Abstract
STUDY DESIGN
Controlled laboratory study.
OBJECTIVES
To determine the effects of pulse duration and stimulation duration on the evoked torque after controlling for the activated area by using magnetic resonance imaging (MRI).
BACKGROUND
Neuromuscular electrical stimulation (NMES) is commonly used in the clinic without considering the physiological implications of its parameters.
METHODS AND MEASURES
Seven able-bodied, college students (mean ± SD age, 28 ± 4 years) participated in this study. Two NMES protocols were applied to the knee extensor muscle group in a random order. Protocol A applied 100-Hz, 450-microsecond pulses for 5 minutes in a 3-seconds-on 3-seconds-off duty cycle. Protocol B applied 60-Hz, 250-microsecond pulses for 5 minutes in a 10-seconds-on 20-seconds-off duty cycle. The amplitude of the current was similar in both protocols. Torque, torque time integral, and normalized torque for the knee extensors were measured for both protocols. MRI scans were taken prior to, and immediately after, each protocol to measure the cross-sectional area of the stimulated muscle.
RESULTS
The skeletal muscle cross-sectional areas activated after both protocols were similar. The longer pulse duration in protocol A elicited 22% greater torque output than that of protocol B (P<.05). After considering the activated area in both protocols, the normalized torque with protocol A was 38% greater than that with protocol B (P<.05). Torque time integral was 21% greater with protocol A (P=.029). Protocol B failed to maintain torque at the start and the end of the 10-second activation.
CONCLUSIONS
Longer pulse duration, but not stimulation duration, resulted in a greater evoked and normalized torque compared to the shorter pulse duration, even after controlling for the activated muscular CSA with both protocols.
LEVEL OF EVIDENCE
Therapy, level 5.
Keywords: electrotherapy, MRI, NMES, quadriceps
Surface neuromuscular electrical stimulation (NMES) is a useful treatment tool in sports medicine and for clinical conditions characterized by motor impairments such as stroke, cerebral palsy, and spinal cord injury.16,18,30,38,39 The common neuromuscular adaptations that characterize the aforementioned conditions are muscle weakness and atrophy resulting from disuse or neurological injury.18,38 NMES protocols consist of a combination of pulse parameters and time modulations to induce muscle contractions that aim to simulate both endurance and resistance training.4,10,15,18 The selection of stimulation parameters is typically based on each patient's rehabilitation goals. For example, selecting a protocol with pulse frequency of less than 15 Hz could help increase aerobic capacity in patients with heart failure.15 On the other hand, a frequency greater than 50 Hz is used to increase muscle strength.16,38 Therefore, studying the parameters that could maximize torque output to attenuate skeletal muscle weakness and atrophy is a key element to the application of NMES.
The pulse parameters that are most commonly adjusted to maximize torque output include amplitude of the current, pulse duration, and frequency of the pulses.8,9,20,23 These parameters characterize the features of a single pulse or series of pulses.34 In a single pulse, the current amplitude, duration of the pulse, and the shape of the waveform determine the magnitude of the pulse charge of a stimulus.24 The pulse charge is defined as the current-time integral of the pulse and determines the strength of the stimulus and the evoked torque.24,34 Laufer et al27 showed that there was no difference in torque production between monophasic and biphasic waveforms; however, both were superior to the polyphasic waveform. Therefore, in a single waveform the interaction between the amplitude and the pulse duration is critical to the pulse charge.3 Unlike single pulse, series of pulses could be determined by adjusting the frequency that controls the number of pulses per unit time or by setting the interpulse intervals.34
Frequency of the pulses has been studied extensively because of its important role in determining the torque development and controlling muscle fatigue.9,13,20,35,36 Increasing the frequency results in a sigmoidal increase in torque production but concurrently accelerates muscle fatigue.9,10,13,25 Also, increasing the frequency from 25 to 100 Hz has been shown to increase the evoked torque without increasing the size of the cross-sectional area (CSA) that was activated.11,20 However, muscle fatigue may limit how much a further increase in frequency can further increase torque output.
The effect that current amplitude has on evoked torque and activated muscle CSA has been previously investigated.1,6,8,20,22 Adams et al1 showed that increasing the current amplitude in a manner to increase stimulation from 25% to 75% of maximum voluntary isometric torque (MVIT) increased the percentage of knee extensor muscle group activated from 18% to 54%. We have recently showed that increasing the current amplitude results in a proportional increase in the torque produced and the size of the activated CSA of the stimulated muscle.20 Rate of muscle strength recovery following anterior cruciate ligament injuries has been associated with the stimulation at higher percentage of MVIT.38 However, increasing current amplitude to maximize torque output and to produce clinically meaningful percentage of MVIT was limited by participants' tolerance to the stimulation, and not all healthy participants were able to tolerate such a high level of stimulation.17,27,32 Patient's pain tolerance to electrical stimulation is considered another limitation to the process of maximizing torque output.
Compared to pulse frequency and current amplitude, the role of pulse duration is less appreciated in its possible influence on maximizing torque output. Alon et al3 showed that motor stimulation could be achieved with pulse durations in the range of 20 to 200 microseconds, without stimulation of pain response. In contrast, Hultman et al23 showed that a pulse duration of 500 microseconds resulted in 40% greater torque output compared to 150 microseconds. Moreover, a pulse duration of 450 microseconds has been shown to be effective in conducting electrically induced resistance training in individuals with spinal cord injury.18,31 However, despite this evidence, most researchers have used pulse durations of 300 microseconds or below in their studies,4,16,29,38 which could potentially limit the outcome of NMES protocols in maximizing elicited torque output. The controversy regarding pulse duration selection reflects the limited amount of knowledge regarding the optimal pulse duration required to maximize torque output. Together with a short pulse duration, a long stimulation duration (5−20 seconds) has often been proposed to augment skeletal muscle torque and strength.27,29,30,38 For example, a stimulation duration of 10 seconds has been previously recommended to evoke skeletal muscle strength in individuals with anterior cruciate ligament tear.38 However, the rationale for selecting stimulation duration to maximize torque output is not clear.
