Abstract
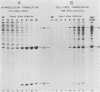
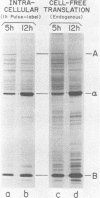
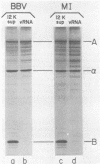
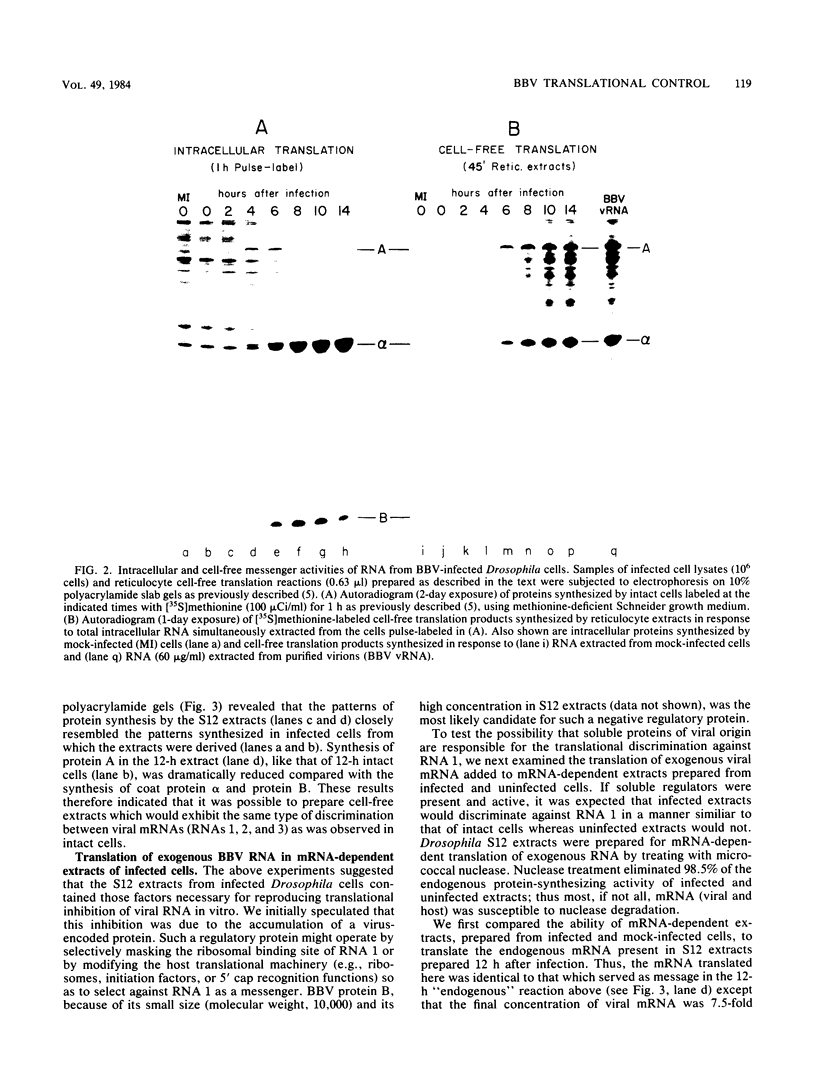
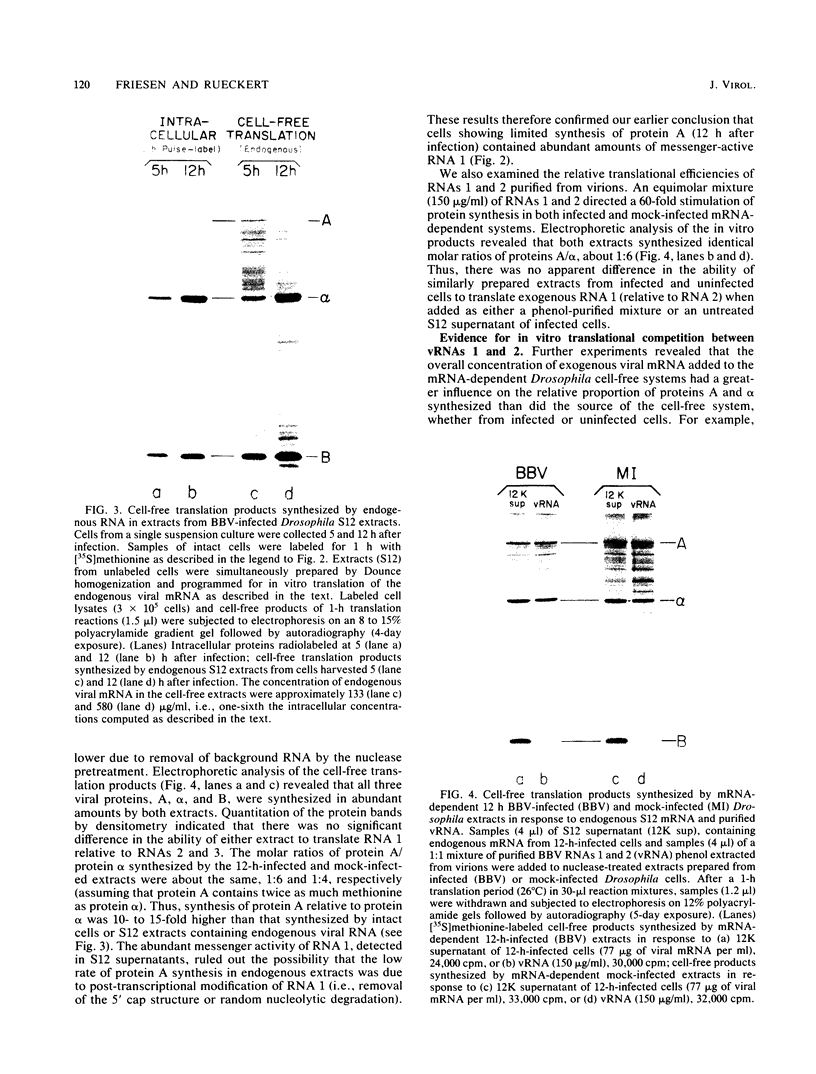
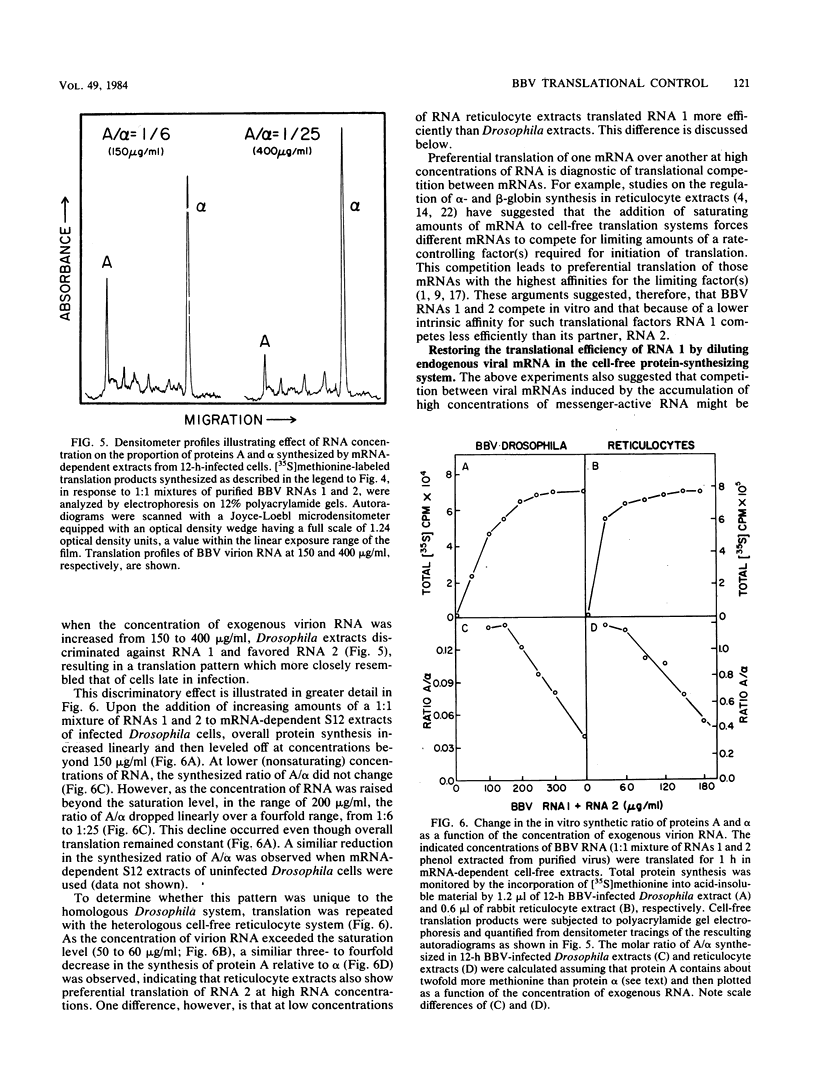
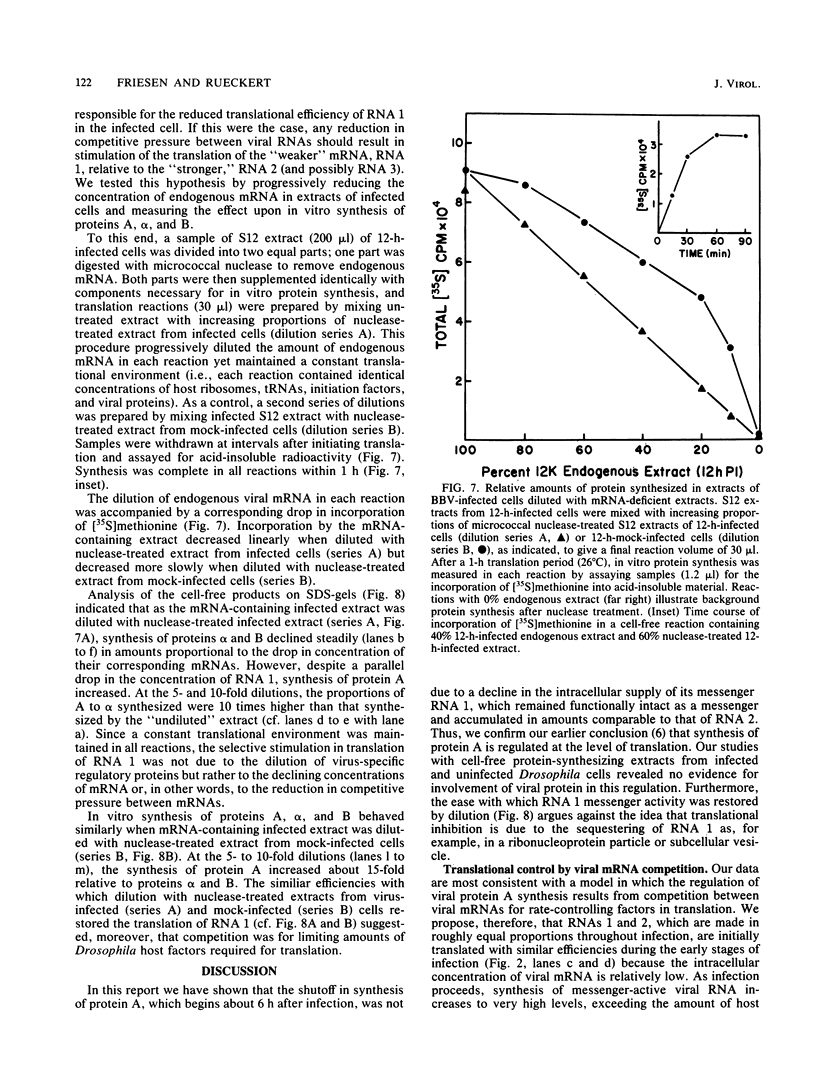
It has been shown previously that Drosophila cells infected with black beetle virus synthesize an early viral protein, protein A, a putative element of the viral RNA polymerase. Synthesis of protein A declines sharply by 6 h postinfection, whereas synthesis of viral coat protein alpha continues for at least 14 h. The early shutoff in protein A synthesis occurred despite the presence of equimolar proportions of the mRNAs for proteins A and alpha, RNAs 1 and 2, respectively. We have now been able to mimic this translational discrimination in a cell-free protein-synthesizing system prepared from infected or uninfected Drosophila cells, thus allowing further analysis of the mechanism by which translation of RNA 1 is selectively turned off. The results revealed no evidence for control by virus-encoded proteins or by virus-induced modification of mRNAs by the cell-free system. Rather, with increasing RNA concentration, viral RNA 1 was outcompeted by its genomic partner, RNA 2. This suggests that the early shutoff in intracellular synthesis of protein A is due to decreasing ability of RNA 1 to compete for a rate-controlling translational factor(s) as the concentration of viral RNAs accumulates within the infected cell.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann J. E., Lodish H. F. A kinetic model of protein synthesis. Application to hemoglobin synthesis and translational control. J Biol Chem. 1979 Dec 10;254(23):11927–11937. [PubMed] [Google Scholar]
