Abstract
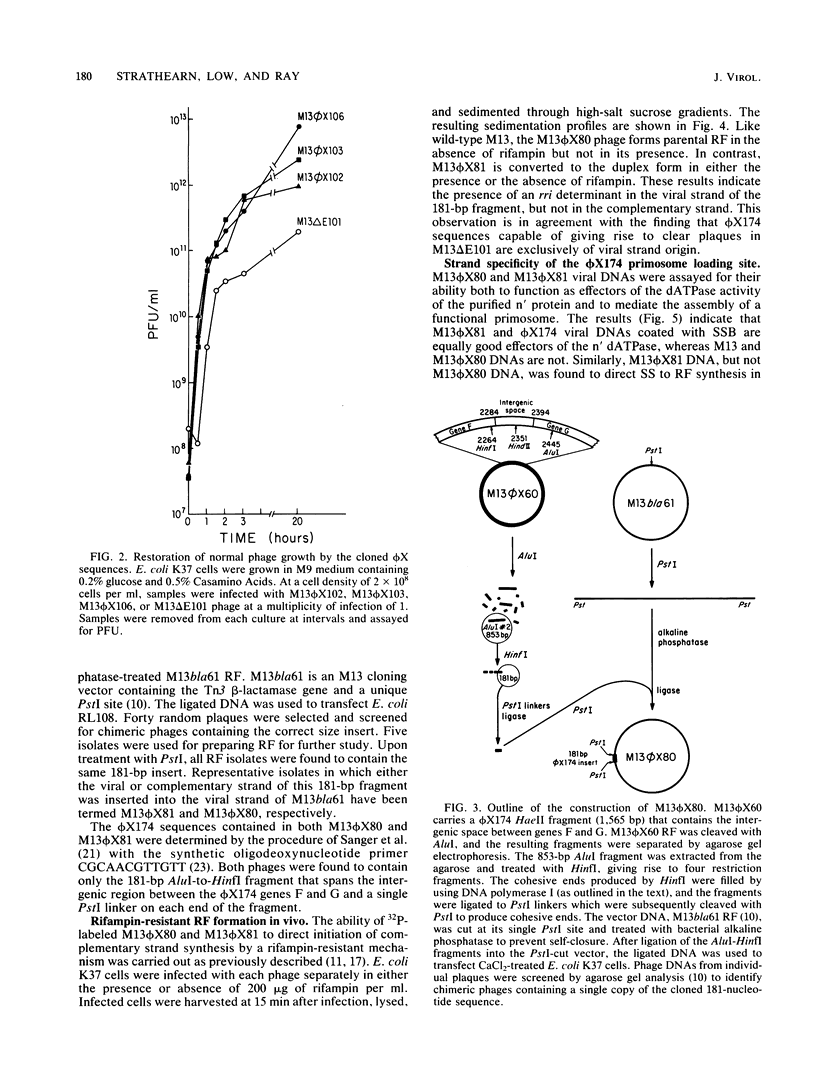
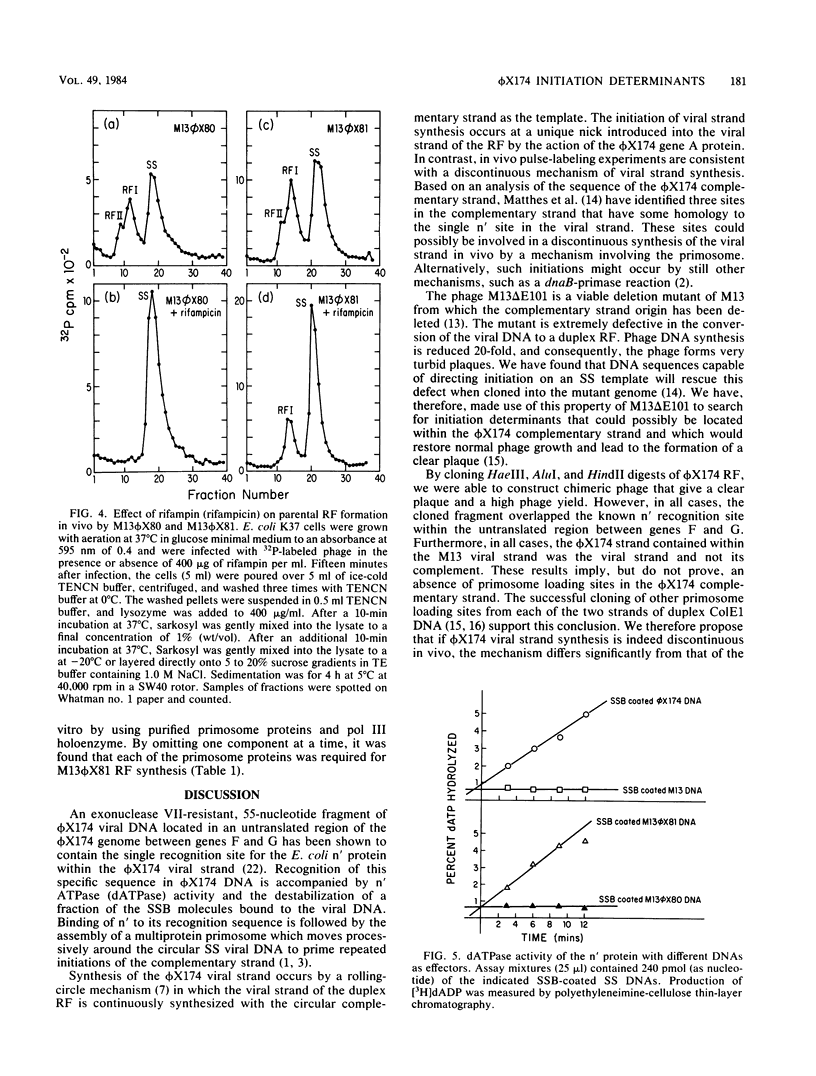
An M13 phage deletion mutant, M13 delta E101, developed as a vector for selecting DNA sequences that direct DNA strand initiation on a single-stranded template, has been used for cloning restriction enzyme digests of phi X174 replicative-form DNA. Initiation determinants, detected on the basis of clear-plaque formation by the chimeric phage, were found only in restriction fragments containing the unique effector site in phi X174 DNA for the Escherichia coli protein n' dATPase (ATPase). Furthermore, these sequences were functional only when cloned in the orientation in which the phi X174 viral strand was joined to the M13 viral strand. A 181-nucleotide viral strand fragment containing this initiation determinant confers a phi X174-type complementary-strand replication mechanism on M13 chimeras. The chimeric phage is converted to the parental replicative form in vivo by a mechanism resistant to rifampin, a specific inhibitor of the normal RNA polymerase-dependent mechanism of M13. In vitro, the chimeric single-stranded DNA promotes the assembly of a functional multiprotein priming complex, or primosome, identical to that utilized by intact phi X174 viral strand DNA. Chimeric phage containing the sequence complementary to the 181-nucleotide viral strand sequence shows no initiation capability, either in vivo or in vitro.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K., Arai N., Shlomai J., Kobori J., Polder L., Low R., Hübscher U., Bertsch L., Kornberg A. Enzyme studies of phi X174 DNA replication. Prog Nucleic Acid Res Mol Biol. 1981;26:9–32. [PubMed] [Google Scholar]
