Abstract
The successful mass cultivation of Entamoeba histolytica strains in pure culture has made it possible to produce pure amoeba antigens, which should improve the prospects for immunodiagnostic research and simplify the interpretation of the results obtained in tests for amoeba antibodies.
The authors have mass-produced immunodiagnostic antigens for amoebiasis serology from 2 axenic strains of E. histolytica and standardized them as dry powders, which upon reconstitution contained approximately 1.8 mg N/ml, or roughly the equivalent of 10 × 106 amoebae per ml.
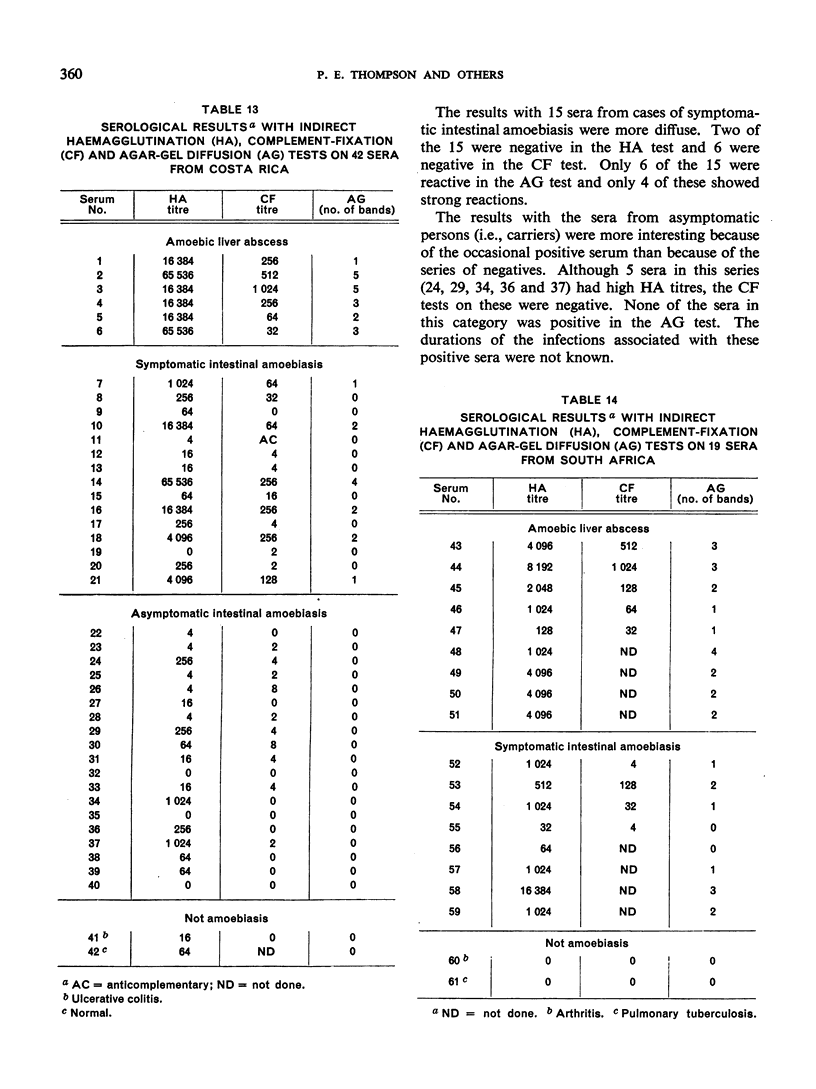
The antigens were evaluated with 121 sera from subjects in Costa Rica, South Africa, Taiwan, Thailand and the USA, 49 of the sera being from cases of amoebic liver abscess, 41 from symptomatic intestinal amoebiasis, 19 from asymptomatic intestinal amoebiasis, and 12 from subjects presumed not to have amoebiasis.
