Abstract
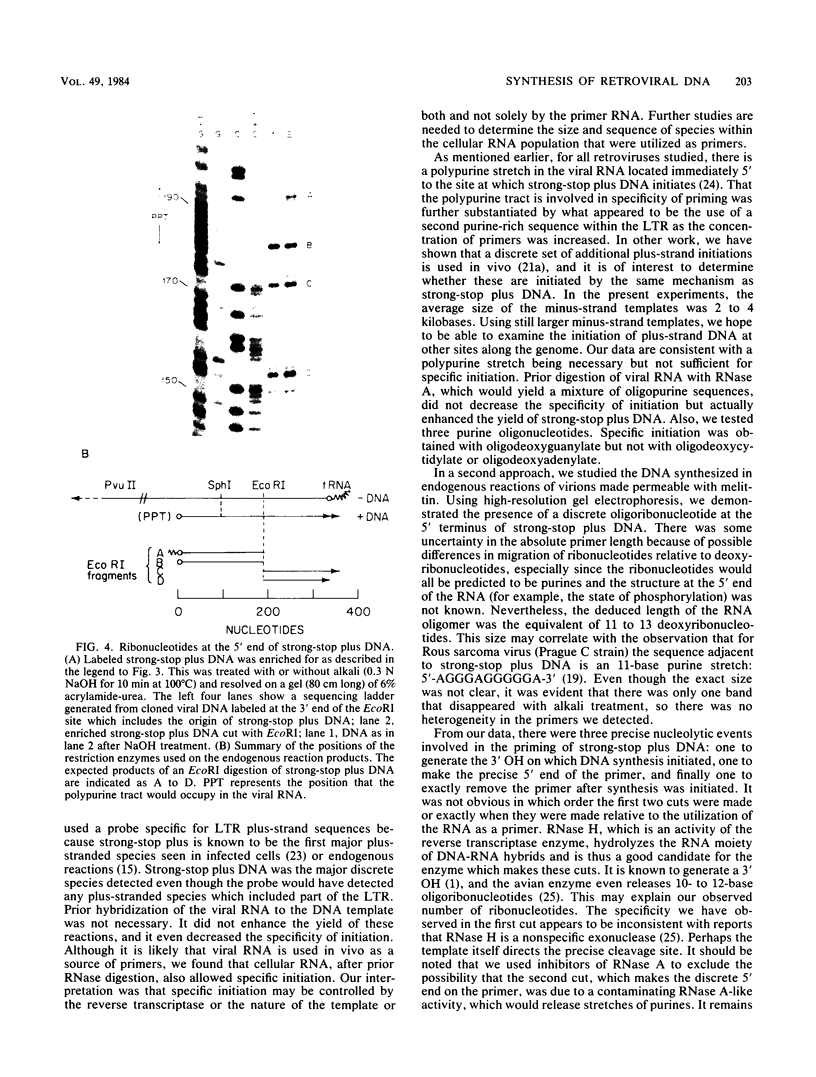
Two in vitro approaches were used to investigate the priming of strong-stop plus DNA by Rous sarcoma virus. This 340-base DNA species is the first major plus-strand DNA product seen in both infected cells and in endogenous reactions of disrupted virions. In the first approach, we set up a reconstructed system in which strong-stop plus DNA was synthesized by reverse transcriptase from a high-molecular-weight minus-strand viral DNA template. This synthesis was shown to be strictly dependent on the addition of primers to the reaction mixture. The addition of high-molecular-weight RNA from both viral and cellular sources, as well as oligodeoxyguanylate, gave specific synthesis of strong-stop plus DNA, whereas the addition of oligodeoxycytidylate-oligodeoxyadenylate and viral 4S RNA did not. In the second approach, strong-stop plus DNA synthesized in melittin-permeabilized virions was examined on a high-resolution polyacrylamide gel. This DNA was shown to have ca. 11 to 13 ribonucleotides at its 5' end. These results indicate that strong-stop plus DNA is initiated on a preformed RNA primer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Smoler D. F. Association of an endoribonuclease with the avian myeloblastosis virus deoxyribonucleic acid polymerase. J Biol Chem. 1972 Nov 25;247(22):7282–7287. [PubMed] [Google Scholar]
- Boone L. R., Skalka A. M. Viral DNA synthesized in vitro by avian retrovirus particles permeabilized with melittin. I. Kinetics of synthesis and size of minus- and plus-strand transcripts. J Virol. 1981 Jan;37(1):109–116. doi: 10.1128/jvi.37.1.109-116.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Taylor J. M., Levinson W. E., Goodman H. M., Bishop J. M. RNA-directed DNA polymerase of Rous sarcoma virus: initiation of synthesis with 70 S viral RNA as template. J Mol Biol. 1973 Sep 5;79(1):163–183. doi: 10.1016/0022-2836(73)90277-5. [DOI] [PubMed] [Google Scholar]
- Guntaka R. V., Richards O. C., Shank P. R., Kung H. J., Davidson N. Covalently closed circular DNA of avian sarcoma virus: purification from nuclei of infected quail tumor cells and measurement by electron microscopy and gel electrophoresis. J Mol Biol. 1976 Sep 15;106(2):337–357. doi: 10.1016/0022-2836(76)90090-5. [DOI] [PubMed] [Google Scholar]
- Hsu T. W., Taylor J. M., Aldrich C., Townsend J. B., Seal G., Mason W. S. Tandem duplication of the proviral DNA in an avian sarcoma virus-transformed quail clone. J Virol. 1981 Apr;38(1):219–223. doi: 10.1128/jvi.38.1.219-223.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T. W., Taylor J. M. Single-stranded regions on unintegrated avian retrovirus DNA. J Virol. 1982 Oct;44(1):47–53. doi: 10.1128/jvi.44.1.47-53.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H. J., Fung Y. K., Majors J. E., Bishop J. M., Varmus H. E. Synthesis of plus strands of retroviral DNA in cells infected with avian sarcoma virus and mouse mammary tumor virus. J Virol. 