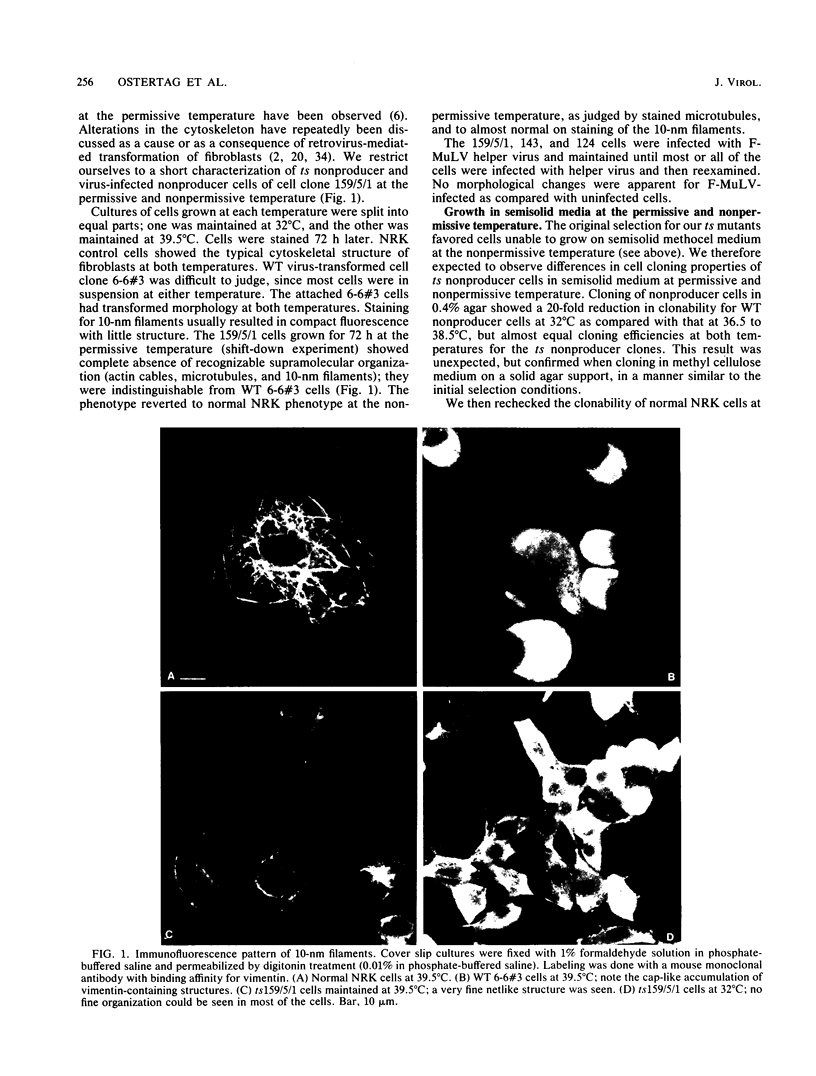
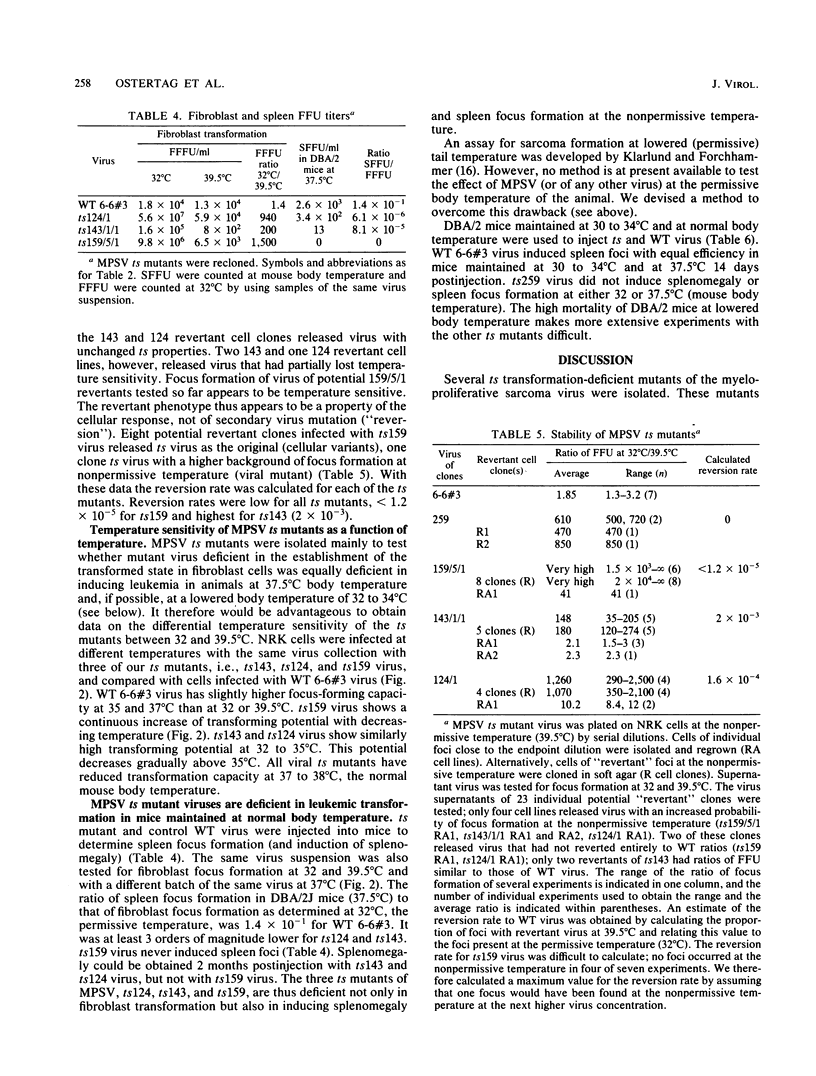
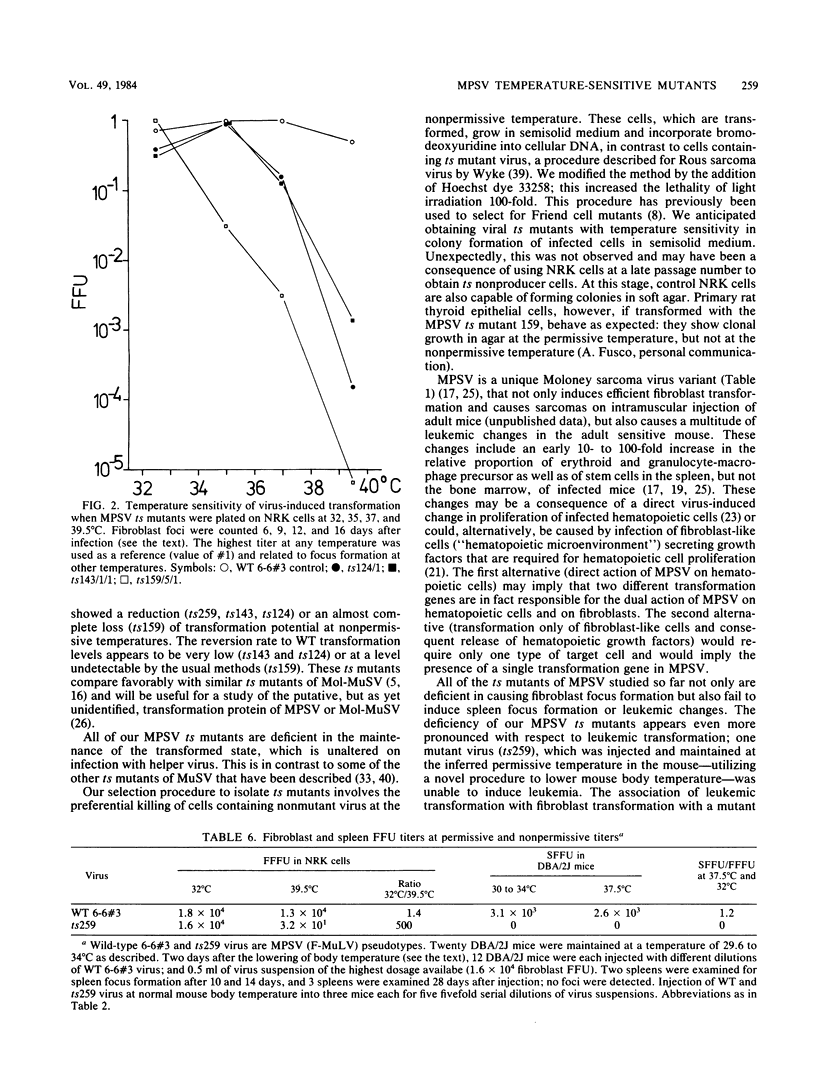
Abstract
The myeloproliferative sarcoma virus is molecularly related to the Moloney sarcoma virus (Pragnell et al., J. Virol. 38:952-957, 1981), but causes both fibroblast transformation in vitro and leukemic changes--including spleen focus formation--in adult mice. The fibroblast transforming properties of myeloproliferative sarcoma virus were used to select viral temperature-sensitive mutants at 39.5 degrees C, the nonpermissive temperature. These mutants are temperature sensitive in the maintenance of the transformed state. This was also shown by cytoskeletal changes of the infected cells at permissive and nonpermissive temperatures. Viruses released from cells maintained at both the permissive and nonpermissive temperature are temperature sensitive in fibroblast transformation functions. All temperature-sensitive mutants show only a low reversion rate to wild-type transforming function. The myeloproliferative sarcoma virus temperature-sensitive mutants are inefficient in causing leukemic transformation (spleen enlargement, focus formation) in mice at the normal temperature. A method to maintain a low body temperature (33 to 34 degrees C) in mice is described. One temperature-sensitive mutant was checked at low body temperature and did not induce leukemia. These data thus indicate that the same or related viral functions are responsible for hematopoietic and fibroblast transformation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P. R., Devare S. G., Tronick S. R., Ellis R. W., Aaronson S. A., Scolnick E. M. Generation of BALB-MuSV and Ha-MuSC by type C virus transduction of homologous transforming genes from different species. Cell. 1981 Oct;26(1 Pt 1):129–134. doi: 10.1016/0092-8674(81)90041-6. [DOI] [PubMed] [Google Scholar]
- Ball E. H., Singer S. J. Association of microtubules and intermediate filaments in normal fibroblasts and its disruption upon transformation by a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6986–6990. doi: 10.1073/pnas.78.11.6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., Kitchener G., Doederlein G., Graf T., Hayman M. J. Mutant of avian erythroblastosis virus defective for erythroblast transformation: deletion in the erb portion of p75 suggests function of the protein in leukemogenesis. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6683–6686. doi: 10.1073/pnas.77.11.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilello J. A., Strand M., August J. T. Murine sarcoma virus gene expression: transformants which express viral envelope glycoprotein in the absence of the major internal protein and infectious particles. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3234–3238. doi: 10.1073/pnas.71.8.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair D. G., Hull M. A., Finch E. A. The isolation and preliminary characterization of temperature-sensitive transformation mutants of Moloney sarcoma virus. Virology. 1979 Jun;95(2):303–316. doi: 10.1016/0042-6822(79)90486-0. [DOI] [PubMed] [Google Scholar]
- Brown R. L., Horn J. P., Wible L., Arlinghaus R. B., Brinkley B. R. Sequence of events in the transformation process in cells infected with a temperature-sensitive transformation mutant of Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5593–5597. doi: 10.1073/pnas.78.9.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirigos M. A., Scott D., Turner W., Perk K. Biological, pathological and physical characterization of a possible variant of a murine sarcoma virus (Moloney). Int J Cancer. 1968 Mar 15;3(2):223–227. doi: 10.1002/ijc.2910030207. [DOI] [PubMed] [Google Scholar]
- Conkie D., Young B. D., Paul J. Friend cell variants temperature-sensitive for growth. Exp Cell Res. 1980 Apr;126(2):439–444. doi: 10.1016/0014-4827(80)90283-9. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Testa N. G., Allen T. D., Rutherford T., Scolnick E. Molecular and cell biologic aspects of erythropoiesis in long-term bone marrow cultures. Blood. 1981 Oct;58(4):699–707. [PubMed] [Google Scholar]
- Duesberg P. H. Transforming genes of retroviruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):13–29. doi: 10.1101/sqb.1980.044.01.005. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Hankins W. D., Kost T. A., Pragnell I. B. The myeloproliferative sarcoma virus causes transformation or erythroid progenitor cells in vitro. Mol Cell Biol. 1982 Feb;2(2):138–146. doi: 10.1128/mcb.2.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins W. D., Scolnick E. M. Harvey and Kirsten sarcoma viruses promote the growth and differentiation of erythroid precursor cells in vitro. Cell. 1981 Oct;26(1 Pt 1):91–97. doi: 10.1016/0092-8674(81)90036-2. [DOI] [PubMed] [Google Scholar]