- Arai K., Kornberg A. A general priming system employing only dnaB protein and primase for DNA replication. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4308–4312. doi: 10.1073/pnas.76.9.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K., Low R., Kobori J., Shlomai J., Kornberg A. Mechanism of dnaB protein action. V. Association of dnaB protein, protein n', and other repriming proteins in the primosome of DNA replication. J Biol Chem. 1981 May 25;256(10):5273–5280. [PubMed] [Google Scholar]
- Arai N., Polder L., Akai K., Kornberg A. Replication of phi X174 DNA with purified enzymes. II. Multiplication of the duplex form by coupling of continuous and discontinuous synthetic pathways. J Biol Chem. 1981 May 25;256(10):5239–5246. [PubMed] [Google Scholar]
- Eisenberg S., Griffith J., Kornberg A. phiX174 cistron A protein is a multifunctional enzyme in DNA replication. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3198–3202. doi: 10.1073/pnas.74.8.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidanián H. M., Ray D. S. Replication of bacteriophage M13. VII. Requirement of the gene 2 protein for the accumulation of a specific RFII species. J Mol Biol. 1972 Dec 14;72(1):51–63. doi: 10.1016/0022-2836(72)90067-8. [DOI] [PubMed] [Google Scholar]
- Hines J. C., Ray D. S. Construction and characterization of new coliphage M13 cloning vectors. Gene. 1980 Nov;11(3-4):207–218. doi: 10.1016/0378-1119(80)90061-x. [DOI] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. H., Hines J. C., Ray D. S. Viable deletions of the M13 complementary strand origin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6784–6788. doi: 10.1073/pnas.78.11.6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes M., Weisbeek P. J., Denhardt D. T. Mechanism of replication of bacteriophage phi X174 XIX. Initiation of phi X174 viral strand DNA synthesis at internal sites on the genome. J Virol. 1982 Apr;42(1):301–305. doi: 10.1128/jvi.42.1.301-305.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura N., Low R. L., Ray D. S. Identification of ColE1 DNA sequences that direct single strand-to-double strand conversion by a phi X174 type mechanism. Proc Natl Acad Sci U S A. 1982 May;79(10):3153–3157. doi: 10.1073/pnas.79.10.