation experiments with anti-Alp11 antibody confirmed the interaction between Alp11B and Alp21E and demonstrated that Alp11B also coprecipitates with α-tubulin (Figure 4C, lanes 7 and 10). The interaction is specific, as no proteins were precipitated without the addition of antibody (Figure 4C, lanes 3 and 8) or when a Δalp11 (our unpublished results) or untagged strain was used (Figure 4C, lane 7). Alp11B and α-tubulin are able to interact in the absence of Alp21E, as these two proteins coimmunoprecipitated in a Δalp21 strain (Figure 4C, lane 9; kept viable by multicopy plasmids containing the alp1+ gene [see below]). On the other hand, Alp11B did not bind Alp1D (Figure 4C, lane 7), nor did we see an interaction between Alp11B/Alp21E and β-tubulin (data not shown). The interaction described above was confirmed by independent immunoprecipitation experiments with the use of Alp1D-HA–tagged strains. As shown in Figure 4D (lanes 5 and 7), Alp1D-HA and Alp21E-GFP, but not Alp11B, coimmunoprecipitated. In summary, Alp11B interacts with α-tubulin and Alp21E but not Alp1D, and Alp21E also interacts with Alp1D. These results are consistent with the notion that cofactor B specifically binds free α-tubulin monomers (Tian et al., 1997; Feierbach et al., 1999) and suggest that Alp21E acts at an intermediate position in the α-tubulin–folding pathway between Alp11B and Alp1D.
Size Fractionation Analysis of Cofactors and Tubulins
We were interested in the native complex state of cofactors and tubulins in the cell. To this end, we analyzed the behavior of cofactors and tubulins by gel filtration chromatography. As shown in Figure 4E, it was found that Alp1D and Alp21E exist predominantly in two fractions, one larger (fractions 4–6, ∼2000 kDa) and the other smaller (fractions 14–16, 400–500 kDa). The ratio of these two complexes appeared equal. In the case of Alp11B and α-tubulin, most of these proteins exist in a complex(es) of a small size (fractions 17–22, 100–200 kDa), although the chromatographic patterns of these two proteins appeared not to be the same. In addition, a relatively minor population (<10%) of both Alp11B and α-tubulin existed in a larger fraction that corresponded in size to Alp1D and Alp21E (fractions 4–6). This result suggests that Alp1D and Alp21E form stable dual complexes in the cell.
The Level of α-Tubulin Is Decreased in the alp1 and alp11 Mutants
The possibility that mutations in cofactors affected the level of free tubulin molecules was examined. It was found that the steady-state level of α-tubulin in temperature-sensitive alp11-924 mutants was reduced at the restrictive temperature (Figure 5A, lanes 1 and 3) and that the level was lower than in the wild type even at the permissive temperature (Figure 5A, lane 2). The level of α-tubulin became comparable to that in wild-type cells when temperature-sensitive alp11-924 mutants were transformed with plasmids containing alp11+ (Figure 5, lane 4), whereas high-dose alp21+ appeared not to restore the amount of α-tubulin (Figure 5, lane 5).
Figure 5.
The level of α-tubulin decreases in the alp11 and alp1 mutants. (A) The level of α-tubulin in the alp11 mutant. Wild-type (lane 1) and alp11-924 (lanes 2–5) strains containing vector (lanes 1–3), pREP-alp11+ (lane 4), or pREP-alp21+ (lane 5) were grown at 26°C in the absence of thiamine for 12 h (lane 2), shifted to 36°C, and incubated for 8 h (lanes 1 and 3–5). Immunoblotting was performed with the indicated antibodies. (B) The level of α-tubulin and Alp11B in the alp1 mutant. Wild-type (lane 1) and alp1-1315 (lanes 2–4; DH2-8D) strains containing vector (lanes 1–3) or pDB-alp1+ (lane 4) were grown and immunoblotted with the indicated antibodies. Densitometric calibration of the data is shown in the lower panels in A and B. The relative levels of α-tubulin and Alp11B were measured with the level of Cdc2 as an internal control.
A similar analysis was performed with a temperature-sensitive alp1 strain. As in the alp11 mutant, it was found that the level of α-tubulin also significantly decreased in this mutant (Figure 5B, lanes 1 and 3). Conversely, Alp11B levels increased significantly (190%) in this mutant. These defects were reversed by the introduction of the wild-type alp1+ gene on plasmids (Figure 5B, lane 4). In contrast, the level of β-tubulin appeared unchanged in both alp1 and alp11 mutants (our unpublished results). These results suggested that unfolded α-tubulin monomers are unstable in the absence of Alp11B or Alp1D function and that Alp1D negatively regulates the level of Alp11B.
