Abstract
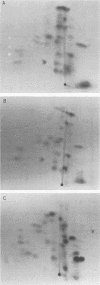
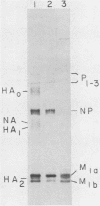
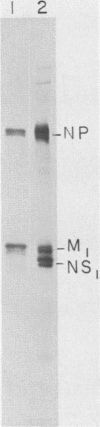
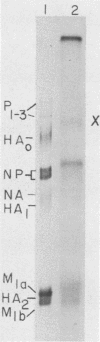
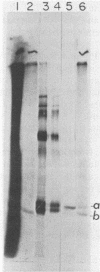
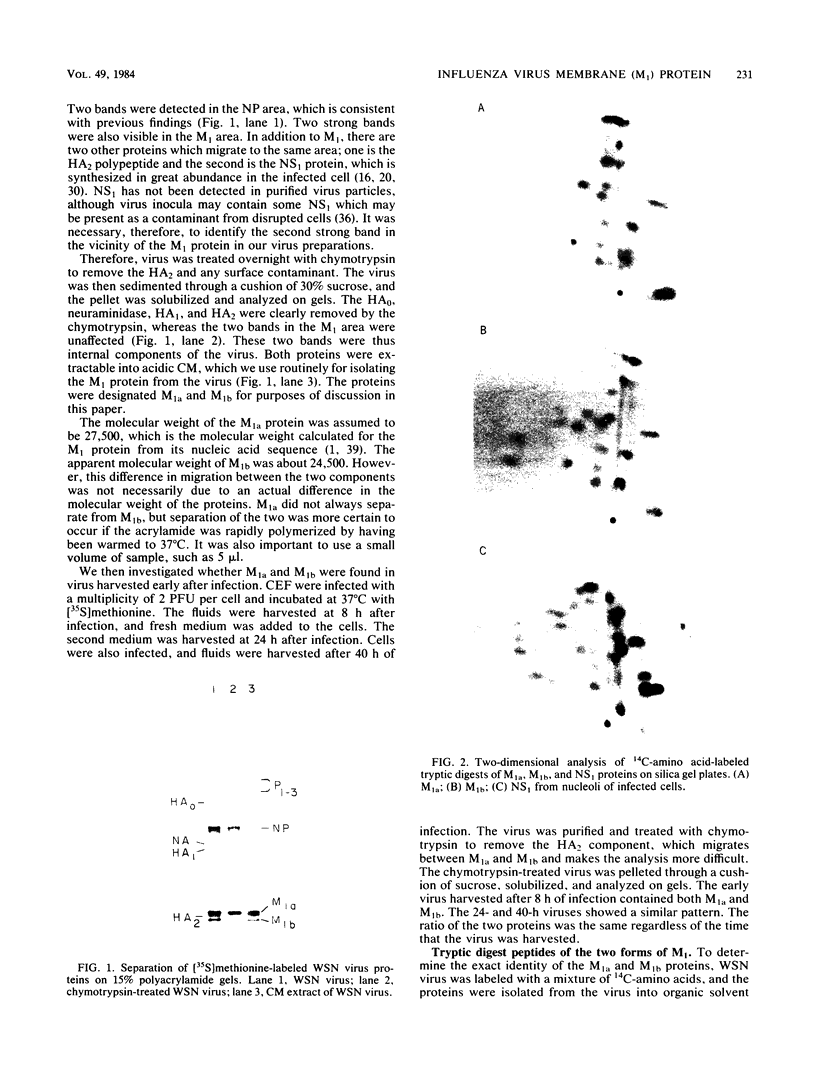
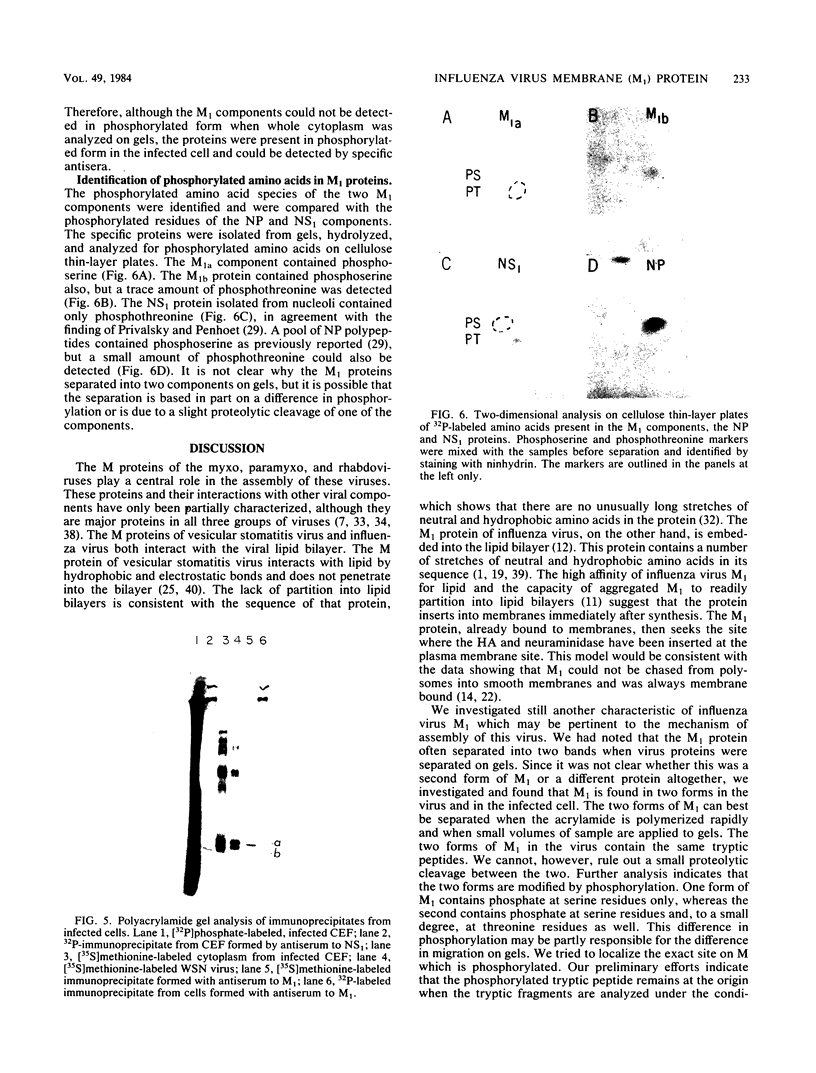
The membrane (M1) protein of influenza virus was found to be heterogenous and to occur in two forms in the virus particle. The two forms of M1 were found in virus which was produced both early and late after infection and in infected cells. The two forms could be separated on polyacrylamide gels under specific conditions. The two components of M1 contained similar tryptic peptides. However, a small proteolytic difference between the two proteins could not be ruled out. Both M1 proteins were present in phosphorylated form in the virus particle. The phosphorylated M1 components were not readily distinguished from phosphorylated nonstructural protein (NS1) when cytoplasm of infected cells was analyzed on polyacrylamide gels. The two phosphorylated M1 components could, however, be detected in infected cells by immunoprecipitation. One M1 component contained only phosphoserine whereas the second contained phosphoserine and a small amount of phosphothreonine as well. In addition to the phosphorylated nucleoprotein and M1, a third phosphorylated protein was routinely detected in virus particles. It was a surface component of the virus, since it could be removed from whole virus with chymotrypsin and contained phosphate at serine residues. The identity of this component was not known.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen H., McCauley J., Waterfield M., Gething M. J. Influenza virus RNA segment 7 has the coding capacity for two polypeptides. Virology. 1980 Dec;107(2):548–551. doi: 10.1016/0042-6822(80)90324-4. [DOI] [PubMed] [Google Scholar]
- Almond J. W., Felsenreich V. Phosphorylation of the nucleoprotein of an avian influenza virus. J Gen Virol. 1982 Jun;60(Pt 2):295–305. doi: 10.1099/0022-1317-60-2-295. [DOI] [PubMed] [Google Scholar]
- Apostolov K., Flewett T. H. Further observations on the structure of influenza viruses A and C. J Gen Virol. 1969 Apr;4(3):365–370. doi: 10.1099/0022-1317-4-3-365. [DOI] [PubMed] [Google Scholar]