- Hankins W. D., Troxler D. Polycythemia- and anemia-inducing erythroleukemia viruses exhibit differential erythroid transforming effects in vitro. Cell. 1980 Dec;22(3):693–699. doi: 10.1016/0092-8674(80)90545-0. [DOI] [PubMed] [Google Scholar]
- Harbers K., Schnieke A., Stuhlmann H., Jähner D., Jaenisch R. DNA methylation and gene expression: endogenous retroviral genome becomes infectious after molecular cloning. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7609–7613. doi: 10.1073/pnas.78.12.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarlund J. K., Forchhammer J. Temperature-sensitive tumorigenicity of cells transformed by a mutant of Moloney sarcoma virus. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1501–1505. doi: 10.1073/pnas.77.3.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein B., Le Bousse C., Fagg B., Smajda-Joffe F., Vehmeyer K., Mori K. J., Jasmin C., Ostertag W. Effects of myeloproliferative sarcoma virus on the pluripotential stem cell and granulocyte precursor cell populations of DBA/2 mice. J Natl Cancer Inst. 1981 May;66(5):935–940. [PubMed] [Google Scholar]
- Lai M. M., Neil J. C., Vogt P. K. Cell-free translation of avian erythroblastosis virus RNA yields two specific and distinct proteins with molecular weights of 75,000 and 40,000. Virology. 1980 Jan 30;100(2):475–483. doi: 10.1016/0042-6822(80)90537-1. [DOI] [PubMed] [Google Scholar]
- Le Bousse-Kerdiles M. C., Smadja-Joffe F., Klein B., Caillou B., Jasmin C. Study of a virus-induced myeloproliferative syndrome associated with tumor formation in mice. Eur J Cancer. 1980 Jan;16(1):43–51. doi: 10.1016/0014-2964(80)90106-1. [DOI] [PubMed] [Google Scholar]
