Abstract
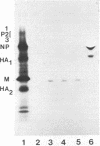
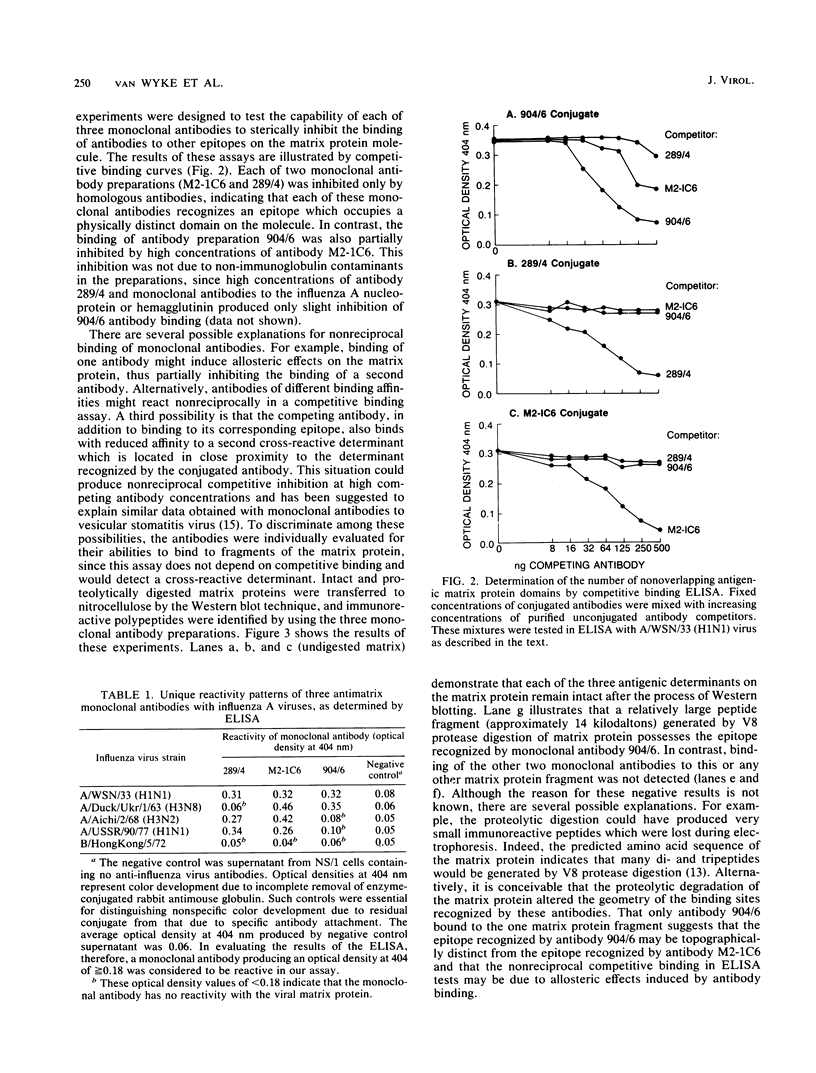
Monoclonal antibodies were used to study antigenic variation in three distinct epitopes on the matrix protein of influenza A viruses. We found that two of these epitopes underwent antigenic variation, but in a very limited number of virus strains. A third epitope appeared to be an invariant type-specific determinant for influenza A viruses. Competitive antibody binding assays and Western blot analysis of proteolytically digested matrix protein indicated that at least two of the three epitopes are located in nonoverlapping domains on the matrix protein molecule.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aymard-Henry M., Coleman M. T., Dowdle W. R., Laver W. G., Schild G. C., Webster R. G. Influenzavirus neuraminidase and neuraminidase-inhibition test procedures. Bull World Health Organ. 1973;48(2):199–202. [PMC free article] [PubMed] [Google Scholar]
- Brand C. M., Stealey V. M., Rowe J. Peptide mapping of 125I-labelled influenza virus proteins. Matrix proteins as markers in recombination. J Gen Virol. 1977 Sep;36(3):385–394. doi: 10.1099/0022-1317-36-3-385. [DOI] [PubMed] [Google Scholar]
- CHOPPIN P. W., TAMM I. Studies of two kinds of virus particles which comprise influenza A2 virus strains. II. Reactivity with virus inhibitors in normal sera. J Exp Med. 1960 Nov 1;112:921–944. doi: 10.1084/jem.112.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dimmock N. J., Carver A. S., Webster R. G. Categorization of nucleoproteins and matrix proteins from type A influenza viruses by peptide mapping. Virology. 1980 Jun;103(2):350–356. doi: 10.1016/0042-6822(80)90193-2. [DOI] [PubMed] [Google Scholar]
- Fazekas de St Groth, Webster R. G. Disquisitions of Original Antigenic Sin. I. Evidence in man. J Exp Med. 1966 Sep 1;124(3):331–345. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard W. The analysis of the monoclonal immune response to influenza virus. II. The antigenicity of the viral hemagglutinin. J Exp Med. 1976 Oct 1;144(4):985–995. doi: 10.1084/jem.144.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourne E. D. Genetic dimorphism in influenza viruses: characterization of stably associated hemagglutinin mutants differing in antigenicity and biological properties. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6258–6262. doi: 10.1073/pnas.75.12.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourne E. D., McGregor S., Easterday B. C. Hemagglutinin mutants of swine influenza virus differing in replication characteristics in their natural host. Infect Immun. 1979 Oct;26(1):197–201. doi: 10.1128/iai.26.1.197-201.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J. Conservation of the influenza virus membrane protein (M1) amino acid sequence and an open reading frame of RNA segment 7 encoding a second protein (M2) in H1N1 and H3N2 strains. Virology. 1981 Jul 30;112(2):746–751. doi: 10.1016/0042-6822(81)90319-6. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. Selection of antigenic mutants of influenza viruses. Isolation and peptide mapping of their hemagglutination proteins. Virology. 1968 Feb;34(2):193–202. doi: 10.1016/0042-6822(68)90230-4. [DOI] [PubMed] [Google Scholar]