Ectopic Overexpression of alp11+ Is Toxic and Rescued by Coexpression of the α-Tubulin Gene
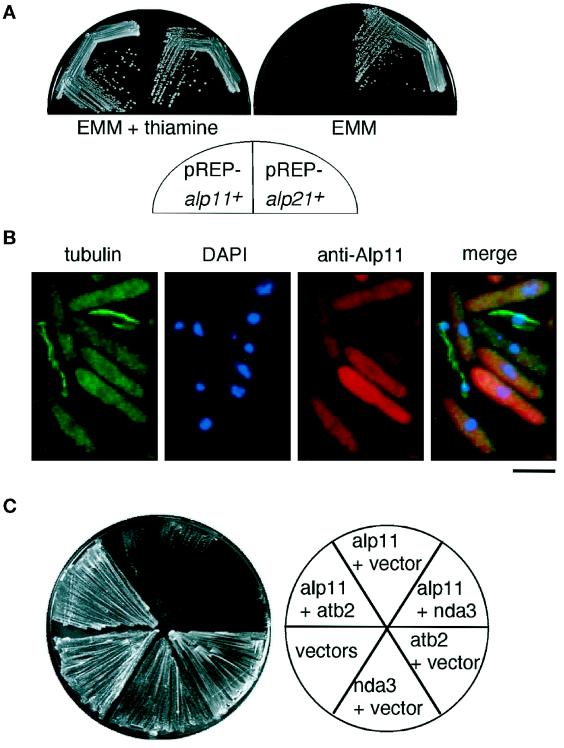
Our previous results showed that ectopic overexpression of cofactor D–encoding alp1+ was lethal (Hirata et al., 1998), suggesting that perturbation of the stoichiometry of cofactors is toxic to the cell. To examine the phenotypes arising from overproduction of fission yeast cofactors B and E, the entire ORFs of the alp11+ and alp21+ genes were inserted into plasmids containing the thiamine-repressible nmt1 promoter (pREP-alp11+ and pREP-alp21+). It was found that alp11+, but not alp21+, was lethal when ectopically overexpressed (Figure 6A).
Figure 6.
Ectopic overexpression of alp11+. (A) Lethal overexpression of alp11+. Cells containing pREP-alp11+ (left on each plate) or pREP-alp21+ (right) were streaked on minimal plates in the presence (left plate) or absence (right) of thiamine and incubated for 3 d at 26°C. Overproduction of each protein has been checked by immunoblotting. (B) Microtubule structures in alp11+-overexpressing cells. Cells containing pREP-alp11+ were grown in the absence of thiamine for 23 h and processed for immunofluorescence microscopy using anti-α-tubulin antibody and anti-Alp11 antibody. Chromosomal DNA was stained with DAPI. The merged image is shown on the right. Note that no MTs were observed in most cells in which Alp11B was overexpressed and that the presence of MTs correlates with lower Alp11B levels. Bar, 10 μm. (C) Suppression of lethal overexpression of alp11+ by coexpression of atb2+. Wild-type cells were doubly transformed with the following plasmids: two empty vectors (pREP1 and pREP2), a vector plus pREP-alp11+, a vector plus pREP2-atb2+, a vector plus pREP2-nda3+, pREP-alp11+ plus pREP2-atb2+, or pREP-alp11+ plus pREP2-nda3+. Transformants were streaked onto minimal media without thiamine and incubated for 3 d at 29°C.
Cells in which alp11+ was overexpressed showed elongated morphology, and prolonged incubation resulted in bent or abnormal morphology, which was similar to that of temperature-sensitive alp11-924. Staining of these alp11+-overexpressing cells with anti-tubulin antibody revealed that in the majority of cells no intact MTs were present; instead, uniform cytoplasmic staining was evident (Figure 6B, left). This result showed that an excess amount of Alp11B abrogates MT structure and function. If this toxicity were due to the binding and absorption of free α-tubulin, co-overexpression of an α-tubulin–encoding gene might rescue the lethality. That was indeed the case. As shown in Figure 6C, cells coexpressing alp11+ and atb2+ (α2-tubulin; Adachi et al., 1986), but not nda3+ (β-tubulin; Hiraoka et al., 1984), were viable. This result suggested that the lethality of excess Alp11B is attributable to the absorption of free α-tubulin molecules.
The Glycine-rich Domain Is Dispensable but Required for the Efficient Interaction between Alp11B and Free α-Tubulins, and Also Alp21E
The Alp11B glycine-rich domain locates in the C-terminal region (Figure 2, A and B, CLIP-170 domain, amino acids 166–216). To test whether this domain is essential for Alp11B function, DNA fragments encoding N-terminal (amino acids 1–163) or C-terminal (amino acids 131–234) portions of Alp11B were subcloned into plasmids under control of the nmt1 promoter (pREP-alp111–163 and pREP-alp11131–234, respectively), and suppression over Δalp11 was examined. As shown in Figure 7A, pREP-alp111–163, but not pREP-alp11131–234, was capable of suppressing the growth defects of Δalp11. It is of note that, unlike intact Alp11, neither Alp111–163 nor Alp11131–234 was toxic when ectopically overproduced. To confirm the dispensability of the CLIP-170 domain, the C-terminal region was deleted in one of the chromosomal alp11+ genes of a diploid strain (truncated Alp11 protein was designated Alp111–163). It was found that the growth of haploid cells containing Alp111–163 was indistinguishable from that of wild-type cells (Figure 7B).
Figure 7.