- McClain D. A., D'Eustachio P., Edelman G. M. Role of surface modulating assemblies in growth control of normal and transformed fibroblasts. Proc Natl Acad Sci U S A. 1977 Feb;74(2):666–670. doi: 10.1073/pnas.74.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K. J., Smadja-Joffe F., Barre-Sinoussi F., Le Bousse-Kerdiles M. C., Klein B., Caillou B., Ostertag W., Jasmin C., Degiorgis V. Myeloproliferative Sarcoma Virus stimulates pluripotent hematopoietic stem cells and provokes tumoral transformation of the hematopoietic microenvironment in vitro. Leuk Res. 1983;7(1):77–86. doi: 10.1016/0145-2126(83)90060-7. [DOI] [PubMed] [Google Scholar]
- Müller R., Slamon D. J., Tremblay J. M., Cline M. J., Verma I. M. Differential expression of cellular oncogenes during pre- and postnatal development of the mouse. Nature. 1982 Oct 14;299(5884):640–644. doi: 10.1038/299640a0. [DOI] [PubMed] [Google Scholar]
- Ostertag W., Odaka T., Smadja-Joffe F., Jasmin C. Inheritance of susceptibility to the myeloproliferative sarcoma virus: effect of the Fv-2 locus and evidence for a myeloproliferative sarcoma virus resistance locus. J Virol. 1981 Feb;37(2):541–548. doi: 10.1128/jvi.37.2.541-548.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostertag W., Pragnell I. B. Differentiation and viral involvement in differentiation of transformed mouse and rat erythroid cells. Curr Top Microbiol Immunol. 1981;94-95:143–208. doi: 10.1007/978-3-642-68120-2_4. [DOI] [PubMed] [Google Scholar]
- Ostertag W., Vehmeyer K., Fagg B., Pragnell I. B., Paetz W., Le Bousse M. C., Smadja-Joffe F., Klein B., Jasmin C., Eisen H. Myeloproliferative virus, a cloned murine sarcoma virus with spleen focus-forming properties in adult mice. J Virol. 1980 Feb;33(2):573–582. doi: 10.1128/jvi.33.2.573-582.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J., Lai M. H., Hunter T., Verma I. M. Analysis of transforming gene products from Moloney murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):109–119. doi: 10.1016/0092-8674(81)90365-2. [DOI] [PubMed] [Google Scholar]
- Pragnell I. B., Fusco A., Arbuthnott C., Smadja-Joffe F., Klein B., Jasmin C., Ostertag W. Analysis of the myeloproliferative sarcoma virus genome: limited changes in the prototype lead to altered target cell specificity. J Virol. 1981 Jun;38(3):952–957. doi: 10.1128/jvi.38.3.952-957.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. A quantitative assay for transformation of bone marrow cells by Abelson murine leukemia virus. J Exp Med. 1976 Jun 1;143(6):1453–1463. doi: 10.1084/jem.143.