1981 Jan;37(1):127–138. doi: 10.1128/jvi.37.1.127-138.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. S., Aldrich C., Summers J., Taylor J. M. Asymmetric replication of duck hepatitis B virus DNA in liver cells: Free minus-strand DNA. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3997–4001. doi: 10.1073/pnas.79.13.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S. W., Chow M., Champoux J., Baltimore D. Synthesis of murine leukemia virus plus strong stop DNA initiates at a unique site. J Biol Chem. 1982 Jun 10;257(11):5983–5986. [PubMed] [Google Scholar]
- Mitra S. W., Goff S., Gilboa E., Baltimore D. Synthesis of a 600-nucleotide-long plus-strand DNA by virions of Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4355–4359. doi: 10.1073/pnas.76.9.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J. C., Watson K. F. Avian retrovirus RNA-directed DNA synthesis by purified reverse transcriptase. Covalent linkage of RNA to plus strand DNA. Biochem Biophys Res Commun. 1980 Dec 31;97(4):1376–1383. doi: 10.1016/s0006-291x(80)80019-2. [DOI] [PubMed] [Google Scholar]
- Omer C. A., Faras A. J. Mechanism of release of the avian rotavirus tRNATrp primer molecule from viral DNA by ribonuclease H during reverse transcription. Cell. 1982 Oct;30(3):797–805. doi: 10.1016/0092-8674(82)90284-7. [DOI] [PubMed] [Google Scholar]
- Sabran J. L., Hsu T. W., Yeater C., Kaji A., Mason W. S., Taylor J. M. Analysis of integrated avian RNA tumor virus DNA in transformed chicken, duck and quail fibroblasts. J Virol. 1979 Jan;29(1):170–178. doi: 10.1128/jvi.29.1.170-178.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Bernstein E. H. Production and purification of large amounts of Rous sarcoma virus. Appl Microbiol. 1973 Mar;25(3):346–353. doi: 10.1128/am.25.3.346-353.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Cywinski A., Smith J. K. Discontinuities in the DNA synthesized by an avian retrovirus. J Virol. 1983 Dec;48(3):654–659. doi: 10.1128/jvi.48.3.654-659.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Hsu T. W. Reverse transcription of avian sarcoma virus RNA into DNA might involve copying of the tRNA primer. J Virol. 1980 Jan;33(1):531–534. doi: 10.1128/jvi.33.1.531-534.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Heasley S., Kung H. J., Oppermann H., Smith V. C., Bishop J. M., Shank P. R. Kinetics of synthesis, structure and purification of avian sarcoma virus-specific DNA made in the cytoplasm of acutely infected cells. J Mol Biol. 1978 Mar 25;120(1):55–82. doi: 10.1016/0022-2836(78)90295-4. [DOI] [PubMed] [Google Scholar]
- Verma I. M. Studies on reverse transcriptase of RNA tumor viruses III. Properties of purified Moloney murine leukemia virus DNA polymerase and associated RNase H. J Virol. 1975 Apr;15(4):843–854. doi: 10.1128/jvi.15.4.843-854.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer E. Genome-linked proteins of viruses. Cell. 1982 Feb;28(2):199–201. doi: 10.1016/0092-8674(82)90335-x. [DOI] [PubMed] [Google Scholar]