The function of the conserved glycine-rich domain of Alp11B. (A) Suppression of the growth defects of alp11-deleted cells. A Δalp11 strain (PR9) was transformed with plasmids containing the alp11+ gene (pREP-alp11+; top right), the C-terminal region (pREP-alp11131–234; bottom right), the N-terminal region (pREP-alp111–163; bottom left), or a vector (top left), streaked on rich medium at 36°C (top right plate) or minimal medium in the absence (lower right plate) or presence (lower left plate) of thiamine at 26°C, and incubated for 4 d. (B) The glycine-rich region of Alp11B is dispensable. The CLIP-170 domain is shown as a hatched box (top). Tetrad analysis was performed with a heterozygous diploid in which onecopy of the alp11 genes was truncated at amino acid 163 (bottom; PR13). (C) The glycine-rich domain is important for efficient binding between Alp11B and α-tubulin. Immunoprecipitation (IP) was performed with anti-Alp11 antibody (lanes 3 and 5) or mock treatment (lane 4) against cell extracts prepared from wild-type (lanes 1, 3, and 4) or a strain containing Alp111–163 (shown as alp11ΔCLIP-170) (lanes 2 and 5; PR14). Total extracts were also run (lanes 1 and 2). (D) The glycine-rich domain is required for association between Alp11B and Alp21E. A strain containing Alp111–163 and integrated alp21+-GFP (PR17) was used for immunoprecipitation with anti-Alp11 antibody, followed by immunoblotting with anti-GFP, anti-α-tubulin, and anti-Alp11 antibodies.
As the CLIP-170 domain appears to be involved in MT binding in other proteins (Rickard and Kreis, 1996) and Alp11B interacts with α-tubulin, the role of the CLIP-170 domain in the binding of α-tubulin was addressed. Cell extracts were prepared from a strain in which this domain was deleted (Alp111–163), and immunoprecipitation was performed using anti-Alp11 antibody. It was found that the binding between Alp111–163 and α-tubulins was greatly diminished (Figure 7C, lane 5). Furthermore, Alp111–163 lost the ability to associate stably not only with α-tubulin but also with Alp21E (Figure 4D, lane 3). Thus, the CLIP-170 domain of Alp11B is dispensable for the essential function of Alp11B but confers a lethal interfering effect when overproduced and, importantly, is required for the efficient binding between Alp11B and α-tubulin, and also Alp21E. In other words, the interaction between Alp11B and both Alp21E and α-tubulin is dependent on the CLIP-170 domain.
The Cellular Localization of Alp11B
The cellular localization of Alp11B was examined in detail by fluorescence microscopy with the use of specific antibodies and GFP. Immunofluorescence microscopy in wild-type cells with anti-Alp11 showed punctate cytoplasmic staining (Figure 8A, left). The staining is specific, as Δalp11 cells showed no signals (Figure 8A, right). Alp21E also showed cytoplasmic staining when overproduced (our unpublished results). A similar pattern was observed with GFP staining in cells carrying multicopy alp11+-GFP. We also examined the localization of Alp111–163 and again observed only cytoplasmic staining (Figure 8B). The same cytoplasmic staining was also observed in cells expressing Alp11131–234-GFP (Figure 8C). These data show that Alp11B localizes to the cytoplasm and that, in the case of this protein, the CLIP-170 domain appears to play no role in MT binding.
Figure 8.
The cellular localization of Alp11. Immunolocalization of Alp11B and truncated Alp11B proteins. (A) Wild-type (WT; left) or Δalp11 (right) cells were processed for immunofluorescence microscopy using anti-Alp11 antibody (top) or DAPI (bottom). (B) Wild-type cells containing a vector (left), pREP-alp11+ (middle), or pREP-alp111–163 (right) were grown in minimal medium for 18 h and processed for immunofluorescence microscopy using anti-tubulin antibody (top), anti-Alp11 antibody (middle), or DAPI (bottom). (C) Wild-type cells containing a vector (left) or pREP42-GFP-alp11131–234 (right) were grown in minimal medium for 18 h and processed for immunofluorescence microscopy using anti-GFP antibody (top) or anti-tubulin antibody (bottom). Bars, 10 μm.
Increased Level of Alp1D Is Sufficient to Rescue the Loss of Alp11B or Alp21E
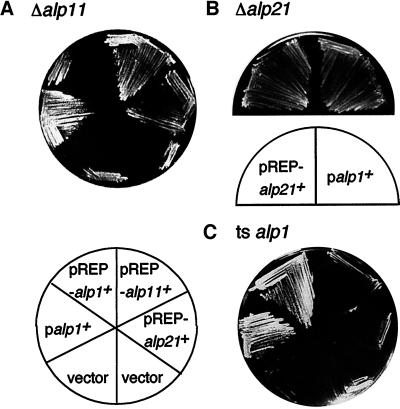
To establish the functional order of cofactors in the tubulin-folding pathway in vivo, multicopy plasmids containing cofactor homologues were introduced into each alp mutant. It transpired that the temperature-sensitive growth defects of an alp11 deletion strain were suppressed by multicopy plasmids containing alp1+, alp11+, or alp21+ (Figure 9A). An identical suppression result was obtained when a diploid heterozygous for alp11 was used (Table 3). Second, the alp21 disruption was rescued by multicopy plasmids containing alp1+ or alp21+ but not alp11+ (Figure 9B, Table 3).
Figure 9.
Functional suppression. (A) Suppression of the alp11 mutation. Δalp11 (PR9) was transformed with the plasmids shown, and transformants were streaked on a minimal plate containing thiamine and incubated at 36°C for 3 d. pREP-alp1+ was not capable of suppressing the alp11 mutant under these conditions (repressed). (B) Suppression of the alp21 disruptant. A diploid heterozygous for alp21 (PR8) was transformed with various plasmids, and random spore analysis was performed. Leu+Ura+ colonies were streaked on minimal plates containing thiamine and incubated at 30°C for 3 d. Haploid Δalp21 strains containing pREP-alp21+ (left) or pDB-alp1+ (right; PR10) are shown. (C) Suppression of the alp1 mutation. alp1-1315 (DH2-8D) was transformed with the plasmids indicated and streaked.