- Crump W. A., Moore N. F. In vivo and in vitro synthesis of the proteins expressed by the RNA of black beetle virus. Arch Virol. 1981;69(2):131–139. doi: 10.1007/BF01315156. [DOI] [PubMed] [Google Scholar]
- Di Segni G., Rosen H., Kaempfer R. Competition between alpha- and beta-globin messenger ribonucleic acids for eucaryotic initiation factor 2. Biochemistry. 1979 Jun 26;18(13):2847–2854. doi: 10.1021/bi00580a027. [DOI] [PubMed] [Google Scholar]
- Friesen P. D., Rueckert R. R. Black beetle virus: messenger for protein B is a subgenomic viral RNA. J Virol. 1982 Jun;42(3):986–995. doi: 10.1128/jvi.42.3.986-995.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen P. D., Rueckert R. R. Synthesis of Black Beetle Virus Proteins in Cultured Drosophila Cells: Differential Expression of RNAs 1 and 2. J Virol. 1981 Mar;37(3):876–886. doi: 10.1128/jvi.37.3.876-886.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen P., Scotti P., Longworth J., Rueckert R. Black beetle virus: propagation in Drosophila line 1 cells and an infection-resistant subline carrying endogenous black beetle virus-related particles. J Virol. 1980 Sep;35(3):741–747. doi: 10.1128/jvi.35.3.741-747.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T. M., Friesen P. D., Rueckert R. R. Autonomous replication and expression of RNA 1 from black beetle virus. J Virol. 1983 May;46(2):481–489. doi: 10.1128/jvi.46.2.481-489.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroy-Colburn T., Thach R. E. The role of mRNA competition in regulating translation. IV. Kinetic model. J Biol Chem. 1981 Nov 25;256(22):11762–11773. [PubMed] [Google Scholar]
