Abstract
We have previously designed in vitro model systems to characterize the herpes simplex virus type 1 (HSV-1) genome during in vitro virus latency. Latency was established by treatment of infected human embryo lung fibroblast (HEL-F) cells or rat fetal neurons with (E)-5-(2-bromovinyl)-2'-deoxyuridine and human leukocyte interferon and was maintained by increasing the incubation temperature after inhibitor removal. Virus was reactivated by reducing the incubation temperature. We have now examined the HSV-1-specific DNA content of latently infected HEL-F cells and rat fetal neurons treated with (E)-5-(2-bromovinyl)-2'-deoxyuridine and human leukocyte interferon and increased temperature. The HEL-F cell population contained, on an average, between 0.25 and 0.5 copies of most, if not all, HSV-1 HindIII and XbaI DNA fragments per haploid cell genome equivalent. In contrast, the latently infected neurons contained, on an average, 8 to 10 copies per haploid cell genome equivalent of most HSV-1 BamHI DNA fragments. There was no detectable alteration in size or molarity of the HSV-1 terminal or junction DNA fragments obtained by HindIII, XbaI, or BamHI digestion of the latently infected neuron or HEL-F cell DNA, as compared with digestion of a reconstruction mixture of purified HSV-1 virion and HEL-F cell DNAs. These data suggest that the predominant form of the HSV-1 genome in either latently infected cell population is nonintegrated, linear, and nonconcatameric.
Full text
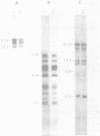
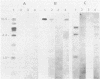
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allaudeen H. S., Chen M. S., Lee J. J., De Clercq E., Prusoff W. H. Incorporation of E-5-(2-halovinyl)-2'-deoxyuridines into deoxyribonucleic acids of herpes simplex virus type 1-infected cells. J Biol Chem. 1982 Jan 25;257(2):603–606. [PubMed] [Google Scholar]
- Allaudeen H. S., Kozarich J. W., Bertino J. R., De Clercq E. On the mechanism of selective inhibition of herpesvirus replication by (E)-5-(2-bromovinyl)-2'-deoxyuridine. Proc Natl Acad Sci U S A. 1981 May;78(5):2698–2702. doi: 10.1073/pnas.78.5.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge M. B., Bunge R. P., Peterson E. R., Murray M. R. A light and electron microscope study of long-term organized cultures of rat dorsal root ganglia. J Cell Biol. 1967 Feb;32(2):439–466. doi: 10.1083/jcb.32.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colberg-Poley A. M., Isom H. C., Rapp F. Involvement of an early human cytomegalovirus function in reactivation of quiescent herpes simplex virus type 2. J Virol. 1981 Mar;37(3):1051–1059. doi: 10.1128/jvi.37.3.1051-1059.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colberg-Poley A. M., Isom H. C., Rapp F. Reactivation of herpes simplex virus type 2 from a quiescent state by human cytomegalovirus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5948–5951. doi: 10.1073/pnas.76.11.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Descamps J., De Somer P., Barr P. J., Jones A. S., Walker R. T. (E)-5-(2-Bromovinyl)-2'-deoxyuridine: a potent and selective anti-herpes agent. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2947–2951. doi: 10.1073/pnas.76.6.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Descamps J., Verhelst G., Walker R. T., Jones A. S., Torrence P. F., Shugar D. Comparative efficacy of antiherpes drugs against different strains of herpes simplex virus. J Infect Dis. 1980 May;141(5):563–574. doi: 10.1093/infdis/141.5.563. [DOI] [PubMed] [Google Scholar]