Table 3.
Suppression analysis among cofactor homologs
| Deleted gene | Gene on plasmid
|
|||
|---|---|---|---|---|
| Vector | alp11+ | alp21+ | alp1+ | |
| Δalp11 | − | + | + | + |
| Δalp21 | − | − | + | + |
| Δalp1 | − | − | − | + |
Heterozygous diploids (ME1, PR7, and PR8; see Table 1) were transformed with a vector (pREP1) or multicopy plasmids containing individual genes. Diploid transformants were allowed to sporulate, and free spores were spread on minimal plates supplemented with histidine and adenine to select viable Leu+Ura+ haploid colonies. (+) We obtained Leu+Ura+ haploid cells that contain the deleted gene carrying the indicated plasmids; (−) we failed to obtain Ura+ haploid.
Finally, temperature-sensitive alp1 mutations were not suppressed by plasmids containing either alp11+ or alp21+ (Figure 9C). Nor could the alp1 deletion be rescued by overexpression of either of these two genes (Table 3). Also, multicopy plasmids containing the α-tubulin–encoding gene (atb2+) are not capable of complementing temperature-sensitive alp1 or alp11 mutants. These results indicate that the essential alp11+ gene becomes dispensable when an excess level of Alp21E or Alp1D is supplied, and similarly that alp21+ is nonessential when Alp1D exists in a larger amount. Therefore, the cofactor homologues appear to operate in a linear manner (Alp11B-Alp21E-Alp1D) in the biogenesis of MTs.
DISCUSSION
An important notion arising from the present study is the existence of a functional hierarchy among cofactors. Biochemical analysis in mammalian systems showed cofactors E and D to have parallel and indispensable roles in the folding of α- and β-tubulins, respectively, in which these cofactors are proposed to form a quadruple complex with α- and β-tubulin upon convergence of the two distinct folding pathways (Tian et al., 1996, 1997). A crucial finding from our study is the bypass of the Alp21E requirement by overproduction of Alp1D (Figure 10A). This situation has never been observed in in vitro tubulin-folding reactions, in which cofactor E is absolutely required for the formation of correctly folded α/β-tubulin heterodimers. It is likely that Alp1D is required for the folding of both α- and β-tubulins, as suggested (Tian et al., 1996, 1997); however, the folding of α-tubulin may be more complex than that of β-tubulin. At present, the importance of the in vivo β-tubulin–folding pathway remains to be determined. Analysis of the recently identified S. pombe homologue of cofactor A (P.A. Radcliffe and T. Toda, unpublished results) and the further analysis of Alp1D should clarify this point.
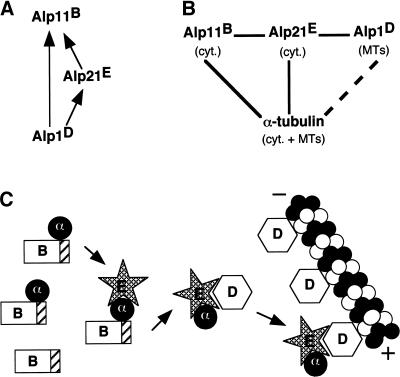
Figure 10.
Model for the sequential operation of cofactors in microtubule biogenesis. (A) Genetic suppression. Arrows (X→Y) indicate that a high level of “X” suppresses loss of “Y” function. (B) Physical interaction. Solid lines (X—Y) indicate interaction between “X” and “Y” by immunoprecipitation. The dashed line indicates interaction by in vitro MT-binding assay (Hirata et al., 1998). cyt., localization in cytoplasm; MTs, colocalization with MTs. (C) A schematic model of cofactor functions. Cofactor B (Alp11) captures α-tubulin molecules released from chaperonins (not shown) and maintains a reservoir of free α-tubulins, which in its absence become unstable. The α-tubulin–binding CLIP-170 domain is depicted by hatched boxes. Cofactor E (Alp21) binds cofactor B, α-tubulin, and cofactor D (Alp1), perhaps via its central region of leucine-rich repeats. It acts as a mediator transporting α-tubulin between cofactors B and D. Interaction between cofactors B and E might be indirect, possibly through α-tubulin. A subpopulation of cofactor D colocalizes with microtubules and delivers α-tubulin (or α/β-heterodimer) to growing microtubule tips.
The most simple explanation of our data would be that cofactors constitute a functionally linear pathway in vivo: Alp11B-Alp21E-Alp1D (Figure 10B), although alternative models remain possible. For example, Alp21E and Alp1D may function together rather than in a sequential manner. Immunoprecipitation and gel filtration analysis support this latter possibility. The quadruple complex (EαDβ) may exist transiently in the cell, but this complex formation is not essential for MT biogenesis, and Alp21E is dispensable if Alp1D is supplied in excess in fission yeast. Of most importance is the genetic epistasis in which multicopy plasmids containing alp1+ suppress alp21-deleted cells. In line with our data, in budding yeast partial suppression of the benomyl sensitivity of mutations in PAC2 (encoding cofactor E homologue) by multicopy CIN1 (cofactor D homologue) has been reported (Hoyt et al., 1997). Because of the difference between the mammalian and yeast systems, a direct comparison might be difficult. Further work will be required to clarify the mode of action of these cofactors in tubulin-folding pathways.