Increasing pulse duration from 150 to 450 microseconds has recently been shown to increase motor unit recruitment by measuring the activated CSA of the stimulated muscle.20 Unlike pulse duration, stimulation duration is known to sustain the activation of the stimulated motor units.34 Increasing the length of the stimulation duration (10 seconds or more) could possibly deliver more pulse trains per stimulation and increase the evoked torque.33 At a constant frequency, a 10-second stimulus has more pulse trains than a 3-second stimulus. Additionally, a longer contraction has been shown to be better than a short one to augment skeletal muscle strength and cause hypertrophy.37 This could lead to believe that a long stimulation duration could possibly sustain the evoked torque, hence offset the effect of short pulse duration. Therefore, the primary purpose of the current study was to examine the effect-dependent interrelationship of pulse duration and stimulation duration at constant stimulation intensity on the evoked torque of skeletal muscle in healthy volunteers after controlling for the activated CSA of the muscle being stimulated. A second purpose is to compare the evoked torque developed over a 10-second contraction at the start and at the end of the contraction. A decline in the evoked torque between the start and the end could mean muscle fatigue, which limits the application of NMES to maximize torque output.
METHODS
Participants
SEVEN ABLE-BODIED COLLEGE STUdents (1 woman, 6 men; mean ± SD age, 28 ± 4 years; body mass, 68 ± 9 kg; height, 173 ± 9 cm) participated and completed this study. They all had previous experience with similar NMES protocols to address different research questions.20 All participants were recruited from the University community by word of mouth and had no history of knee or hip pathologies. All participants were right handed and they were asked to refrain from any strenuous activities for the 48 hours prior to their participation in the study. Detailed descriptions for all procedures and risks of participation in this study were given to each potential participant. All subjects gave written informed consent prior to the study. The Institutional Review Board of The University of Georgia approved this study.
Procedures
Each participant underwent 2 separate test sessions, one for familiarization with the testing protocol and the second, performed at the magnetic resonance imaging (MRI) facility, to determine the activated CSA. The 2 sessions were separated by a 1-week period. Both left and right knee extensor muscle groups were involved in the testing procedures. Two protocols (A and B) for NMES were administered: one protocol was randomly assigned to the right knee extensors and the other protocol was administered to the knee extensors of the opposite lower extremity. Four of the participants received protocol A on their right side and the other 3 on their left side, and vice versa for protocol B. This design was applied to avoid the effect of muscle fatigue on 1 muscle group and to save time with the MRI unit. At least 1 hour separated both protocols.
Familiarization Session
Seven days prior to the testing day, each participant performed a familiarization session for 30 to 60 minutes. The participant was asked to perform 3 MVIT efforts with each knee extensor muscle group and to demonstrate tolerance to NMES with each thigh. All participants received 5 minutes of stimulation to each knee extensor muscle group to ensure their eligibility to join the study. There were 3 eligible participants who were excluded because they could not tolerate the assigned protocols.
Determination of MVIT
MVIT of each knee extensor muscle group was determined for each subject. Testing was performed with the subject sitting in a custom-built chair and the subject's lower leg strapped at 60° below horizontal to an immovable wooden lever arm, so that an isometric action could be performed. The lever arm was established by mounting a load cell perpendicular to, and 33 cm away from, the axis of rotation. The dynamometer was calibrated by hanging known weights on the load cell (model RL 2000; Rice Lake Weighing Systems, Rice Lake, WI). Each subject was asked to extend the knee against resistance as fast and forcefully as possible. The effort was maintained for 3 seconds. A 1-minute rest was given between trials. The participant was asked to perform an additional trial if the difference between 2 of the 3 trials was greater than 5%. The load cell, interfaced with a personal computer, was used to measure knee extension torque expressed in Nm. All force data were compensated for gravity.20,21,27,33
Instrumentation
A Theratouch 4.7 electric stimulation unit (Rich-Mar Corporation, Inola, OK) was used to determine the current amplitude required to elicit 45% of the predetermined MVIT of each thigh. Two commercially available 7 × 10-cm self-adhesive electrodes were placed on the skin over each quadriceps muscle. One was placed 2 to 3 cm above the proximal aspect of the patella over the vastus medialis muscle and the other 30 cm proximal to the patella over the vastus lateralis muscle.6,7,18,20 To determine the current amplitude required to reach 45% of the MVIT for both protocols, NMES, using a 100-Hz pulse rate and 450-microsecond pulse duration, was applied to the quad-riceps femoris muscles for 1 second, while the subject was asked to completely relax and the current progressively increased. We started by determining the current amplitude required for the right knee extensors then determined the amplitude for the left knee extensors. After achieving 45% of MVIT, the current amplitudes (mA) for the right and left knee extensor muscle groups were recorded. This percentage of MVIT was chosen because it is comfortable and tolerated by most participants and it has been recommended previously to induce muscle strength.28,38
NMES Protocols
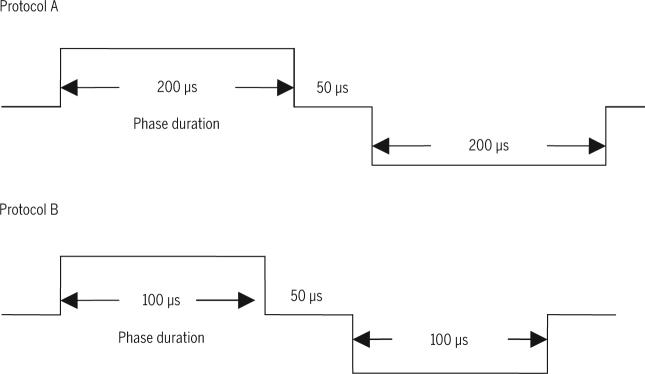
After determining the required current amplitude to achieve 45% of MVIT, a rest of 3 minutes was given to ensure no muscle fatigue before stimulation. Two stimulation protocols were randomly applied. Common to both protocols was the use of symmetric biphasic waveform, current amplitude set at an intensity predetermined to induce a quadriceps contraction at 45% of MVIT, and a session duration of 5 minutes. Contrasting elements between the protocols were that protocol A delivered a 100-Hz pulse rate, 450-microsecond pulse duration, and a stimulation duration lasting 3 seconds, followed by 3-second relaxation; protocol B delivered a 60-Hz pulse rate, a 250-microsecond pulse duration, and a 10-second stimulation duration followed by a 20-second relaxation. FIGURE 1 shows the symmetrical biphasic pulses that were used for each protocol.