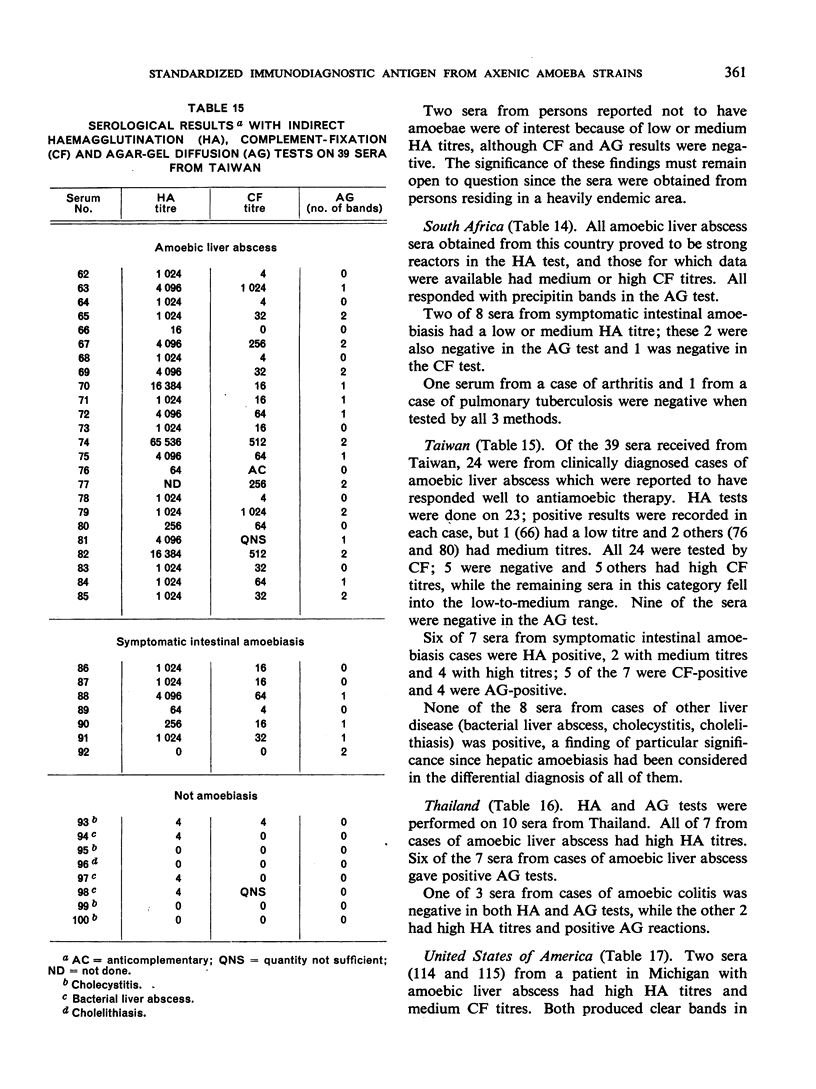
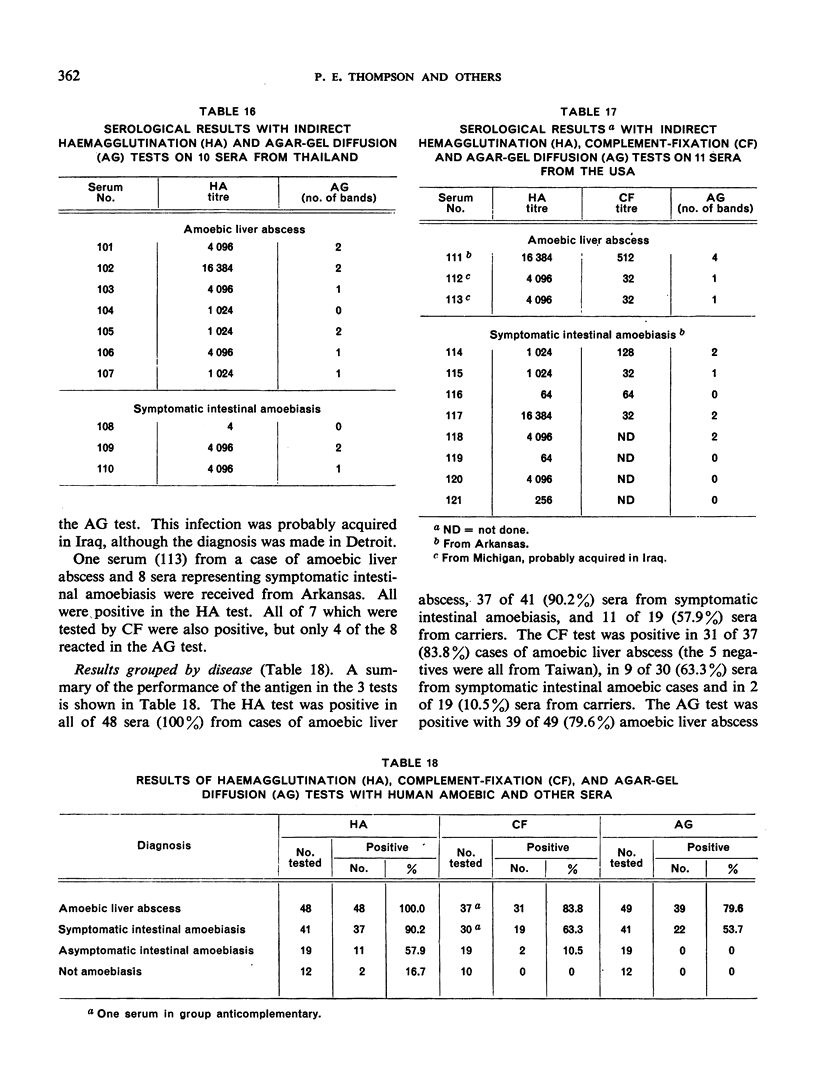
Positive results with the indirect haemagglutination test were obtained with 100% of amoebic liver abscess sera, 90.2% of those from symptomatic intestinal amoebiasis, 57.9% of those from asymptomatic intestinal amoebiasis, and 16.7% of the normal sera. Positive complement-fixation tests for the respective groups were obtained in 83.8%, 63.3%, 10.5% and 0%, and positive agar-gel diffusion in 79.6%, 53.7%, 0% and 0%.
These results compare favourably with earlier reports of similar studies in which non-axenic cultures were used and agree well with those of investigators in different parts of the world to whom samples of the axenic antigens were sent.