Distinct Roles of Cofactors in MT Biogenesis
Our analysis is consistent with the idea that Alp11B captures newly synthesized α-tubulin molecules that are released from chaperonins and maintains a reservoir of partially folded α-tubulin in the cell (Figure 10C) (Tian et al., 1997; Feierbach et al., 1999). It binds and perhaps protects unfolded α-tubulin from degradation, although the stable binding between Alp11B and α-tubulin appears not to be essential for Alp11B function. Alp21E, which interacts with both Alp11B and Alp1D as well as α-tubulin, may function as a mediator that transfers α-tubulin from the pool maintained by Alp11B to Alp1D. It should be noted that interaction between Alp11B and Alp21E requires the CLIP-170 domain of Alp11B. This suggests either that the CLIP-170 domain has a dual binding capacity for both α-tubulin and Alp21E or that interaction between Alp11B and Alp21E is indirect and occurs via α-tubulin. Although we cannot rule out the former possibility, suppression of lethal Alp11B overproduction by simultaneous expression of α-tubulin alone suggests that Alp11B and Alp21E interact indirectly. Some population of Alp1D colocalizes with MTs in vivo and cosediments with polymerized porcine brain MTs in vitro (Hirata et al., 1998). This behavior is unique among the cofactors; as shown in this study, we failed to see colocalization of either Alp11B or Alp21E with MTs. It is possible that cofactor D functions to deliver a cargo of α/β-heterodimers to the “plus” end of MTs via MTs (see Figure 10C). Cofactor D may also function to preserve MT integrity. We note that Alp1D and mammalian cofactor D contain two repeats of HEAT motifs that are involved in protein-protein interactions (Andrade and Bork, 1995; Groves et al., 1999).
That cells have such an elaborate mechanism for the folding and transport of tubulin molecules indicates that an abundance of free tubulin molecules is likely to be deleterious. This implies that flux through the folding pathway, especially the later stages, may be tightly coordinated with the changing needs of the cell. In this way, the cell is able to quickly mobilize this supply to maximize heterodimer availability at times of high demand. In this context, it is likely that activities of cofactors, either quantitatively or qualitatively, are regulated during the cell cycle or developmental stage. Up-regulation of Alp11B in the temperature-sensitive alp1 mutant suggests that a negative feedback mechanism may exist that regulates the relative abundance of each cofactor.
Regulation of Tubulin Levels by Cofactors
Ectopic overproduction of Alp11B, like that of Alp1D (Hirata et al., 1998), is toxic. We note that this toxic effect is enhanced at lower temperatures (26°C). Observation of overproducing cells revealed phenotypes similar to those of alp11 mutants, including polarity defects and loss of MTs. This indicates that severe perturbation of Alp11B levels results in a lethal shortage of correctly folded α-tubulin molecules. It is likely that excess Alp11B captures α-tubulin irreversibly, thereby inhibiting subsequent reactions by Alp21E. Consistent with this effect, an increased level of α-tubulin in Alp11B-overproducing cells rescues the lethality. The endogenous level of Alp11B in the cell is much higher (>10-fold) than that of Alp21E, which would correlate well with the roles of cofactors B and E as a reservoir and as a mediator, respectively, of free α-tubulin.
As shown in this study, the steady-state levels of α-tubulin decrease substantially in the temperature-sensitive alp1 and alp11 mutants. In agreement with this phenomenon, in in vitro systems perturbation of the stoichiometry of cofactors results in the instability of tubulin molecules (Tian et al., 1997). As cofactor D is involved in the folding of both α- and β-tubulins (Tian et al., 1996, 1997), one might expect that in alp1 mutants the levels of β-tubulins also decrease, but this was not the case. It appears that the physical states of α- and β-tubulin in the absence of Alp1D function differ in terms of protein stability. This implies that α-tubulin is the major target for regulation of the level of free tubulin pools via cofactors. It may also explain why an excess of β-tubulin is more harmful to the cell than an excess of α-tubulin (Hiraoka et al., 1984; Toda et al., 1984).
Structural Conservation and Diversity of Cofactor E
The LRR motif is a conserved structural feature present in cofactor E from all species. Although mammalian cofactor E contains the CLIP-170 domain (as does the budding yeast homologue Pac2; Hoyt et al., 1997; Tian et al., 1997), fission yeast Alp21E does not. It is possible that fission yeast has evolved differently from other organisms, such that Alp21E has lost a crucial function that both budding yeast Pac2 and mammalian cofactor E retain. Alternatively, as in the case of Alp11B, the CLIP-170 domain in cofactor E may play an accessory role in its interaction with α-tubulin, with the LRRs crucial for the function of cofactor E. Thus, it is possible that the CLIP-170 domain has been lost from Alp21E during evolution because it is not essential.