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura N., Low R. L., Ray D. S. Selective cloning of Co1E1 DNA initiation sequences using the cloning vector M13 delta E101. Gene. 1982 Jun;18(3):239–246. doi: 10.1016/0378-1119(82)90161-5. [DOI] [PubMed] [Google Scholar]
- Nomura N., Ray D. S. Expression of a DNA strand initiation sequence of ColE1 plasmid in a single-stranded DNA phage. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6566–6570. doi: 10.1073/pnas.77.11.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D. S., Hines J. C., Kim M. H., Imber R., Nomura N. M13 vectors for selective cloning of sequences specifying initiation of DNA synthesis on single-stranded templates. Gene. 1982 Jun;18(3):231–238. doi: 10.1016/0378-1119(82)90160-3. [DOI] [PubMed] [Google Scholar]
- Ray D. S., Kook K. Insertion of the Tn3 transposon into the genome of the single-stranded DNA phage M13. Gene. 1978 Oct;4(2):109–119. doi: 10.1016/0378-1119(78)90024-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomai J., Kornberg A. An Escherichia coli replication protein that recognizes a unique sequence within a hairpin region in phi X174 DNA. Proc Natl Acad Sci U S A. 1980 Feb;77(2):799–803. doi: 10.1073/pnas.77.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Suggs S. V., Miyoshi K., Bhatt R., Itakura K. A set of synthetic oligodeoxyribonucleotide primers for DNA sequencing in the plasmid vector pBR322. Gene. 1981 Dec;16(1-3):21–26. doi: 10.1016/0378-1119(81)90057-3. [DOI] [PubMed] [Google Scholar]
- de Vries F. A., Collins C. J., Jackson D. A. Joining of simian virus 40 DNA molecules at endonuclease R Eco Ri sites by polynucleotide ligase and analysis of the products by agarose gel electrophoresis. Biochim Biophys Acta. 1976 Jul 2;435(3):213–227. doi: 10.1016/0005-2787(76)90103-9. [DOI] [PubMed] [Google Scholar]