The authors conclude that the axenic antigens from either strain used, or from the 2 strains pooled, are of broad usefulness in the detection of amoebic antibodies, but point out that the limitations of the tests now used in amoeba serology are not yet clearly understood and that the pattern of antibody persistence after infection requires further study.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DIAMOND L. S. Axenic cultivation of Entamoeba hitolytica. Science. 1961 Aug 4;134(3475):336–337. doi: 10.1126/science.134.3475.336. [DOI] [PubMed] [Google Scholar]
- Diamond L. S. Improved method for the monoxenic cultivation of Entamoeba histolytica Schaudinn, 1903 and E. histolytica-like amebae with trypanosomatids. J Parasitol. 1968 Aug;54(4):715–719. [PubMed] [Google Scholar]
- GEIMAN Q. M., BECKER C. E. In vitro growth and metabolism of Endamoeba histolytica. Ann N Y Acad Sci. 1953 Oct 14;56(5):1048–1056. doi: 10.1111/j.1749-6632.1953.tb30285.x. [DOI] [PubMed] [Google Scholar]
- GENTRY R. F. Differentiation of avian PPLO and bacterial L forms. Ann N Y Acad Sci. 1960 Jan 15;79:403–409. doi: 10.1111/j.1749-6632.1960.tb42705.x. [DOI] [PubMed] [Google Scholar]