FIGURE 1.
Illustration of the durations (450 and 250 microseconds) of the symmetrical biphasic pulse used in protocols A and B. Both pulses have interphase period of 50 microseconds.
Torque Analysis
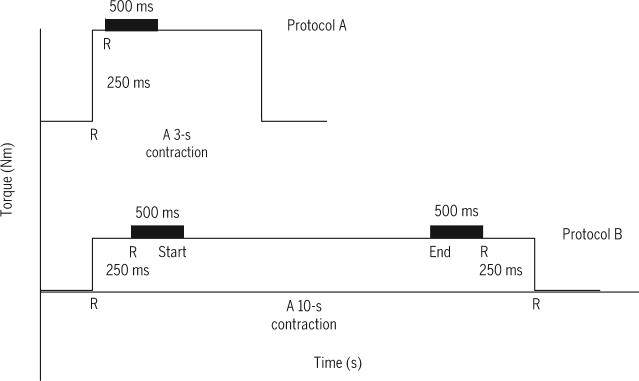
Peak torque and torque time integral (TTI) were determined for the first contraction for both protocols. Peak torque was defined as the maximum torque produced over a 500-millisecond window and covered the plateau phase after a 250-millisecond rise time above baseline. The 500-millisecond window of each corresponding contraction was used to establish peak torque (FIGURE 2).20 For protocol B, two 500-millisecond windows, one beginning 250 milliseconds after torque rose above baseline and the other 750 milliseconds before torque returned to baseline, were used to measure the torque at the start and at the end of a given 10 seconds contraction (FIGURE 2). Additionally, the peak torque for contractions 3, 7, and 10 for protocol B were measured to determine the effect of 10-second stimulus on muscle fatigue. The TTIs over the 500-millisecond window in protocol A and B were calculated. To adjust for the duty cycles between both protocols, the TTI was adjusted relative to the on-time in each protocol, a similar approach was adopted in NMES protocols with different duty cycles.33 The TTI of protocol A was equal to the torque integrated over the 500-millisecond window divided by 150 seconds. The TTI of protocol B was equal to the torque integrated over the 500-millisecond window divided by 100 seconds.
FIGURE 2.
A schematic diagram of the 3- and 10-second stimulation durations used in protocols A and B, respectively. Letter R denotes rise time with interval of 250 milliseconds to standardize the starting point of measuring the evoked torque in both protocols. The evoked torque was the torque maintained over a 500-millisecond window started directly after the rise time. For protocol B, the evoked torque was measured for a 500-millisecond window after the initial 250-millisecond rise time and for a 500-millisecond window ending 250 milliseconds prior to the end of 10-second stimulus.
Magnetic Resonance Imaging (MRI)
Standard spin echo images of the thighs were collected using a 1.5-T superconducting magnet (Signa, General Electric, Milwaukee, WI).6,20,22 After 30 minutes of lying down supine to avoid body fluid shift, subjects were positioned within the magnet using the whole body coil. Transaxial MR images were obtained before NMES, the participant was then moved out of the magnet to a separate room to apply NMES. After the NMES protocol, the subject was asked to hop to the MRI unit without bearing weight on the stimulated lower extremity so as to repeat the imaging within 3 minutes after ending the electrical stimulation. The total time of the scan was around 4 minutes and 40 seconds. The scout view time and subsequent imaging adjustments (mean ± SD, 120 ± 23 seconds) made the total imaging time almost 7 minutes. The transaxial T2 images (TR/TE, 2000/30, 60) were 1 cm thick and 1 cm apart. They had a 40-cm field of view, with a 256 × 256-pixel matrix size, and used 1 number of excitation (NEX). Fourteen to 18 slices for each subject were analyzed for the knee extensors, beginning with the first slice containing the 4 heads of the quad-riceps femoris muscle group and continued distally until the slice just before the proximal pole of the patella. Images were analyzed and T2 values calculated in NIH Image 1.62 (http://rsb.info.nih.gov/nih-image/download.html).
Calculation of Activated Skeletal Muscle
MR images were transferred to a computer for calculation of the activated skeletal muscle CSA (FIGURE 3). A region of interest was defined by tracing the outline of the quadriceps femoris muscle within the subfascial fat. A T2 for each pixel within the region of interest was determined from the native images excluding the fat content within the region on interest. Pixels with a T2 signal intensity between 20 and 35 milliseconds were used to represent quadriceps muscle in the pre-electrical stimulation images. The pattern and extent of stimulation was assessed, as reflected by pixels with an elevated T2 in the postelectrical stimulation images. The mean and SD of the T2of the pixels in each pre-electrical stimulation image were calculated. Pixels in matching postelectrical stimulation images with a T2 greater than the mean plus 1 SD of the T2 of pre-electrically stimulated images were considered elevated or as having an increased T2 signal intensity (FIGURE 3). The CSA of such pixels was determined. Values were averaged over slices to determine the average absolute and relative CSA of skeletal muscle that was stimulated.6,20,22
FIGURE 3.

Representative binary T2 maps of quadriceps femoris muscle from 1 participant before and after the neuromuscular electrical stimulation (NMES) protocols. (A) Pre-NMES T2 map for a single slice of left thigh. (B) T2 map for the same slice after stimulation with protocol A. (C) Pre-NMES T2 map for a single slice of right thigh. (D) T2 map for the same slice after stimulation with protocol B. (E) The activated measured cross-sectional area in each slice was multiplied by slice thickness (1 cm) and interslice space (1 cm) to calculate muscle volume that was activated (volume 1 + volume 2 +....+ volume 14).