Function of the Glycine-rich (CLIP-170) Domain in Binding Tubulin and MTs
We have shown here that the CLIP-170 domain of Alp11B is required for the efficient binding of α-tubulin by the cofactor. It has been proposed and generally assumed that the CLIP-170 domain is a MT-binding motif (Riehemann and Sorg, 1993; Rickard and Kreis, 1996). There is solid evidence to support the notion that all of the CLIP-170-domain–containing proteins are indeed involved in MT-related processes; however, not all of these proteins are MT-associated proteins. For example, p150Glued and CLIP-170 do not always colocalize with MTs in the cell (Dujardin et al., 1998; Robinson et al., 1998). Also, vertebrate cofactors B and E and fission yeast Alp11B are not MT-binding proteins (Tian et al., 1997; this study). It is nonetheless possible that MT binding of Alp11B was not detected for some technical reason or that only a subpopulation of Alp11B colocalizes with MTs. In budding yeast, Alf1B has been shown to localize with MTs when overexpressed (Feierbach et al., 1999). Alternatively, the CLIP-170 domain is not sufficient for MT binding but is an archetypal motif for α-tubulin binding, as has been suggested (Lewis et al., 1997).
In line with this idea, it has recently been shown that budding yeast Alf1B also binds free α-tubulin (Feierbach et al., 1999). It is possible that the CLIP-170-domain–containing proteins that bind MTs require additional amino acid sequences other than the CLIP-170 domain to ensure MT binding. For instance, many of these CLIP-170-domain–containing proteins consist of regions with coiled-coil structures and/or those rich in basic amino acid residues adjacent to the CLIP-170 domain (Rickard and Kreis, 1996). Alternatively, minor evolutionary modification of this domain or adjacent regions may have conferred the ability to discriminate between the various forms of α-tubulin in the cell (i.e., monomers and MTs). Detailed domain analysis of these proteins or domain-swapping experiments between different proteins will be required for further clarification of the molecular role of the CLIP-170 domain.
Note added in proof
While this paper was under review, Grishchuk and McIntosh published the paper on Alp21 (called Sto1, Sto1p a fission yeast protein similar to tubulin folding Cofactor E, plays an essential role in mitotic microtubules assembly [J Cell Sci. (1999). 112, 1979–1988], in which the authors show A1p21 also localizes in the cytoplasm.
ACKNOWLEDGMENTS
We thank Drs. Keith Gull, Kenneth Sawin, and Hiroyuki Yamano for providing materials used in this study. We are grateful to Drs. Ekaterina L. Grishchuk, J. Richard McIntosh, and Masayuki Yamamoto for sharing results before publication. We thank Drs. Chris Norbury and Itziar Ochotorena for instruction and help in gel filtration. We thank Drs. Jacqueline Hayles, Paul Nurse, and Graham Warren for critical reading of the manuscript and useful suggestions. This article is dedicated to the memory of Kazuhiko Umesono.
Abbreviations used:
- GFP
green fluorescent protein
- HA
hemagglutinin
- MT
microtubule
REFERENCES
- Adachi Y, Toda T, Yanagida M. Differential expression of essential and nonessential α-tubulin genes in Schizosaccharomyces pombe. Mol Cell Biol. 1986;6:2168–2178. doi: 10.1128/mcb.6.6.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade MA, Bork P. HEAT repeats in the Huntington’s disease protein. Nat Genet. 1995;11:115–116. doi: 10.1038/ng1095-115. [DOI] [PubMed] [Google Scholar]
- Archer JE, Vega LR, Solomon F. Rbl2p, a yeast protein that binds to β-tubulin and participates in microtubule function in vivo. Cell. 1995;82:425–434. doi: 10.1016/0092-8674(95)90431-x. [DOI] [PubMed] [Google Scholar]
- Bachurski C, Theodorakis N, Coulson RM, Cleveland DW. An amino-terminal tetrapeptide specifies cotranslational degradation of β-tubulin but not α-tubulin mRNA. Mol Cell Biol. 1994;14:4076–4086. doi: 10.1128/mcb.14.6.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler J, Wu J, Longtine MS, Shah NG, McKenzie A, III, Steever AB, Wach A, Philippsen P, Pringle JR. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–951. doi: 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Belmont LD, Mitchison TJ. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 1996;84:623–631. doi: 10.1016/s0092-8674(00)81037-5. [DOI] [PubMed] [Google Scholar]
- Bilbe G, et al. Restin: a novel intermediate filament-associated protein highly expressed in the Reed-Sternberg cells of Hodgkin’s disease. EMBO J. 1992;11:2103–2113. doi: 10.1002/j.1460-2075.1992.tb05269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven RA, Griffiths DJF, Sheldrick KS, Randall RE, Hagan IM, Carr AM. Vectors for the expression of tagged proteins in Schizosaccharomyces pombe. Gene. 1998;221:59–68. doi: 10.1016/s0378-1119(98)00434-x. [DOI] [PubMed] [Google Scholar]
