Abstract
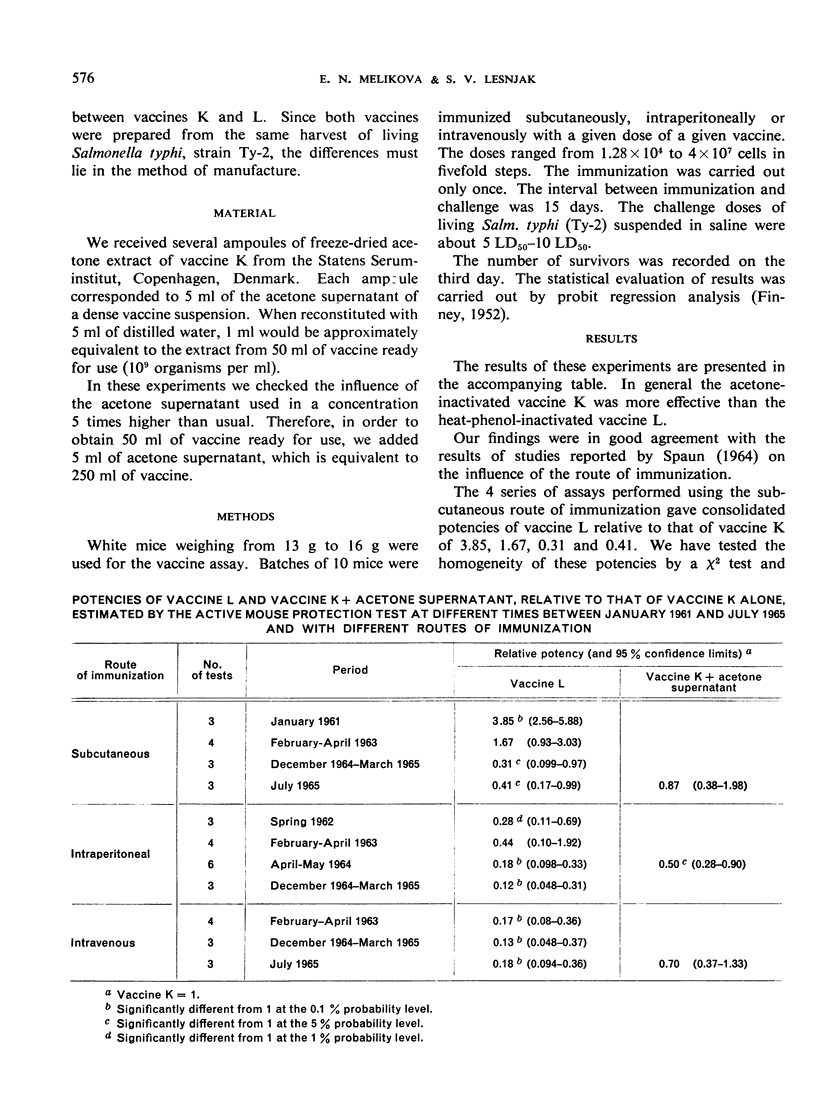
International collaborative laboratory studies on the International Reference Preparations of Typhoid Vaccine have so far failed to provide data on which international units for these vaccines can be based. Further assays carried out using the active mouse protection test, with immunization by the subcutaneous, intraperitoneal or intravenous route, confirmed the findings by some workers that the International Reference Preparation of Typhoid Vaccine (Acetone-Inactivated) (vaccine K) was more effective than the International Reference Preparation of Typhoid Vaccine (Heat-Phenol-Inactivated) (vaccine L), and indicated that intraperitoneal immunization was the most promising method. Vaccine K, together with the material extracted by the acetone in the preparation of the vaccine, had a significantly lower effectiveness (at the 5% probability level) only when intraperitoneal immunization was used. The reasons for the differences found between the various vaccines and routes of immunization are discussed at length.
It is suggested that challenge with a strain of Salmonella moscow instead of the strain of Salm. typhi used until now gives a true infection and forms the basis of a reliable method for the potency assay of typhoid vaccines.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Melikova E. N., Vasil'eva I. G., Lesniak S. V. Sravnitel'naia kharakteristika metodik primenennykh pri laboratornoi otsenke effektivnostisukhikh briushnotifoznykh vaktsii voz. Zh Mikrobiol Epidemiol Immunobiol. 1965 Mar;42(3):58–65. [PubMed] [Google Scholar]
- Pittman M., Bohner H. J. Laboratory assays of different types of field trial typhoid vaccines and relationship to efficacy in man. J Bacteriol. 1966 May;91(5):1713–1723. doi: 10.1128/jb.91.5.1713-1723.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPAUN J. STUDIES ON THE INFLUENCE OF THE ROUTE OF IMMUNIZATION IN THE ACTIVE MOUSE PROTECTION TEST WITH INTRAPERITONEAL CHALLENGE FOR POTENCY ASSAY OF TYPHOID VACCINES. Bull World Health Organ. 1964;31:793–798. [PMC free article] [PubMed] [Google Scholar]
- SPAUN J., UEMURA K. INTERNATIONAL REFERENCE PREPARATIONS OF TYPHOID VACCINE. A REPORT ON INTERNATIONAL COLLABORATIVE LABORATORY STUDIES. Bull World Health Organ. 1964;31:761–791. [PMC free article] [PubMed] [Google Scholar]