The activated volume (cm3) of each axial section was calculated by multiplying the CSA of the section by slice thickness (1 cm) and interslice space (1 cm). The activated volumes of each section and spacing were summed to calculate a volume of the entire knee extensor muscle group along the length of the femur (FIGURE 3E).
Normalized Torque
Knee extensors normalized torque was calculated by dividing the highest torque (Nm) achieved during either protocol by the total activated skeletal muscle volume (cm3); the results were normalized torque in Nm/ cm3. The normalized torque was defined as the torque per unit volume of active motor units.
Data Analysis
Differences in peak torque, TTI, activated CSA, activated volume, and normalized torque between the 2 NMES protocols were tested using paired t tests. Two-way repeated-measures ANOVA was used to test for differences in torque and fatigue between the start and the end of each of the 4 contractions of protocol B (contractions 1, 3, 7, and 10). Statistical significance was set at a level of P<.05.
RESULTS
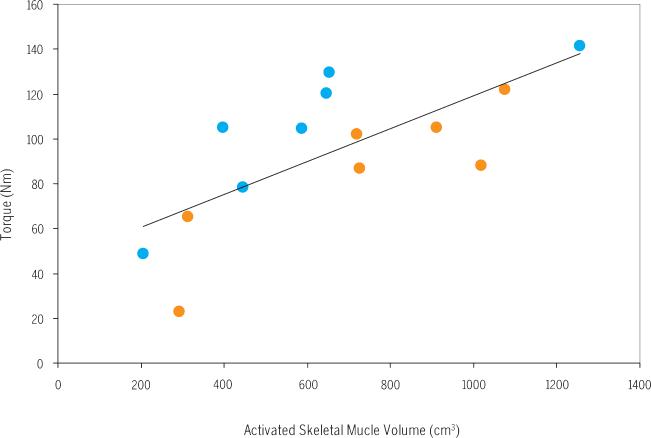
A SUMMARY OF THE RESULTS FOR THE 2 protocols is presented in TABLE 1. The mean ± SD amplitude of the current delivered was not different (P = .2) between protocols A and B (56 ± 13 and 59 ± 15 mA, respectively). The amplitude of the current in both protocols initially produced 45% of MVIT (ie, when they had a 450-microsecond pulse). However, after shortening the pulse duration to 250 microseconds, protocol A resulted in a 22% greater absolute torque output compared to protocol B (P<.05). The CSA of the knee extensors activated was not different between the protocols (P = .7) (FIGURE 3). Thus, normalized torque (Nm/cm3) for protocol A was 38% greater than that of protocol B (P<.05). To offset for the difference in the duty cycles between both protocols, TTI was 21% greater in protocol A compared to protocol B (P = .029) (FIGURE 4). However, after adjusting for the on-time, the difference in TTI disappeared (P = .3), reflecting the difference in the on-time between both protocols (TABLE 1). Simple linear regression analysis showed positive increase between the activated skeletal muscle volume and torque output after both protocols (r2= 0.51, P = .004) (FIGURE 5).
TABLE 1.
Torque and Muscle Activation (Mean ± SD) Produced With Protocols A and B
| NMES Protocols | ||
|---|---|---|
| A | B | |
| MVIT (Nm) | 239 ± 60 | 241 ± 66 |
| Torque (Nm) | 109 ± 57* | 85 ± 53 |
| Activated CSA (cm2) | 22 ± 11 | 25 ± 10 |
| Activated muscle volume (cm3) | 599 ± 330 | 722 ± 316 |
| Normalized torque (Nm/cm3) | 0.21 ± 0.07* | 0.12 ± 0.04 |
| Adjusted TTI (Nm·s/s) | 0.39 ± 0.1 | 0.42 ± 0.1 |
Abbreviations: CSA, cross-sectional area; MVIT, maximum voluntary isometric torque; NMES, neuromuscular electrical stimulation; TTI, torque time integral.
Significant difference between protocols (P<.05).
FIGURE 4.
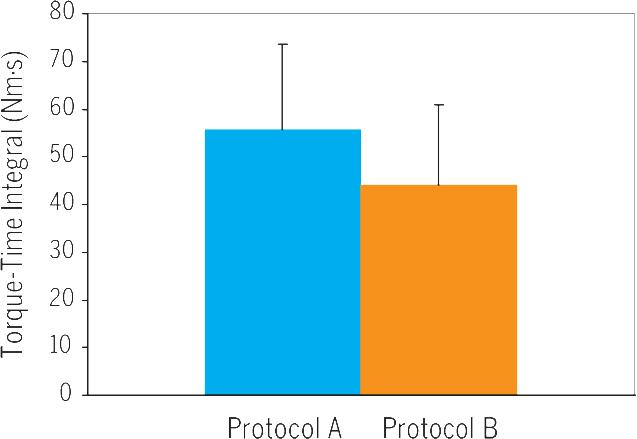
Torque time integral (mean ± SD) of the first contraction in both protocols (A versus B). Significant differences between both protocols (P = .029).
FIGURE 5.
Peak torque (Nm) versus activated skeletal muscle volume (cm3). The peak torque values of both protocols (A, blue circles; B, orange circles) were plotted against the corresponding activated skeletal muscle volume. The peak torque produced is equal to 0.08 times the activated volume plus 45 [Torque = (0.08 × activated volume) + 45] (r2 = 0.51, P = .004).
A summary of the torque values at the start and the end of 4 contractions with protocol B is presented in TABLE 2. Protocol B fails to show maintenance of torque at the start and the end of the 10-second contractions over the 5 minutes. At contraction 10, torque decreased (P = .0001) compared to the torque of the first contraction by a mean ± SD of 31% ± 8% (start) and 50% ± 11% (end). The decline in torque was different between the start and the end of protocol B (P.05). Over a 10-second contraction, the decline in torque was not different between the start and end of the evoked torque for contractions 1, 3, and 7 (P = .3). For contraction 10, there was a decline in torque by 24% at the end compared to the start (P = .06).