- Dujardin D, Wacker UI, Moreau A, Schroer TA, Rickard JE, De Mey JR. Evidence for a role of CLIP-170 in the establishment of metaphase chromosome alignment. J Cell Biol. 1998;141:849–862. doi: 10.1083/jcb.141.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierbach B, Nogales E, Downing KH, Stearns T. Alf1p, a CLIP-170 domain-containing protein, is functionally and physically associated with α-tubulin. J Cell Biol. 1999;144:113–124. doi: 10.1083/jcb.144.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Melki R, Walden PD, Lewis SA, Ampe C, Rommelaere H, Vandekerckhove J, Cowan NJ. A novel cochaperonin that modulates the ATPase activity of cytoplasmic chaperonin. J Cell Biol. 1994;125:989–996. doi: 10.1083/jcb.125.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Thomas JO, Chow RL, Lee GH, Cowan NJ. A cytoplasmic chaperonin that catalyzes β-actin folding. Cell. 1992;69:1043–1050. doi: 10.1016/0092-8674(92)90622-j. [DOI] [PubMed] [Google Scholar]
- Gao Y, Vainberg IE, Chow RL, Cowan NJ. Two cofactors and cytoplasmic chaperonin are required for the folding of α- and β-tubulin. Mol Cell Biol. 1993;13:2478–2485. doi: 10.1128/mcb.13.4.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler S, Siegers K, Schiebel E. A novel protein complex promoting formation of functional α- and γ-tubulin. EMBO J. 1998;17:952–966. doi: 10.1093/emboj/17.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SR, Schroer TA, Szilak I, Steuer ER, Sheetz MP, Cleveland DW. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J Cell Biol. 1991;115:1639–1650. doi: 10.1083/jcb.115.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Garay ML, Cabral F. alpha-Tubulin limits its own synthesis: evidence for a mechanism involving translational repression. J Cell Biol. 1996;135:1525–1534. doi: 10.1083/jcb.135.6.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves MR, Hanlon N, Turowski P, Hemmings BA, Barford D. The structure of the protein phosphatase 2A PR65/A subunit reveals the conformation of its 15 tandemly repeated HEAT motifs. Cell. 1999;96:99–110. doi: 10.1016/s0092-8674(00)80963-0. [DOI] [PubMed] [Google Scholar]
- Hiraoka Y, Toda T, Yanagida M. The NDA3 gene of fission yeast encodes β-tubulin: a cold-sensitive nda3 mutation reversibly blocks spindle formation and chromosome movement in mitosis. Cell. 1984;39:349–358. doi: 10.1016/0092-8674(84)90013-8. [DOI] [PubMed] [Google Scholar]
- Hirata D, Masuda H, Eddison M, Toda T. Essential role of tubulin-folding cofactor D in microtubule assembly and its association with microtubules in fission yeast. EMBO J. 1998;17:658–666. doi: 10.1093/emboj/17.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- Horio T, Hotani H. Visualization of the dynamic instability of individual microtubules by dark-field microscopy. Nature. 1986;321:605–607. doi: 10.1038/321605a0. [DOI] [PubMed] [Google Scholar]
- Hoyt MA, Macke JP, Roberts BT, Geiser JR. Saccharomyces cerevisiae PAC2 functions with CIN1, 2 and 4 in a pathway leading to normal microtubule stability. Genetics. 1997;146:849–857. doi: 10.1093/genetics/146.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt MA, Stearns T, Botstein D. Chromosome instability mutants of Saccharomyces cerevisiae that are defective in microtubule-mediated processes. Mol Cell Biol. 1990;10:223–234. doi: 10.1128/mcb.10.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman AA, Karsenti E. Morphogenetic properties of microtubules and mitotic spindle assembly. Cell. 1996;84:401–410. doi: 10.1016/s0092-8674(00)81285-4. [DOI] [PubMed] [Google Scholar]
- Kobe B, Deisenhofer J. The leucine-rich repeat: a versatile binding motif. Trends Biochem Sci. 1994;19:415–421. doi: 10.1016/0968-0004(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Kominami K, Seth-Smith H, Toda T. Apc10 and Ste9/Srw1, two regulators of the APC-cyclosome, as well as the CDK inhibitor Rum1 are required for G1 cell cycle arrest in fission yeast. EMBO J. 1998;17:5388–5399. doi: 10.1093/emboj/17.18.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H, Hynes G, Carne A, Ashworth A, Willison K. Identification of six Tcp-1-related genes encoding divergent subunits of the TCP-1-containing chaperonin. Curr Biol. 1994;4:89–99. doi: 10.1016/s0960-9822(94)00024-2. [DOI] [PubMed] [Google Scholar]
- Lewis SA, Tian G, Cowan NJ. The α- and β-tubulin folding pathways. Trends Cell Biol. 1997;7:479–484. doi: 10.1016/S0962-8924(97)01168-9. [DOI] [PubMed] [Google Scholar]
- Lewis VA, Hynes GM, Zheng D, Saibil H, Willison K. T-complex polypeptide-1 is a subunit of a heteromeric particle in the eukaryotic cytosol. Nature. 1992;358:249–252. doi: 10.1038/358249a0. [DOI] [PubMed] [Google Scholar]
- Li HP, Liu ZM, Nirenberg M. Kinesin-73 in the nervous system of Drosophila embryos. Proc Natl Acad Sci USA. 1997;94:1086–1091. doi: 10.1073/pnas.94.4.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llosa M, Aloria K, Campo R, Padilla R, Avila J, Sánchez-Pulido L, Zabala JC. The β-tubulin monomer release factor (p14) has homology with a region of the DnaJ protein. FEBS Lett. 1996;397:283–289. doi: 10.1016/s0014-5793(96)01198-2. [DOI] [PubMed] [Google Scholar]
- Mandelkow E, Mandelkow E-M. Microtubules and microtubule-associated proteins. Curr Opin Cell Biol. 1995;7:72–81. doi: 10.1016/0955-0674(95)80047-6. [DOI] [PubMed] [Google Scholar]