- Golini F., Thach S. S., Birge C. H., Safer B., Merrick W. C., Thach R. E. Competition between cellular and viral mRNAs in vitro is regulated by a messenger discriminatory initiation factor. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3040–3044. doi: 10.1073/pnas.73.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino L. A., Hruby D. E., Ball L. A., Kaesberg P. Translation of black beetle virus RNA and heterologous viral RNAs in cell-free lysates derived from Drosophila melanogaster. J Virol. 1981 Jan;37(1):500–505. doi: 10.1128/jvi.37.1.500-505.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino L. A., Kaesberg P. Isolation and Characterization of an RNA-Dependent RNA Polymerase from Black Beetle Virus-Infected Drosophila melanogaster Cells. J Virol. 1981 Nov;40(2):379–386. doi: 10.1128/jvi.40.2.379-386.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herson D., Schmidt A., Seal S., Marcus A., van Vloten-Doting L. Competitive mRNA translation in an in vitro system from wheat germ. J Biol Chem. 1979 Sep 10;254(17):8245–8249. [PubMed] [Google Scholar]
- Kabat D., Chappell M. R. Competition between globin messenger ribonucleic acids for a discriminating initiation factor. J Biol Chem. 1977 Apr 25;252(8):2684–2690. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Froshauer S. Rates of initiation of protein synthesis by two purified species of vesicular stomatitis virus messenger RNA. J Biol Chem. 1977 Dec 25;252(24):8804–8811. [PubMed] [Google Scholar]
- Lodish H. F. Translational control of protein synthesis. Annu Rev Biochem. 1976;45:39–72. doi: 10.1146/annurev.bi.45.070176.000351. [DOI] [PubMed] [Google Scholar]