- Fraser N. W., Lawrence W. C., Wroblewska Z., Gilden D. H., Koprowski H. Herpes simplex type 1 DNA in human brain tissue. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6461–6465. doi: 10.1073/pnas.78.10.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., Fenoglio C. M., McDougall J. K. Limited transcription of the herpes simplex virus genome when latent in human sensory ganglia. J Virol. 1982 Feb;41(2):686–691. doi: 10.1128/jvi.41.2.686-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J. C., Marsden H. S., Cook M. L., Stevens J. G. Acute infection of differentiated neuroblastoma cells by latency-positive and latency-negative herpes simplex virus ts mutants. Virology. 1979 Apr 30;94(2):430–441. doi: 10.1016/0042-6822(79)90473-2. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R. J., Morse L. S., Roizman B. Anatomy of herpes simplex virus DNA. XII. Accumulation of head-to-tail concatemers in nuclei of infected cells and their role in the generation of the four isomeric arrangements of viral DNA. J Virol. 1979 Feb;29(2):448–457. doi: 10.1128/jvi.29.2.448-457.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Batterson W., Nosal C., Roizman B., Buchan A. Molecular genetics of herpes simplex virus. VI. Characterization of a temperature-sensitive mutant defective in the expression of all early viral gene products. J Virol. 1981 May;38(2):539–547. doi: 10.1128/jvi.38.2.539-547.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M. Cell growth transformation by herpes simplex virus. Prog Med Virol. 1982;28:114–144. [PubMed] [Google Scholar]
- Kudler L., Jones T. R., Russell R. J., Hyman R. W. Heteroduplex analysis of cloned fragments of herpes simplex virus DNAs. Virology. 1983 Jan 15;124(1):86–99. doi: 10.1016/0042-6822(83)90292-1. [DOI] [PubMed] [Google Scholar]
- Levine M., Goldin A. L., Glorioso J. C. Persistence of herpes simplex virus genes in cells of neuronal origin. J Virol. 1980 Jul;35(1):203–210. doi: 10.1128/jvi.35.1.203-210.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey J. K., Brackmann K. H., Green M. R., Green M. Preparation and characterization of highly radioactive in vitro labeled adenovirus DNA and DNA restriction fragments. Biochemistry. 1977 Oct 4;16(20):4478–4483. doi: 10.1021/bi00639a023. [DOI] [PubMed] [Google Scholar]
- Mancini W. R., De Clercq E., Prusoff W. H. The relationship between incorporation of E-5-(2-Bromovinyl)-2'-deoxyuridine into herpes simplex virus type 1 DNA with virus infectivity and DNA integrity. J Biol Chem. 1983 Jan 25;258(2):792–795. [PubMed] [Google Scholar]
- Miller R. H., Russell R. J., Hyman R. W. Physical map of the short foldback sequences of herpes simplex virus type 1 DNA. Virology. 1982 Feb;117(1):70–80. doi: 10.1016/0042-6822(82)90508-6. [DOI] [PubMed] [Google Scholar]
- Mocarski E. S., Roizman B. Structure and role of the herpes simplex virus DNA termini in inversion, circularization and generation of virion DNA. Cell. 1982 Nov;31(1):89–97. doi: 10.1016/0092-8674(82)90408-1. [DOI] [PubMed] [Google Scholar]
- O'Neill F. J., Goldberg R. J., Rapp F. Herpes simplex virus latency in cultured human cells following treatment with cytosine arabinoside. J Gen Virol. 1972 Feb;14(2):189–197. doi: 10.1099/0022-1317-14-2-189. [DOI] [PubMed] [Google Scholar]
- O'Neill F. J., Rapp F. Early events required for induction of chromosome abnormalities in human cells by herpes simplex virus. Virology. 1971 Jun;44(3):544–553. doi: 10.1016/0042-6822(71)90368-0. [DOI] [PubMed] [Google Scholar]
- O'Neill F. J., Rapp F. Synergistic effect of herpes simplex virus and cytosine arabinoside on human chromosomes. J Virol. 1971 May;7(5):692–695. doi: 10.1128/jvi.7.5.692-695.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden K., Mounts P., Hayward G. S. Homology between mammalian cell DNA sequences and human herpesvirus genomes detected by a hybridization procedure with high-complexity probe. Cell. 1982 Nov;31(1):71–80. doi: 10.1016/0092-8674(82)90406-8. [DOI] [PubMed] [Google Scholar]
- Post L. E., Conley A. J., Mocarski E. S., Roizman B. Cloning of reiterated and nonreiterated herpes simplex virus 1 sequences as BamHI fragments. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4201–4205. doi: 10.1073/pnas.77.7.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R. W., Rubenstein R., Khan A. Herpes simplex virus infection of isolated autonomic neurons in culture: viral replication and spread in a neuronal network. Arch Virol. 1982;71(2):127–140. doi: 10.1007/BF01314882. [DOI] [PubMed] [Google Scholar]
