Abstract
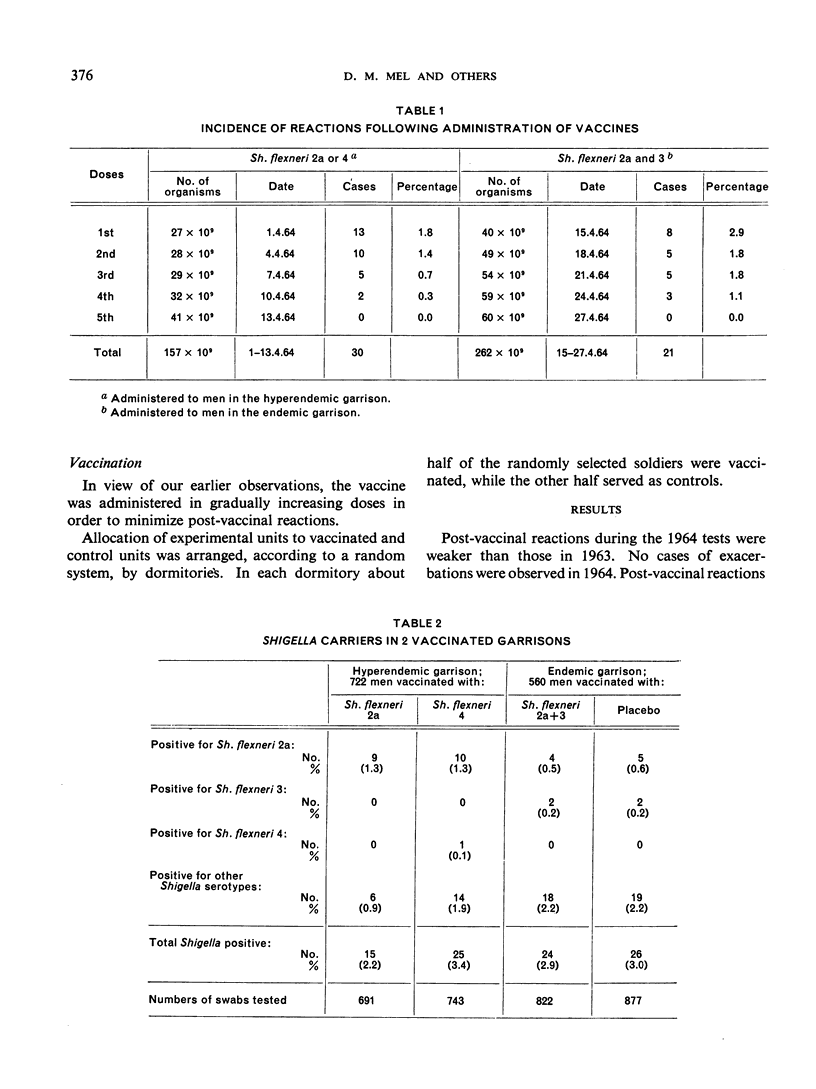
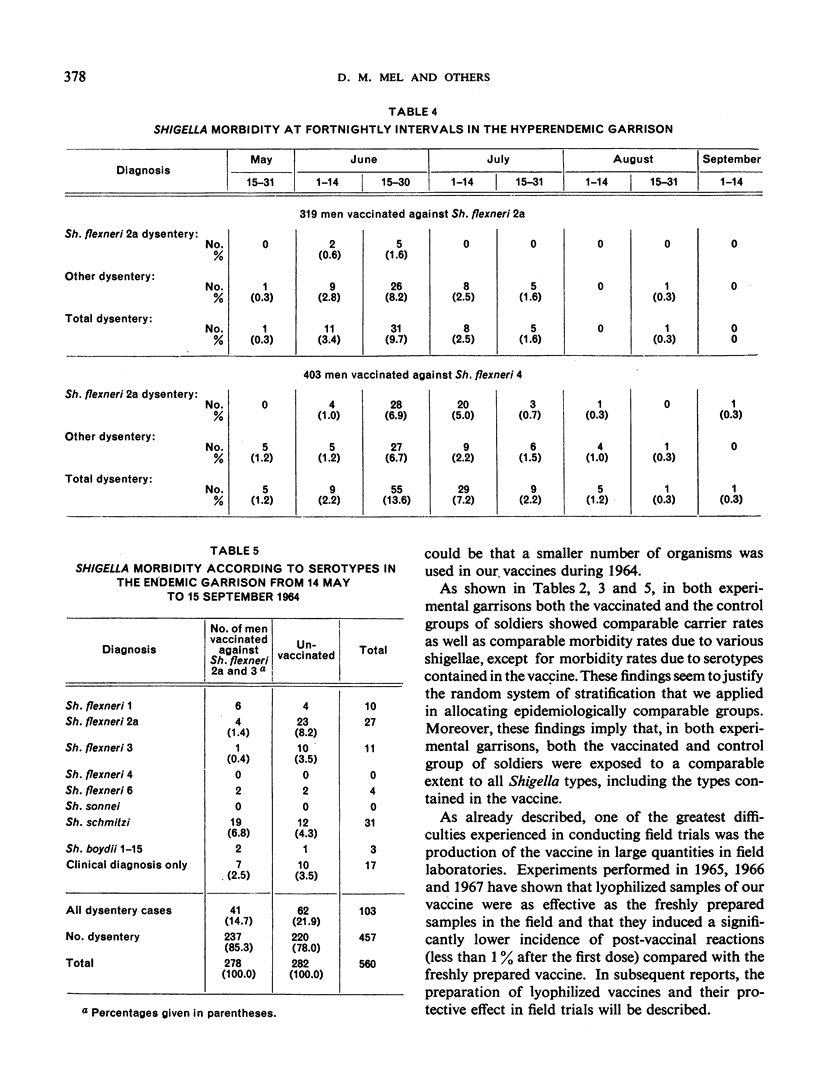
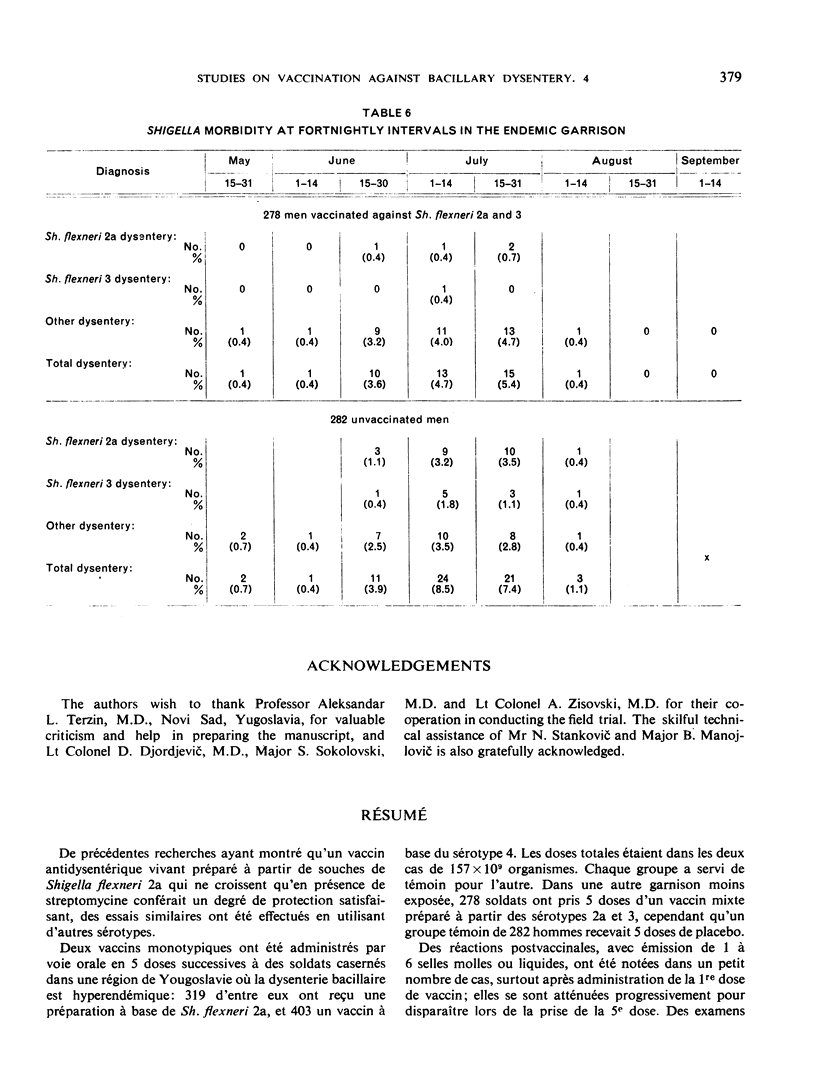
Results of tests made in 1964 confirm the previous findings that live oral vaccine, prepared from streptomycin-dependent strains of shigellae, confers a strong, type-specific protection against acute bacillary dysentery. This vaccine did not reduce the carrier rate of shigellae. Observations on soldiers treated with a vaccine of Shigella flexneri serotypes 2a and 3 combined revealed no antagonizing effects from the type 3 component upon the protective effect of the 2a component contained in the same vaccine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Mel D. M., Papo R. G., Terzin A. L., Vuksić L. Studies on vaccination against bacillary dysentery. 2. Safety tests and reactogenicity studies on a live dysentery vaccine intended for use in field trials. Bull World Health Organ. 1965;32(5):637–645. [PMC free article] [PubMed] [Google Scholar]
- Mel D. M., Terzin A. L., Vuksić L. Studies on vaccination against bacillary dysentery. 3. Effective oral immunization against Shigella flexneri 2a in a field trial. Bull World Health Organ. 1965;32(5):647–655. [PMC free article] [PubMed] [Google Scholar]