- Longworth J. F., Carey G. P. A small RNA virus with a divided genome from Heteronychus arator (F.) [Coleoperai Scarabaeidae]. J Gen Virol. 1976 Oct;33(1):31–40. doi: 10.1099/0022-1317-33-1-31. [DOI] [PubMed] [Google Scholar]
- Longworth J. F. Small isometric viruses of invertebrates. Adv Virus Res. 1978;23:103–157. doi: 10.1016/s0065-3527(08)60099-8. [DOI] [PubMed] [Google Scholar]
- Maitra U., Stringer E. A., Chaudhuri A. Initiation factors in protein biosynthesis. Annu Rev Biochem. 1982;51:869–900. doi: 10.1146/annurev.bi.51.070182.004253. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L. Regulation of hemoglobin synthesis. Effect of concentration of messenger ribonucleic acid, ribosome subunits, initiation factors, and salts on ratio of alpha and beta chains synthesized in vitro. J Biol Chem. 1974 Oct 25;249(20):6517–6526. [PubMed] [Google Scholar]
- Newman J. F., Matthews T., Omilianowski D. R., Salerno T., Kaesberg P., Rueckert R. In vitro translation of the Two RNAs of Nodamura virus, a novel mammalian virus with a divided genome. J Virol. 1978 Jan;25(1):78–85. doi: 10.1128/jvi.25.1.78-85.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ray B. K., Brendler T. G., Adya S., Daniels-McQueen S., Miller J. K., Hershey J. W., Grifo J. A., Merrick W. C., Thach R. E. Role of mRNA competition in regulating translation: further characterization of mRNA discriminatory initiation factors. Proc Natl Acad Sci U S A. 1983 Feb;80(3):663–667. doi: 10.1073/pnas.80.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel M., Groner Y. Post-transcriptional and translational controls of gene expression in eukaryotes. Annu Rev Biochem. 