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer-Pokora B., Beug H., Claviez M., Winkhardt H. J., Friis R. R., Graf T. Transformation parameters in chicken fibroblasts transformed by AEV and MC29 avian leukemia viruses. Cell. 1978 Apr;13(4):751–760. doi: 10.1016/0092-8674(78)90225-8. [DOI] [PubMed] [Google Scholar]
- Rutter G., Mannweiler K. Alterations of actin-containing structures in BHK21 cells infected with Newcastle disease virus and vesicular stomatitis virus. J Gen Virol. 1977 Nov;37(2):233–242. doi: 10.1099/0022-1317-37-2-233. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Siegler R. Direct transformation of 3T3 cells by Abelson murine leukaemia virus. Nature. 1975 Feb 27;253(5494):729–731. doi: 10.1038/253729a0. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Stephenson J. R., Aaronson S. A. Isolation of temperature-sensitive mutants of murine sarcoma virus. J Virol. 1972 Oct;10(4):653–657. doi: 10.1128/jvi.10.4.653-657.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A., Todaro G. J., Blair D. G., Robey W. G. Thermolabile protein kinase molecules in a temperature-sensitive murine sarcoma virus pseudotype. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3617–3621. doi: 10.1073/pnas.76.8.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler D. H., Ruscetti S. K., Scolnick E. M. The molecular biology of Friend virus. Biochim Biophys Acta. 1980 Sep 22;605(3):305–324. doi: 10.1016/0304-419x(80)90014-1. [DOI] [PubMed] [Google Scholar]
- Vande Woude G. F., Oskarsson M., Enquist L. W., Nomura S., Sullivan M., Fischinger P. J. Cloning of integrated Moloney sarcoma proviral DNA sequences in bacteriophage lambda. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4464–4468. doi: 10.1073/pnas.76.9.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- Wyke J. A. Temperature sensitive mutants of avian sarcoma viruses. Biochim Biophys Acta. 1975 Jul 11;417(2):91–121. doi: 10.1016/0304-419x(75)90001-3. [DOI] [PubMed] [Google Scholar]
- Yuasa Y., Shimojo H. Isolation and preliminary characterization of temperature-sensitive mutants of the murine sarcoma leukaemia virus complex. J Gen Virol. 1977 Aug;36(2):257–266. doi: 10.1099/0022-1317-36-2-257. [DOI] [PubMed] [Google Scholar]