- Price R. W., Walz M. A., Wohlenberg C., Notkins A. L. Latent infection of sensory ganglia with herpes simplex virus: efficacy of immunization. Science. 1975 May 30;188(4191):938–940. doi: 10.1126/science.166432. [DOI] [PubMed] [Google Scholar]
- Puga A., Cantin E. M., Notkins A. L. Homology between murine and human cellular DNA sequences and the terminal repetition of the S component of herpes simplex virus type 1 DNA. Cell. 1982 Nov;31(1):81–87. doi: 10.1016/0092-8674(82)90407-x. [DOI] [PubMed] [Google Scholar]
- Rapp F. Viral carcinogenesis. Int Rev Cytol Suppl. 1983;15:203–244. doi: 10.1016/b978-0-12-364376-6.50014-4. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Tompkins W. A., Melnick J. L. The association of herpesvirus type 2 and carcinoma of the uterine cervix. Am J Epidemiol. 1969 May;89(5):547–554. doi: 10.1093/oxfordjournals.aje.a120967. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Fraser N. W. Detection of HSV-1 genome in central nervous system of latently infected mice. Nature. 1983 Apr 7;302(5908):523–525. doi: 10.1038/302523a0. [DOI] [PubMed] [Google Scholar]
- Russell R. J., Kudler L., Miller R. H., Hyman R. W. Stability of the cloned 'joint region' of herpes simplex virus DNA. Intervirology. 1982;18(1-2):98–104. doi: 10.1159/000149312. [DOI] [PubMed] [Google Scholar]
- Seed B. Diazotizable arylamine cellulose papers for the coupling and hybridization of nucleic acids. Nucleic Acids Res. 1982 Mar 11;10(5):1799–1810. doi: 10.1093/nar/10.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skare J., Summers W. C. Structure and function of herpesvirus genomes. II. EcoRl, Sbal, and HindIII endonuclease cleavage sites on herpes simplex virus. Virology. 1977 Feb;76(2):581–595. doi: 10.1016/0042-6822(77)90240-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Warren K. G., Brown S. M., Wroblewska Z., Gilden D., Koprowski H., Subak-Sharpe J. Isolation of latent herpes simplex virus from the superior cervical and vagus ganglions of human beings. N Engl J Med. 1978 May 11;298(19):1068–1069. doi: 10.1056/NEJM197805112981907. [DOI] [PubMed] [Google Scholar]
- Wigdahl B. L., Isom H. C., De Clercq E., Rapp F. Activation of herpes simplex virus (HSV) type 1 genome by temperature-sensitive mutants of HSV type 2. Virology. 1982 Jan 30;116(2):468–479. doi: 10.1016/0042-6822(82)90140-4. [DOI] [PubMed] [Google Scholar]
- Wigdahl B. L., Isom H. C., Rapp F. Repression and activation of the genome of herpes simplex viruses in human cells. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6522–6526. doi: 10.1073/pnas.78.10.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigdahl B. L., Scheck A. C., De Clercq E., Rapp F. High efficiency latency and activation of herpes simplex virus in human cells. Science. 1982 Sep 17;217(4565):1145–1146. doi: 10.1126/science.6180477. [DOI] [PubMed] [Google Scholar]
- Wigdahl B. L., Ziegler R. J., Sneve M., Rapp F. Herpes simplex virus latency and reactivation in isolated rat sensory neurons. Virology. 1983 May;127(1):159–167. doi: 10.1016/0042-6822(83)90380-x. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Wood P. M. Separation of functional Schwann cells and neurons from normal peripheral nerve tissue. Brain Res. 1976 Oct 22;115(3):361–375. doi: 10.1016/0006-8993(76)90355-3. [DOI] [PubMed] [Google Scholar]
- Youssoufian H., Hammer S. M., Hirsch M. S., Mulder C. Methylation of the viral genome in an in vitro model of herpes simplex virus latency. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2207–2210. doi: 10.1073/pnas.79.7.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler R. J., Herman R. E. Peripheral infection in culture of rat sensory neurons by herpes simplex virus. Infect Immun. 1980 May;28(2):620–623. doi: 10.1128/iai.28.2.620-623.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler R. J., Pozos R. S. Effects of lectins on peripheral infections by herpes simplex virus of rat sensory neurons in culture. Infect Immun. 1981 Nov;34(2):588–595. doi: 10.1128/iai.34.2.588-595.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]