- Maundrell K. nmt1 of fission yeast. J Biol Chem. 1990;265:10857–10864. [PubMed] [Google Scholar]
- McNally FJ, Vale RD. Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell. 1993;75:419–429. doi: 10.1016/0092-8674(93)90377-3. [DOI] [PubMed] [Google Scholar]
- Melki R, Rommelaere H, Leguy R, Vandekerckhove J, Ampe C. Cofactor A is a molecular chaperone required for β-tubulin folding: functional and structural characterization. Biochemistry. 1996;35:10422–10435. doi: 10.1021/bi960788r. [DOI] [PubMed] [Google Scholar]
- Melki R, Vainberg IE, Chow RL, Cowan NJ. Chaperonin-mediated folding of vertebrate actin-related protein and γ-tubulin. J Cell Biol. 1993;122:1301–1310. doi: 10.1083/jcb.122.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T, Kirschner MW. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Mitchison T, Kirschner MW. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analyses of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:773–782. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Pierre P, Scheel J, Rickard JE, Kreis TE. CLIP-170 links endocytotic vesicles to microtubules. Cell. 1992;70:887–900. doi: 10.1016/0092-8674(92)90240-d. [DOI] [PubMed] [Google Scholar]
- Radcliffe P, Hirata D, Childs D, Vardy L, Toda T. Identification of novel temperature-sensitive lethal alleles in essential β-tubulin and nonessential α2-tubulin genes as fission yeast polarity mutants. Mol Biol Cell. 1998;9:1757–1771. doi: 10.1091/mbc.9.7.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard JE, Kreis TE. CLIPs for organelle-microtubule interactions. Trends Cell Biol. 1996;6:178–183. doi: 10.1016/0962-8924(96)10017-9. [DOI] [PubMed] [Google Scholar]
- Riehemann K, Sorg C. Sequence homologies between four cytoskeleton-associated proteins. Trends Biochem Sci. 1993;18:82–83. doi: 10.1016/0968-0004(93)90159-k. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Stippec SA, Goldsmith E, White MA, Cobb MH. A constitutively active and nuclear form of the MAP kinase ERK2 is sufficient for neurite outgrowth and cell transformation. Curr Biol. 1998;8:1141–1150. doi: 10.1016/s0960-9822(07)00485-x. [DOI] [PubMed] [Google Scholar]
- Rommelaere H, Van Troys M, Gao Y, Melki R, Cowan NJ, Vandekerckhove J, Ampe C. Eukaryotic cytoplasmic chaperonin contains t-complex polypeptide 1 and seven related subunits. Proc Natl Acad Sci USA. 1993;90:11975–11981. doi: 10.1073/pnas.90.24.11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Stearns T, Hoyt MA, Botstein D. Yeast mutants sensitive to antimicrotubule drugs define three genes that affect microtubule function. Genetics. 1990;124:251–262. doi: 10.1093/genetics/124.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian G, Huang Y, Rommelaere H, Vandekerckhove J, Ampe C, Cowan NJ. Pathway leading to correctly folded β-tubulin. Cell. 1996;86:287–296. doi: 10.1016/s0092-8674(00)80100-2. [DOI] [PubMed] [Google Scholar]
- Tian G, Lewis SA, Feierbach B, Stearns T, Rommelaere H, Ampe C, Cowan NJ. Tubulin subunits exist in an activated conformational state generated and maintained by protein cofactors. J Cell Biol. 1997;138:821–832. doi: 10.1083/jcb.138.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Adachi Y, Hiraoka Y, Yanagida M. Identification of the pleiotropic cell cycle gene NDA2 as one of two different α-tubulin genes in Schizosaccharomyces pombe. Cell. 1984;37:233–242. doi: 10.1016/0092-8674(84)90319-2. [DOI] [PubMed] [Google Scholar]
- Ursic D, Culbertson MR. The yeast homolog to mouse Tcp-1 affects microtubule-mediated processes. Mol Cell Biol. 1991;11:2629–2640. doi: 10.1128/mcb.11.5.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainberg IE, Lewis SA, Rommelaere H, Ampe C, Vandekerckhove J, Klein HL, Cowan NJ. Prefoldin, a chaperone that delivers unfolded proteins to cytosolic chaperonin. Cell. 1998;93:863–873. doi: 10.1016/s0092-8674(00)81446-4. [DOI] [PubMed] [Google Scholar]
- Vega LR, Fleming J, Solomon F. An α-tubulin mutant destabilizes the heterodimer: phenotypic consequences and interactions with tubulin-binding proteins. Mol Biol Cell. 1998;9:2349–2360. doi: 10.1091/mbc.9.9.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon ED. Microtubule dynamics: treadmilling comes around again. Curr Biol. 1997;7:R360–R372. doi: 10.1016/s0960-9822(06)00177-1. [DOI] [PubMed] [Google Scholar]
- Yaffe MB, Farr GW, Miklos D, Horwich AL, Sternlicht ML, Sternlicht H. TCP1 complex is a molecular chaperone in tubulin biogenesis. Nature. 1992;358:245–248. doi: 10.1038/358245a0. [DOI] [PubMed] [Google Scholar]
- Yaffe MP, Hirata D, Verde F, Eddison M, Toda T, Nurse P. Microtubules mediate mitochondrial distribution in fission yeast. Proc Natl Acad Sci USA. 1996;93:11664–11668. doi: 10.1073/pnas.93.21.11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Watanabe Y, Yamamoto M. Microtubule-associated coiled-coil protein Ssm4 is involved in the meiotic development in fission yeast. Genes Cells. 1997;2:155–166. doi: 10.1046/j.1365-2443.1997.1100307.x. [DOI] [PubMed] [Google Scholar]