1978;47:1079–1126. doi: 10.1146/annurev.bi.47.070178.005243. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER I. DIFFERENTIATION OF LARVAL DROSOPHILA EYE-ANTENNAL DISCS IN VITRO. J Exp Zool. 1964 Jun;156:91–103. doi: 10.1002/jez.1401560107. [DOI] [PubMed] [Google Scholar]
- Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol. 1972 Apr;27(2):353–365. [PubMed] [Google Scholar]
- Scott M. P., Pardue M. L. Translational control in lysates of Drosophila melanogaster cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3353–3357. doi: 10.1073/pnas.78.6.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Kaesberg P. Translation of the RNAs of brome mosaic virus: the monocistronic nature of RNA1 and RNA2. J Mol Biol. 1976 May 5;103(1):77–88. doi: 10.1016/0022-2836(76)90053-x. [DOI] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Kew O., Pallansch M., Rueckert R., Kaesberg P. Cell-free synthesis and processing of the proteins of poliovirus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5807–5811. doi: 10.1073/pnas.75.12.5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Zimmern D., Rueckert R. R., Kaesberg P. Translation of encephalomyocarditis virus RNA in reticulocyte lysates: kinetic analysis of the formation of virion proteins and a protein required for processing. J Virol. 1979 May;30(2):472–480. doi: 10.1128/jvi.30.2.472-480.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagórski W. Translational regulation of expression of the brome-mosaic-virus RNA genome in vitro. Eur J Biochem. 1978 May 16;86(2):465–472. doi: 10.1111/j.1432-1033.1978.tb12329.x. [DOI] [PubMed] [Google Scholar]