TABLE 2.
Torque (Mean ± SD) Produced at the Start and the End of Protocol B*
| Protocol B | ||
|---|---|---|
| Contraction | Starting Torque (Nm) | Ending Torque (Nm) |
| 1 | 85 ± 33 | 89 ± 36 |
| 3 | 71 ± 26 | 67 ± 25 |
| 7 | 63 ± 23 | 55 ± 25 |
| 10 | 57 ± 24 | 47 ± 24 |
The decline in “starting” and “ending” torque between the first and tenth contractions was 31% and 50%, respectively. The tenth contraction showed a decline in torque by 24% from the beginning to the end of the contraction.
DISCUSSION
THE ROLE OF PULSE DURATION IN maximizing torque output has been overlooked in previous research trials when compared to the attention given to the frequency or the amplitude characteristics of the NMES.9,16,17,35,38 In fact, a short pulse duration combined with a long stimulation duration has been proposed to achieve skeletal muscle strength.4,29,30,38 This would suggest that a long stimulation duration could offset the effect of a short pulse duration, by possibly adding more pulses during the stimulation period, hence sustain the evoked torque output during NMES. To test this hypothesis, 2 protocols with different pulse durations (250 versus 450 microseconds) and stimulation durations (10 versus 3 seconds) were used to examine if a long stimulation duration could maximize the evoked torque relative to the activated muscle volume. The major findings of the current study did not support this hypothesis and showed that increasing pulse duration but not stimulation duration is responsible for maximizing torque output.
One of the major limitations to the application of NMES is the lack of evidence-based knowledge on how to maximize elicited torque output.2 Maximizing the torque output generated by electrical stimulation is critical, especially when the goals are to achieve muscle strengthening and hypertrophy as an outcome of treatment.4,16,18,30,38 Various clinical trials have shown that increasing the frequency and the amplitude of the current are limited by the rapid onset of muscle fatigue and the subject's tolerance to the delivered current, respectively.8,15,25,32 This would sugest that both parameters can not always be used to maximize torque output. Recently, we have shown that increasing the pulse duration from 150 to 450 microseconds has resulted in increasing the peak torque generated by the electrical stimulus by 55%, independent of the current amplitude and frequency.20 Our current findings reaffirm previous findings about the role of pulse duration in augmenting the evoked torque. Hultman et al23 showed that increasing pulse duration from 150 to 500 microseconds resulted in increasing the evoked torque by 40%. Despite these findings, there are several research and clinical trials that are still using pulse durations of 100 or 250 microseconds and a stimulation duration of 10 seconds or longer to achieve strength training.27,29,38
The physiological mechanisms underlying the effect of pulse duration in maximizing torque need to be examined. A progressive increase from short to long pulse durations can result in more motor units being recruited; this may lead to steep rise in the evoked torque.13,25 This was just confirmed when increasing pulse duration from 150 to 450 microseconds resulted in increasing the activated muscle CSA by 40% as determined by MRI.20 The activated area represents the motor units recruitment during the process of stimulation.1,6,20,22 Conversely, further increase in pulse duration above 500 microseconds results in less motor units being recruited and torque reaches a plateau.25 However, others have shown that long pulse durations greater than 500 to 1000 microseconds may result in increasing torque output via antidromic stimulation of the alpha motor neurons and contribution of the central nervous system.14 In the current study, long pulse duration with a short stimulation duration evoked greater normalized torque compared to a short one. This could demonstrate the ability of long pulse duration to recruit additional motor units. However, we could not confirm this observation by measuring the activated area using MRI, which showed no difference between protocols A and B. A possible mechanism is that the long pulse duration has recruited large motor units or result in nonlinear summation of motor units compared to the short one13,40; this results in 20% increase in the evoked torque during protocol A compared to B. Another alternative explanation is that the product of the frequency and pulse duration, which has been recently suggested to strongly explain the variance in torque output, could have played a role in maximizing torque output in protocol A.21
The waveform used in the current study is symmetrical biphasic in nature. A symmetrical biphasic pulse is equal to the sum of 2 phases (FIGURE 2). A symmetrical biphasic waveform was found preferable over a monophasic or asymmetrical biphasic waves during stimulation of the knee extensor muscle.24,34 Kantor et al24 explained this observation by showing that symmetrical biphasic form has a low total pulse charge. Therefore, it is important to use a biphasic form to minimize the charges delivered and this might help reduce the pain clinically reported with long pulse durations. Moreover, increasing pulse duration tends to be less painful compared to increasing the current amplitude, because less electrical charges develop under the electrodes.20 It is known that the strength of a single pulse is the product of the amplitude and the duration of each pulse. Alon et al3 calculated the difference in the amplitude between motor and sensory thresholds after changing the length of pulse duration at logarithmic intervals from 5 to 1000 microseconds. The findings showed that as the pulse duration becomes longer, the absolute difference in the intensity required to cause motor stimulation decreases.3 The reduction in the amplitude is associated with insufficient charges to stimulate the pain fibers at longer pulse duration.
A number of factors complicate the appropriate selection of pulse duration in clinical settings. The difference between phase duration and pulse duration continues to confuse both clinical and research inquiries. Because of erroneous and inconsistent use of these 2 terms, it is impossible to reach meaningful consensus regarding the importance of these variables. For example, Alon et al3 used monophasic pulse where phase duration and pulse duration are the same and can be termed either phase or pulse duration. Laufer et al27 used symmetric biphasic pulse with a phase duration of 200- and 50-microsecond intrapulse interval, making the pulse duration 450 microseconds. In the current study, pulse durations of 450 and 250 microseconds were used; but the phase durations were 200 and 100 microseconds, respectively. Therefore, it is erroneous to report that the pulse duration was 200 microseconds. Moreover, it is well established that if the stimulator is designed as a constant voltage, there is a discrepancy between the reported and the actual values of pulse duration.24,34 The effective phase and pulse duration of the current waveform are shorter than the phase and pulse duration of the voltage waveform, due to the exponential decline associated with the capacitative properties of the biological conductive medium.24 Therefore, future trials need to consider the difference between phase and pulse durations as well as the type of stimulators.