- Bucher D. J., Kharitonenkov I. G., Zakomirdin J. A., Grigoriev V. B., Klimenko S. M., Davis J. F. Incorporation of influenza virus M-protein into liposomes. J Virol. 1980 Nov;36(2):586–590. doi: 10.1128/jvi.36.2.586-590.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Burge B. W., Huang A. S. Effects of phosphorylation and pH on the association of NS protein with vesicular stomatitis virus cores. J Virol. 1978 Aug;27(2):340–346. doi: 10.1128/jvi.27.2.340-346.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Huang A. S. Distribution of phosphoserine, phosphothreonine and phosphotyrosine in proteins of vesicular stomatitis virus. Virology. 1981 Jan 30;108(2):510–514. doi: 10.1016/0042-6822(81)90459-1. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Klenk H. D., Caliguiri L. A., Choppin P. W. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970 Dec;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- Gazitt Y., Ohad I., Loyter A. Phosphorylation and dephosphorylation of membrane proteins as a possible mechanism for structural rearrangement of membrane components. Biochim Biophys Acta. 1976 Jun 4;436(1):1–14. doi: 10.1016/0005-2736(76)90214-5. [DOI] [PubMed] [Google Scholar]
- Gregoriades A., Frangione B. Insertion of influenza M protein into the viral lipid bilayer and localization of site of insertion. J Virol. 1981 Oct;40(1):323–328. doi: 10.1128/jvi.40.1.323-328.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriades A. Influenza virus-induced proteins in nuclei and cytoplasm of infected cells. Virology. 1977 Jun 15;79(2):449–454. doi: 10.1016/0042-6822(77)90372-5. [DOI] [PubMed] [Google Scholar]
- Gregoriades A. Interaction of influenza M protein with viral lipid and phosphatidylcholine vesicles. J Virol. 1980 Nov;36(2):470–479. doi: 10.1128/jvi.36.2.470-479.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriades A., Markarian K. Anomalous electrophoretic behavior of the membrane (M) protein of influenza virus in polyacrylamide gels. Arch Virol. 1983;76(3):263–267. doi: 10.1007/BF01311110. [DOI] [PubMed] [Google Scholar]
- Gregoriades A. The membrane protein of influenza virus: extraction from virus and infected cell with acidic chloroform-methanol. Virology. 1973 Aug;54(2):369–383. doi: 10.1016/0042-6822(73)90150-5. [DOI] [PubMed] [Google Scholar]
- Hay A. J. Studies on the formation of the influenza virus envelope. Virology. 1974 Aug;60(2):398–418. doi: 10.1016/0042-6822(74)90335-3. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., Brown C. M. Spliced and unspliced RNAs encoded by virion RNA segment 7 of influenza virus. Nucleic Acids Res. 1981 Jun 25;9(12):2727–2740. doi: 10.1093/nar/9.12.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis S. C., Carroll A. R., Lamb R. A., Mahy B. W. Polypeptides specified by the influenza virus genome I. Evidence for eight distinct gene products specified by fowl plague virus. Virology. 1976 Oct 15;74(2):489–503. doi: 10.1016/0042-6822(76)90355-x. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov I. G., ElKaradahgi S., Bucher D. J., Zakomirdin J. A., Tverdislov V. A. Interaction of influenza virus proteins with planar lipid bilayers: a model for virion assembly. Biochem Biophys Res Commun. 1981 Sep 16;102(1):308–314. doi: 10.1016/0006-291x(81)91522-9. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Identification of a second protein (M2) encoded by RNA segment 7 of influenza virus. Virology. 1981 Jul 30;112(2):729–737. doi: 10.1016/0042-6822(81)90317-2. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. The synthesis of Sendai virus polypeptides in infected cells. III. Phosphorylation of polypeptides. Virology. 1977 Sep;81(2):382–397. doi: 10.1016/0042-6822(77)90154-4. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology. 1971 Dec;46(3):830–843. doi: 10.1016/0042-6822(71)90084-5. [DOI] [PubMed] [Google Scholar]
- Makino S., Woolford J. L., Jr, Tanford C., Webster R. E. Interaction of deoxycholate and of detergents with the coat protein of bacteriophage f1. J Biol Chem. 1975 Jun 10;250(11):4327–4332. [PubMed] [Google Scholar]
- Meier-Ewert H., Compans R. W. Time course of synthesis and assembly of influenza virus proteins. J Virol. 1974 Nov;14(5):1083–1091. doi: 10.1128/jvi.14.5.1083-1091.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrongiello M. P., Dales S. Characterization of cytoplasmic inclusions formed during influenza/WSN virus infection of chick embryo fibroblast cells. Intervirology. 1977;8(5):281–293. doi: 10.1159/000148903. [DOI] [PubMed] [Google Scholar]
- Neer E. J. Size and detergent binding of adenylate cyclase from bovine cerebral cortex. J Biol Chem. 1978 Mar 10;253(5):1498–1502. [PubMed] [Google Scholar]
- Pepinsky R. B., Vogt V. M. Identification of retrovirus matrix proteins by lipid-protein cross-linking. J Mol Biol. 1979 Jul 15;131(4):819–837. doi: 10.1016/0022-2836(79)90203-1. [DOI] [PubMed] [Google Scholar]
- Petri T., Dimmock N. J. Phosphorylation of influenza virus nucleoprotein in vivo. J Gen Virol. 1981 Nov;57(Pt 1):185–190. doi: 10.1099/0022-1317-57-1-185. [DOI] [PubMed] [Google Scholar]
- Privalsky M. L., Penhoet E. E. Influenza virus proteins: identity, synthesis, and modification analyzed by two-dimensional gel electrophoresis. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3625–3629. doi: 10.1073/pnas.75.8.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalsky M. L., Penhoet E. E. Phosphorylated protein component present in influenza virions. J Virol. 1977 Oct;24(1):401–405. doi: 10.1128/jvi.24.1.401-405.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalsky M. L., Penhoet E. E. The structure and synthesis of influenza virus phosphoproteins. J Biol Chem. 1981 Jun 10;256(11):5368–5376. [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Schulman J. L. Difference in protein patterns of influenza A viruses. Virology. 1977 Jan;76(1):122–128. doi: 10.1016/0042-6822(77)90289-6. [DOI] [PubMed] [Google Scholar]
