Abstract
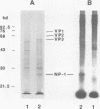
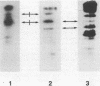
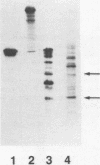
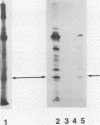
A phosphorylated protein (NP-1) with an Mr of 28,000 has been detected in nuclei of bovine parvovirus (BPV)-infected cells in association with chromatin. No protein in this size range was detected after infection of appropriate cells with several autonomous rodent parvoviruses although the BPV-specific protein is similar in size to noncapsid proteins associated with rabbit parvovirus or adeno-associated virus infection. Structural homology between NP-1 and a BPV capsid protein could be detected by electrophoretic analysis of the products of proteolysis with chymotrypsin. This protein can be detected after in vitro translation of RNA from BPV-infected cells and BPV-specific RNA. Homology between the in vivo- and in vitro-synthesized species was shown by the similarity of the chymotryptic products.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astell C. R., Thomson M., Merchlinsky M., Ward D. C. The complete DNA sequence of minute virus of mice, an autonomous parvovirus. Nucleic Acids Res. 1983 Feb 25;11(4):999–1018. doi: 10.1093/nar/11.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Identification of a nonvirion protein of Aleutian disease virus: mink with Aleutian disease have antibody to both virion and nonvirion proteins. J Virol. 1982 Aug;43(2):608–616. doi: 10.1128/jvi.43.2.608-616.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Rose J. A. Characterization of adenovirus-associated virus-induced polypeptides in KB cells. J Virol. 1978 Jan;25(1):331–338. doi: 10.1128/jvi.25.1.331-338.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. L., Hansen U., Handa H., Sharp P. A. Sequential transcription-translation of simian virus 40 by using mammalian cell extracts. Mol Cell Biol. 1981 Oct;1(10):919–931. doi: 10.1128/mcb.1.10.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lederman M., Bates R. C., Stout E. R. In vitro and in vivo studies of bovine parvovirus proteins. J Virol. 1983 Oct;48(1):10–17. doi: 10.1128/jvi.48.1.10-17.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubeck M. D., Lee H. M., Hoggan M. D., Johnson F. B. Adenovirus-associated virus structural protein sequence homology. J Gen Virol. 1979 Oct;45(1):209–216. doi: 10.1099/0022-1317-45-1-209. [DOI] [PubMed] [Google Scholar]
- Matsunaga Y., Matsuno S. Structural and nonstructural proteins of a rabbit parvovirus. J Virol. 1983 Feb;45(2):627–633. doi: 10.1128/jvi.45.2.627-633.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Wali T., Valdez V., Fabisch P., Salzman L. A. Transcription and translation in the autonomous parvovirus KRV. Virology. 1983 Mar;125(2):349–360. doi: 10.1016/0042-6822(83)90207-6. [DOI] [PubMed] [Google Scholar]
- Moss L. G., Moore J. P., Chan L. A simple, efficient method for coupling DNA to cellulose. Development of the method and application to mRNA purification. J Biol Chem. 1981 Dec 25;256(24):12655–12658. [PubMed] [Google Scholar]
- Parris D. S., Bates R. C. Effect of bovine parvovirus replication on DNA, RNA, and protein synthesis in S phase cells. Virology. 1976 Aug;73(1):72–78. doi: 10.1016/0042-6822(76)90061-1. [DOI] [PubMed] [Google Scholar]
- Pintel D., Dadachanji D., Astell C. R., Ward D. C. The genome of minute virus of mice, an autonomous parvovirus, encodes two overlapping transcription units. Nucleic Acids Res. 1983 Feb 25;11(4):1019–1038. doi: 10.1093/nar/11.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard C., Bates R. C., Stout E. R. Levels of cellular DNA polymerases in synchronized bovine paravovirus-infected cells. J Virol. 1978 Jul;27(1):258–261. [PMC free article] [PubMed] [Google Scholar]
- Revie D., Tseng B. Y., Grafstrom R. H., Goulian M. Covalent association of protein with replicative form DNA of parvovirus H-1. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5539–5543. doi: 10.1073/pnas.76.11.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Paradiso P. R. Parvovirus genome: nucleotide sequence of H-1 and mapping of its genes by hybrid-arrested translation. J Virol. 1983 Jan;45(1):173–184. doi: 10.1128/jvi.45.1.173-184.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm I. Definition of subclasses of nucleoplasmic RNA. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5011–5015. doi: 10.1073/pnas.74.11.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Shatkin A. J., Ward D. C. Sequence homology between the structural polypeptides of minute virus of mice. J Mol Biol. 1977 Apr 25;111(4):375–394. doi: 10.1016/s0022-2836(77)80060-0. [DOI] [PubMed] [Google Scholar]
- Tremblay G. Y., Daniels M. J., Schaechter M. Isolation of a cell membrane-DNA-nascent RNA complex from bacteria. J Mol Biol. 1969 Feb 28;40(1):65–76. doi: 10.1016/0022-2836(69)90296-4. [DOI] [PubMed] [Google Scholar]