The rationale for using long stimulation duration in different NMES protocols is not clearly justified. The length of the stimulation duration contributes to the overall training by maintaining the activation of the stimulated motor units.34 For example, it has been suggested that long contraction evokes skeletal muscle strength and hypertrophy after 14 weeks of voluntary isometric training.37 Additionally, the authors37 showed a drop in the pH and phosphocreatine and greater rise in inorganic phosphate with long voluntary contraction time. These metabolic changes may definitely cause muscle fatigue.37 Moreover, a long contraction time could interrupt circulation and cause ischemic muscle fatigue.5 After stimulation, a 3-second stimulation caused more fatigue than 1.6 seconds during NMES.5 Owing to the fact that minimizing muscle fatigue may be desired consequence of applying NMES, the use of long contraction time may accentuate such process. The major objective of the current study was to compare the outcomes of 2 protocols with different lengths of pulse durations and stimulation durations. A simple debate is that no relation exists between pulse duration and stimulation duration, and short pulse durations have been previously used because they are comfortable at high current amplitude.3 Another explanation is that using short pulse duration may have had different clinical goals (ie, muscle re-education or submaximal training). Therefore, those parameters may still have valid rationales for use in clinical practice. For example, short pulse duration is required to selectively stimulate sensory, motor, and pain fibers.3
The current study used MRI to account for the activated area. T2-weighted MRI has been previously used to calculate the amount of activated muscle during NMES.1,6,20,22 The increase in signal intensity after stimulation can be used to delineate between activated and nonactivated muscle areas. Previously, we have shown that altering NMES parameters affect the activated area.20 Therefore, the same current amplitude was used in both protocols to maintain a constant activation. A recent study showed that using similar amplitudes of the current could match the activated areas between left and right knee extensors.11 However, it was surprising that protocol B resulted in more activation compared to protocol A. It is possible that the 10-second stimulation was not comfortable to our participants and caused them to become tense during the stimulation process.5
There are few important features that need to be considered pertaining to the selection of the parameters in both protocols. Both protocols were set to deliver matched absolute amplitude that could generate 45% of MVIT and had a total duration of 5 minutes. Additionally, a stimulus generating 45% of MVIT is known to be well tolerated by most participants and is recommended for increasing muscle strength.28 However, both protocols were different in the frequency (100 versus 60 Hz), pulse duration (450 versus 150 microseconds), and stimulation duration (3 versus 10 seconds). This could possibly limit the interpretation of the current study and challenge the validity of comparing the protocols. We originally set both protocols to deliver 100 Hz to evoke maximum tension relative to the activated area.20 However, the combination of 100 Hz and 10 seconds was painful to most of the participants. Therefore, the 100 Hz was modified to 60 Hz. Even with such modifications, 3 participants were excluded for failing to tolerate the assigned protocols. However, it is well established that both frequencies (60 and 100 Hz) are at the plateau phase of the force-frequency curve, so that any difference in the evoked torque, based on the frequency, should not exceed 5%.9,12,36 Therefore, a difference in the evoked torque of more than 20% can not be solely explained by variations in the frequency between both protocols. This would suggest that the pulse duration is responsible for the difference in the evoked torque between both protocols. Additionally, to account for the difference in stimulation duration between both protocols, the TTIs of the initial contraction were used, so that any difference in the evoked torque would be attributed to the change in pulse duration.
Major limitations to the applications of NMES are pain and fatigue.6,7,10,17,27 As the amplitude of the current continues to increase, painful stimulus increases and participants do not tolerate further increase due to stimulation of pain free nerve endings. Fatigue is a common neuromuscular adaptation to electrical stimulation. Fatigue during NMES arises as a result of repeated activation of the same motor units.1,8,26 If increasing the pulse duration serves to increase the recruitment of the motor units,20 then this could serve to spread the work over many motor units and attenuate fatigue. However, this assumption needs to be further investigated. In previous studies, a 10-second stimulation duration has been recommended to induce muscle strength without occurrence of fatigue.38 Lyons et al29 found that a protocol similar to protocol B maintained torque during 10-second contraction. The longer contraction time in protocol B required to measure torque at the start and at the end of the contraction to determine the changes in torque over a 10-second contraction. Contrary to previous findings, a 10-second stimulation fails to maintain performance over repeated contractions and fatigue ensued. The difference between findings could possibly be explained by the use of battery power in the previous study and an AC-powered stimulator in the current study.
CONCLUSION
ALONGER STIMULATION DURATION fails to offset the effect of short pulse duration and could not maintain performance over repeated contractions. Longer pulse duration evokes greater peak torque and normalized torque compared to short pulse. Therefore, clinicians are encouraged to reconsider the use of pulse duration and stimulation duration when they are designing protocols to augment muscle strength. Further studies are warranted to determine the effectiveness of different parameters of NMES in clinical settings and on training programs as well as the effects of pulse durations and stimulation durations without confounding effects from the frequency. T
KEY POINTS
FINDINGS
With NMES, long pulse duration evokes greater peak torque and normalized torque compared to short pulse duration with long stimulation duration.
IMPLICATION
Long pulse duration is recommended to increase muscle strength or cause hypertrophy, because it results in a greater peak torque, normalized torque, as well as torque-time integral.
CAUTON
The study examined healthy individuals with no pathologies. Therefore, direct extrapolation to specific clinical population is not feasible.
ACKNOWLEDGEMENTS
The authors would like to thank all the participants who volunteered in the study. We would like to thank Shepherd Center for giving us the opportunity to use their MRI facility to conduct the current study.
This study was supported by NIH grants to GAD (HD39679 and HD39676S2). The Institutional Review Board of The University of Georgia approved the current study.