- Robinson N. C., Tanford C. The binding of deoxycholate, Triton X-100, sodium dodecyl sulfate, and phosphatidylcholine vesicles to cytochrome b5. Biochemistry. 1975 Jan 28;14(2):369–378. doi: 10.1021/bi00673a025. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Schulze I. T. The structure of influenza virus. I. The polypeptides of the virion. Virology. 1970 Dec;42(4):890–904. doi: 10.1016/0042-6822(70)90338-7. [DOI] [PubMed] [Google Scholar]
- Schulze I. T. The structure of influenza virus. II. A model based on the morphology and composition of subviral particles. Virology. 1972 Jan;47(1):181–196. doi: 10.1016/0042-6822(72)90251-6. [DOI] [PubMed] [Google Scholar]
- Shaw M. W., Lamon E. W., Compans R. W. Surface expression of a nonstructural antigen on influenza A virus-infected cells. Infect Immun. 1981 Dec;34(3):1065–1067. doi: 10.1128/iai.34.3.1065-1067.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama K., Kamada T., Shimizu K., Watanabe Y. Preferential phosphorylation of NP-protein of influenza A2 virus by virion-associated protein kinase. Jpn J Microbiol. 1976 Jun;20(3):227–232. doi: 10.1111/j.1348-0421.1976.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Winter G., Fields S. Cloning of influenza cDNA ino M13: the sequence of the RNA segment encoding the A/PR/8/34 matrix protein. Nucleic Acids Res. 1980 May 10;8(9):1965–1974. doi: 10.1093/nar/8.9.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakowski J. J., Petri W. A., Jr, Wagner R. R. Role of matrix protein in assembling the membrane of vesicular stomatitis virus: reconstitution of matrix protein with negatively charged phospholipid vesicles. Biochemistry. 1981 Jun 23;20(13):3902–3907. doi: 10.1021/bi00516a037. [DOI] [PubMed] [Google Scholar]
- Zhirnov O. P., Bukrinskaya A. G. Two forms of influenza virus nucleoprotein in infected cells and virions. Virology. 1981 Feb;109(1):174–179. doi: 10.1016/0042-6822(81)90482-7. [DOI] [PubMed] [Google Scholar]
- Zvonarjev A. Y., Ghendon Y. Z. Influence of membrane (M) protein on influenza A virus virion transcriptase activity in vitro and its susceptibility to rimantadine. J Virol. 1980 Feb;33(2):583–586. doi: 10.1128/jvi.33.2.583-586.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]