- Lefrancios L., Lyles D. S. The interactionof antiody with the major surface glycoprotein of vesicular stomatitis virus. I. Analysis of neutralizing epitopes with monoclonal antibodies. Virology. 1982 Aug;121(1):157–167. [PubMed] [Google Scholar]
- Lenard J., Compans R. W. The membrane structure of lipid-containing viruses. Biochim Biophys Acta. 1974 Apr 8;344(1):51–94. doi: 10.1016/0304-4157(74)90008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubeck M., Gerhard W. Conformational changes at topologically distinct antigenic sites on the influenza A/PR/8/34 virus HA molecule are induced by the binding of monoclonal antibodies. Virology. 1982 Apr 15;118(1):1–7. doi: 10.1016/0042-6822(82)90313-0. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Schild G. C. Evidence for a new type-specific structural antigen of the influenza virus particle. J Gen Virol. 1972 Apr;15(1):99–103. doi: 10.1099/0022-1317-15-1-99. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Rohde W., Von Hoyningen V., Rott R. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology. 1978 Jun 1;87(1):13–20. doi: 10.1016/0042-6822(78)90153-8. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Hinshaw V. S., Laver W. G. Selection and analysis of antigenic variants of the neuraminidase of N2 influenza viruses with monoclonal antibodies. Virology. 1982 Feb;117(1):93–104. doi: 10.1016/0042-6822(82)90510-4. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Kendal A. P., Gerhard W. Analysis of antigenic drift in recently isolated influenza A (H1N1) viruses using monoclonal antibody preparations. Virology. 1979 Jul 15;96(1):258–264. doi: 10.1016/0042-6822(79)90189-2. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G., Air G. M., Ward C., Gerhard W., van Wyke K. L. The mechanism of antigenic drift in influenza viruses: analysis of Hong Kong (H3N2) variants with monoclonal antibodies to the hemagglutinin molecule. Ann N Y Acad Sci. 1980;354:142–161. doi: 10.1111/j.1749-6632.1980.tb27964.x. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G. Determination of the number of nonoverlapping antigenic areas on Hong Kong (H3N2) influenza virus hemagglutinin with monoclonal antibodies and the selection of variants with potential epidemiological significance. Virology. 1980 Jul 15;104(1):139–148. doi: 10.1016/0042-6822(80)90372-4. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Frank E., Gerhard W. Expression of influenza A virus internal antigens on the surface of infected P815 cells. J Immunol. 1981 May;126(5):1814–1819. [PubMed] [Google Scholar]
- van Wyke K. L., Bean W. J., Jr, Webster R. G. Monoclonal antibodies to the influenza A virus nucleoprotein affecting RNA transcription. J Virol. 1981 Jul;39(1):313–317. doi: 10.1128/jvi.39.1.313-317.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyke K. L., Hinshaw V. S., Bean W. J., Jr, Webster R. G. Antigenic variation of influenza A virus nucleoprotein detected with monoclonal antibodies. J Virol. 1980 Jul;35(1):24–30. doi: 10.1128/jvi.35.1.24-30.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]