REFERENCES
- 1.Adams GR, Harris RT, Woodard D, Dudley GA. Mapping of electrical muscle stimulation using MRI. J Appl Physiol. 1993;74:532–537. doi: 10.1152/jappl.1993.74.2.532. [DOI] [PubMed] [Google Scholar]
- 2.Alon G. Use of neuromuscular electrical stimulation in neureorehabilitation: a challenge to all. J Rehabil Res Dev. 2003;40:ix–xii. doi: 10.1682/jrrd.2003.11.0009. [DOI] [PubMed] [Google Scholar]
- 3.Alon G, Allin J, Inbar GF. Optimizing of pulse duration and pulse charge during transcutaneous electrical nerve stimulation. Aust J Physiother. 1983;29:196–210. doi: 10.1016/S0004-9514(14)60670-X. [DOI] [PubMed] [Google Scholar]
- 4.Bax L, Staes F, Verhagen A. Does neuromuscular electrical stimulation strengthen the quadriceps femoris? A systematic review of randomised controlled trials. Sports Med. 2005;35:191–212. doi: 10.2165/00007256-200535030-00002. [DOI] [PubMed] [Google Scholar]
- 5.Bergstrom M, Hultman E. Energy cost and fatigue during intermittent electrical stimulation of human skeletal muscle. J Appl Physiol. 1988;65:1500–1505. doi: 10.1152/jappl.1988.65.4.1500. [DOI] [PubMed] [Google Scholar]
- 6.Bickel CS, Slade JM, Dudley GA. Long-term spinal cord injury increases susceptibility to isometric contraction-induced muscle injury. Eur J Appl Physiol. 2004;91:308–313. doi: 10.1007/s00421-003-0973-5. http://dx.doi.org/10.1007/s00421-003-0973-5. [DOI] [PubMed]
- 7.Bickel CS, Slade JM, Warren GL, Dudley GA. Fatigability and variable-frequency train stimulation of human skeletal muscles. Phys Ther. 2003;83:366–373. [PubMed] [Google Scholar]
- 8.Binder-Macleod SA, Halden EE, Jungles KA. Effects of stimulation intensity on the physiological responses of human motor units. Med Sci Sports Exerc. 1995;27:556–565. [PubMed] [Google Scholar]
- 9.Binder-Macleod SA, McDermond LR. Changes in the force-frequency relationship of the human quadriceps femoris muscle following electrically and voluntarily induced fatigue. Phys Ther. 1992;72:95–104. doi: 10.1093/ptj/72.2.95. [DOI] [PubMed] [Google Scholar]
- 10.Binder-Macleod SA, Snyder-Mackler L. Muscle fatigue: clinical implications for fatigue assessment and neuromuscular electrical stimulation. Phys Ther. 1993;73:902–910. doi: 10.1093/ptj/73.12.902. [DOI] [PubMed] [Google Scholar]
- 11.Black CD, Elder CP, Gorgey A, Dudley GA. High specific torque is related to lengthening contraction-induced skeletal muscle injury. J Appl Physiol. 2008;104:639–647. doi: 10.1152/japplphysiol.00322.2007. http://dx.doi.org/10.1152/japplphysiol.00322.2007. [DOI] [PubMed]
- 12.Celichowski J. Mechanisms underlying the regulation of motor unit contraction in the skeletal muscle. J Physiol Pharmacol. 2000;51:17–33. [PubMed] [Google Scholar]
- 13.Chou LW, Binder-Macleod SA. The effects of stimulation frequency and fatigue on the force-intensity relationship for human skeletal muscle. Clin Neurophysiol. 2007;118:1387–1396. doi: 10.1016/j.clinph.2007.02.028. http://dx.doi.org/10.1016/j.clinph.2007.02.028. [DOI] [PMC free article] [PubMed]
- 14.Collins DF, Burke D, Gandevia SC. Sustained contractions produced by plateau-like behaviour in human motoneurones. J Physiol. 2002;538:289–301. doi: 10.1113/jphysiol.2001.012825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deley G, Kervio G, Verges B, et al. Comparison of low-frequency electrical myostimulation and conventional aerobic exercise training in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2005;12:226–233. doi: 10.1097/01.hjr.0000166455.23346.a5. [DOI] [PubMed] [Google Scholar]
- 16.Delitto A, Brown M, Strube MJ, Rose SJ, Lehman RC. Electrical stimulation of quadriceps femoris in an elite weight lifter: a single subject experiment. Int J Sports Med. 1989;10:187–191. doi: 10.1055/s-2007-1024898. [DOI] [PubMed] [Google Scholar]
- 17.Delitto A, Strube MJ, Shulman AD, Minor SD. A study of discomfort with electrical stimulation. Phys Ther. 1992;72:410–421. doi: 10.1093/ptj/72.6.410. discussion on 421−414. [DOI] [PubMed] [Google Scholar]
- 18.Dudley GA, Castro MJ, Rogers S, Apple DF., Jr. A simple means of increasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol Occup Physiol. 1999;80:394–396. doi: 10.1007/s004210050609. [DOI] [PubMed] [Google Scholar]
- 19.Fukunaga T, Miyatani M, Tachi M, Kouzaki M, Kawakami Y, Kanehisa H. Muscle volume is a major determinant of joint torque in humans. Acta Physiol Scand. 2001;172:249–255. doi: 10.1046/j.1365-201x.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- 20.Gorgey AS, Mahoney E, Kendall T, Dudley GA. Effects of neuromuscular electrical stimulation parameters on specific tension. Eur J Appl Physiol. 2006;97:737–744. doi: 10.1007/s00421-006-0232-7. http://dx.doi. org/10.1007/s00421-006-0232-7. [DOI] [PubMed]
- 21.Gregory CM, Dixon W, Bickel CS. Impact of varying pulse frequency and duration on muscle torque production and fatigue. Muscle Nerve. 2007;35:504–509. doi: 10.1002/mus.20710. http://dx.doi.org/10.1002/ mus.20710. [DOI] [PubMed]