- MADDISON S. E., ELSDON-DEW R. Non-specific antibodies in amebiasis. Exp Parasitol. 1961 Feb;11:90–92. doi: 10.1016/0014-4894(61)90011-x. [DOI] [PubMed] [Google Scholar]
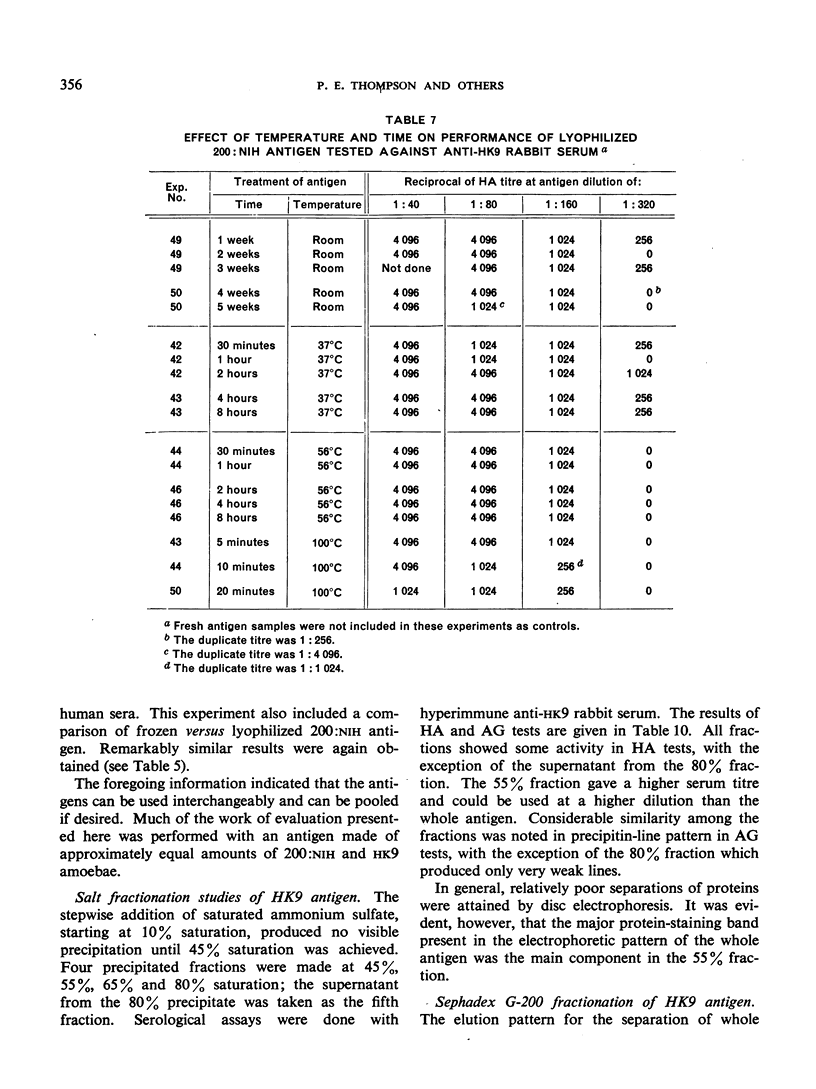
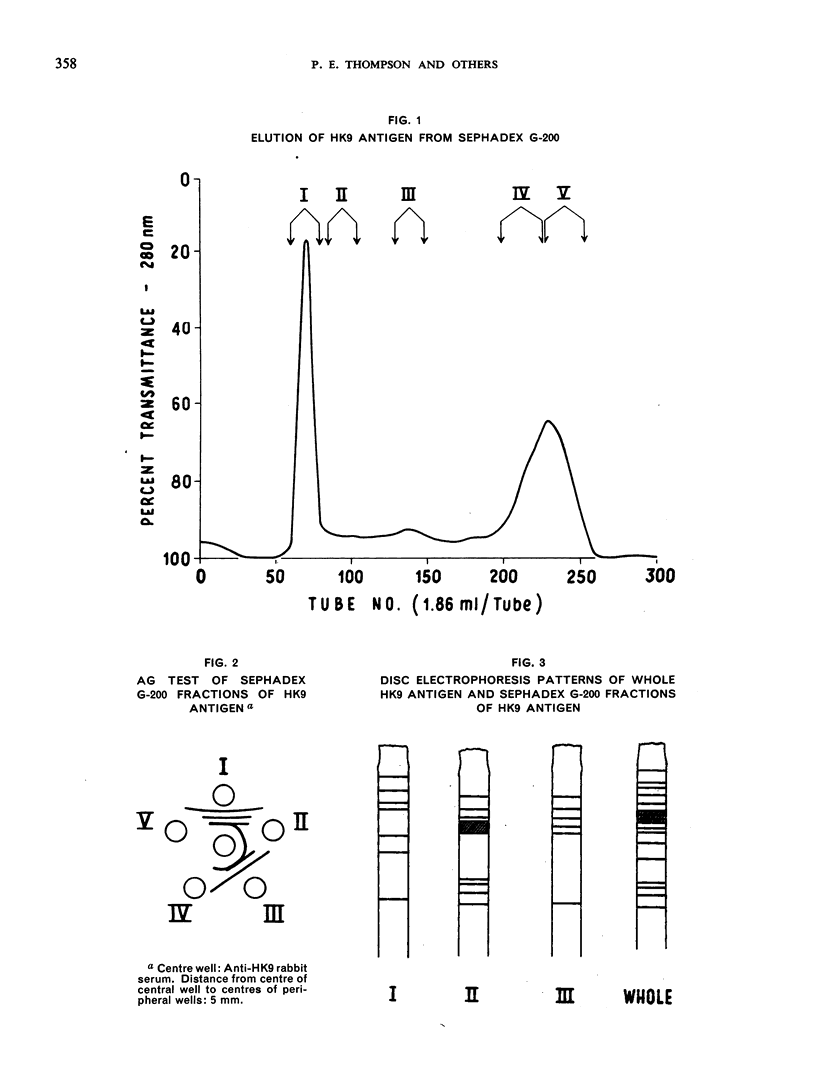
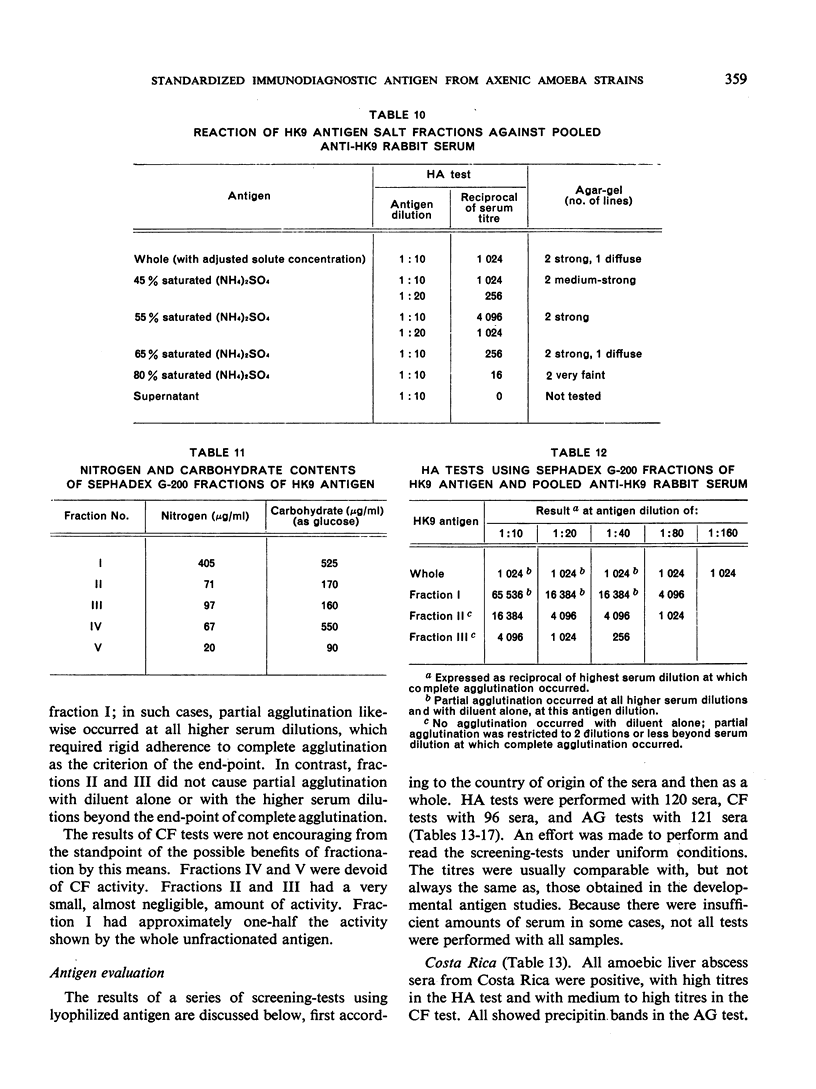
- Schneider C. R., Gordon R. M. The effect of medium components on the specificity of axenic Entamoeba histolytica antigen. J Parasitol. 1968 Aug;54(4):711–714. [PubMed] [Google Scholar]
- TOBIE J. E. Experimental infection of the rabbit with Endamoeba histolytica. Am J Trop Med Hyg. 1949 Nov;29(6):859-70, illust. doi: 10.4269/ajtmh.1949.s1-29.859. [DOI] [PubMed] [Google Scholar]