- 22.Hillegass EA, Dudley GA. Surface electrical stimulation of skeletal muscle after spinal cord injury. Spinal Cord. 1999;37:251–257. doi: 10.1038/sj.sc.3100792. [DOI] [PubMed] [Google Scholar]
- 23.Hultman E, Sjoholm H, Jaderholm-Ek I, Krynicki J. Evaluation of methods for electrical stimulation of human skeletal muscle in situ. Pflugers Arch. 1983;398:139–141. doi: 10.1007/BF00581062. [DOI] [PubMed] [Google Scholar]
- 24.Kantor G, Alon G, Ho HS. The effects of selected stimulus waveforms on pulse and phase characteristics at sensory and motor thresholds. Phys Ther. 1994;74:951–962. doi: 10.1093/ptj/74.10.951. [DOI] [PubMed] [Google Scholar]
- 25.Kesar T, Chou LW, Binder-Macleod SA. Effects of stimulation frequency versus pulse duration modulation on muscle fatigue. J Electromyogr Kinesiol. 2007 doi: 10.1016/j.jelekin.2007.01.001. http://dx.doi.org/10.1016/j.jelekin.2007.01.001. [DOI] [PMC free article] [PubMed]
- 26.Knaflitz M, Merletti R, De Luca CJ. Inference of motor unit recruitment order in voluntary and electrically elicited contractions. J Appl Physiol. 1990;68:1657–1667. doi: 10.1152/jappl.1990.68.4.1657. [DOI] [PubMed] [Google Scholar]
- 27.Laufer Y, Ries JD, Leininger PM, Alon G. Quad-riceps femoris muscle torques and fatigue generated by neuromuscular electrical stimulation with three different waveforms. Phys Ther. 2001;81:1307–1316. [PubMed] [Google Scholar]
- 28.Lieber RL, Kelly MJ. Torque history of electrically stimulated human quadriceps: implications for stimulation therapy. J Orthop Res. 1993;11:131–141. doi: 10.1002/jor.1100110115. http://dx.doi.org/10.1002/jor.1100110115. [DOI] [PubMed]
- 29.Lyons CL, Robb JB, Irrgang JJ, Fitzgerald GK. Differences in quadriceps femoris muscle torque when using a clinical electrical stimulator versus a portable electrical stimulator. Phys Ther. 2005;85:44–51. [PubMed] [Google Scholar]
- 30.Maffiuletti NA, Cometti G, Amiridis IG, Martin A, Pousson M, Chatard JC. The effects of electro-myostimulation training and basketball practice on muscle strength and jumping ability. Int J Sports Med. 2000;21:437–443. doi: 10.1055/s-2000-3837. [DOI] [PubMed] [Google Scholar]
- 31.Mahoney ET, Bickel CS, Elder C, et al. Changes in skeletal muscle size and glucose tolerance with electrically stimulated resistance training in subjects with chronic spinal cord injury. Arch Phys Med Rehabil. 2005;86:1502–1504. doi: 10.1016/j.apmr.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 32.McLoda TA, Carmack JA. Optimal burst duration during a facilitated quadriceps femoris contraction. J Athl Train. 2000;35:145–150. [PMC free article] [PubMed] [Google Scholar]
- 33.Miller M, Downham D, Lexell J. Superimposed single impulse and pulse train electrical stimulation: a quantitative assessment during submaximal isometric knee extension in young, healthy men. Muscle Nerve. 1999;22:1038–1046. doi: 10.1002/(sici)1097-4598(199908)22:8<1038::aid-mus5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 34.Robinson AJ. Basic concepts in electricity and contemporary terminology in electrotherapy. In: Robinson AJ, Snyder-Mackler L, editors. Clinical Electrophysiology. Williams & Wilkins; Philadelphia, PA: 2003. pp. 1–26. [Google Scholar]
- 35.Russ DW, Vandenborne K, Walter GA, Elliott M, Binder-Macleod SA. Effects of muscle activation on fatigue and metabolism in human skeletal muscle. J Appl Physiol. 2002;92:1978–1986. doi: 10.1152/japplphysiol.00483.2001. http://dx.doi.org/10.1152/japplphysiol.00483.2001. [DOI] [PubMed]
- 36.Sale D, Quinlan J, Marsh E, McComas AJ, Belanger AY. Influence of joint position on ankle plantarflexion in humans. J Appl Physiol. 1982;52:1636–1642. doi: 10.1152/jappl.1982.52.6.1636. [DOI] [PubMed] [Google Scholar]
- 37.Schott J, McCully K, Rutherford OM. The role of metabolites in strength training. II. Short versus long isometric contractions. Eur J Appl Physiol Occup Physiol. 1995;71:337–341. doi: 10.1007/BF00240414. [DOI] [PubMed] [Google Scholar]
- 38.Snyder-Mackler L, Delitto A, Stralka SW, Bailey SL. Use of electrical stimulation to enhance recovery of quadriceps femoris muscle force production in patients following anterior cruciate ligament reconstruction. Phys Ther. 1994;74:901–907. doi: 10.1093/ptj/74.10.901. [DOI] [PubMed] [Google Scholar]
- 39.Stackhouse SK, Binder-Macleod SA, Stackhouse CA, McCarthy JJ, Prosser LA, Lee SC. Neuro-muscular electrical stimulation versus volitional isometric strength training in children with spastic diplegic cerebral palsy: a preliminary study. Neurorehabil Neural Repair. 2007;21:475–485. doi: 10.1177/1545968306298932. http://dx.doi.org/10.1177/1545968306298932. [DOI] [PMC free article] [PubMed]
- 40.Troiani D, Filippi GM, Bassi FA. Nonlinear tension summation of different combinations of motor units in the anesthetized cat peroneus longus muscle. J Neurophysiol. 1999;81:771–780. doi: 10.1152/jn.1999.81.2.771. [DOI] [PubMed] [Google Scholar]