Abstract
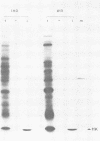
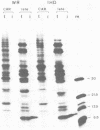
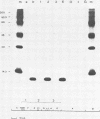
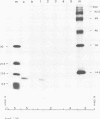
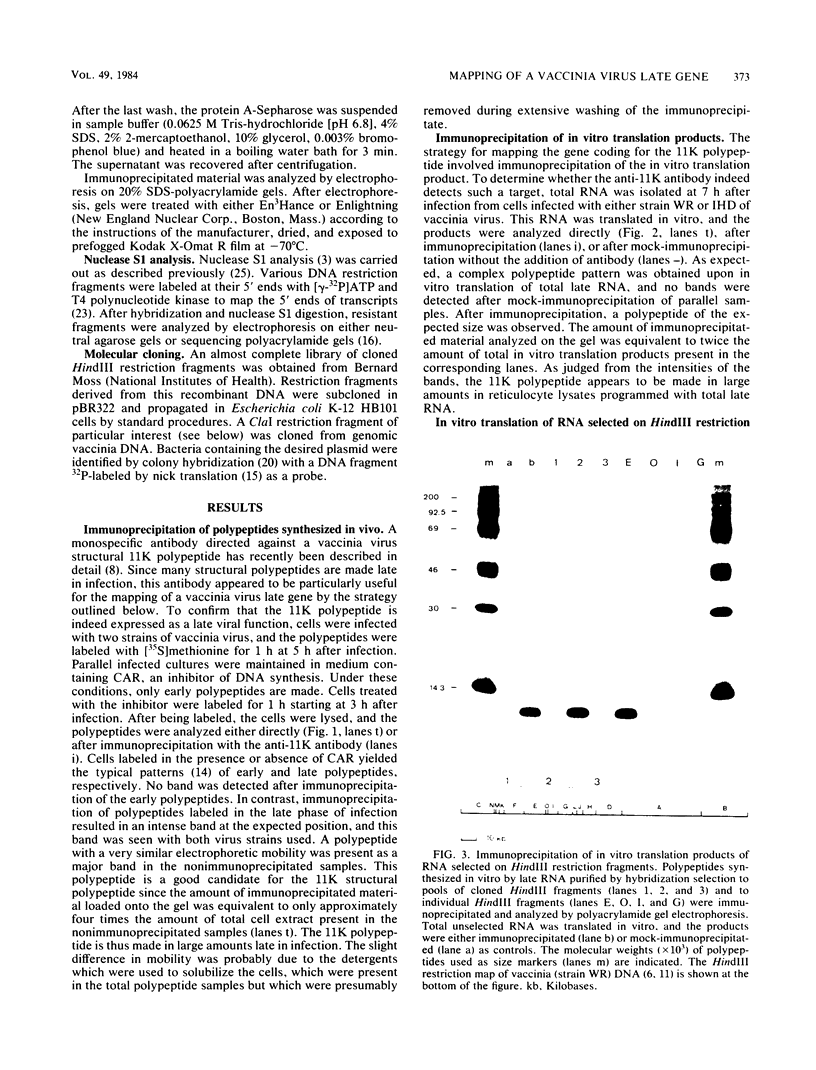
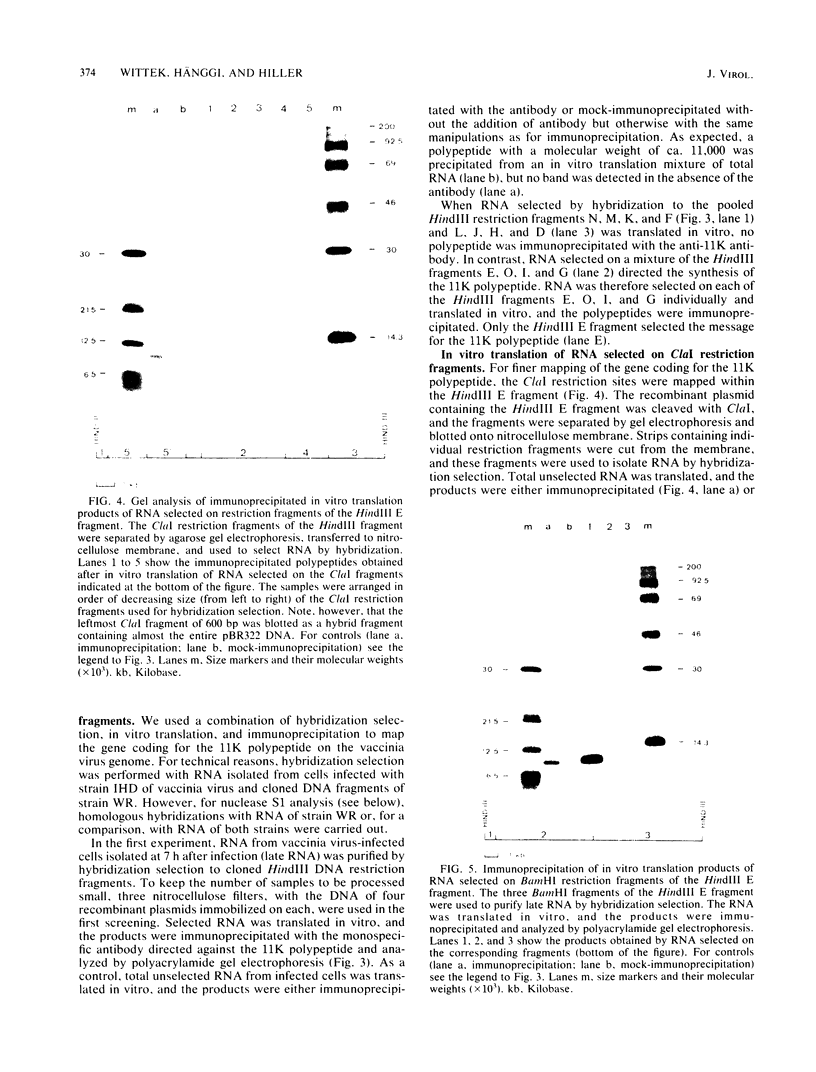
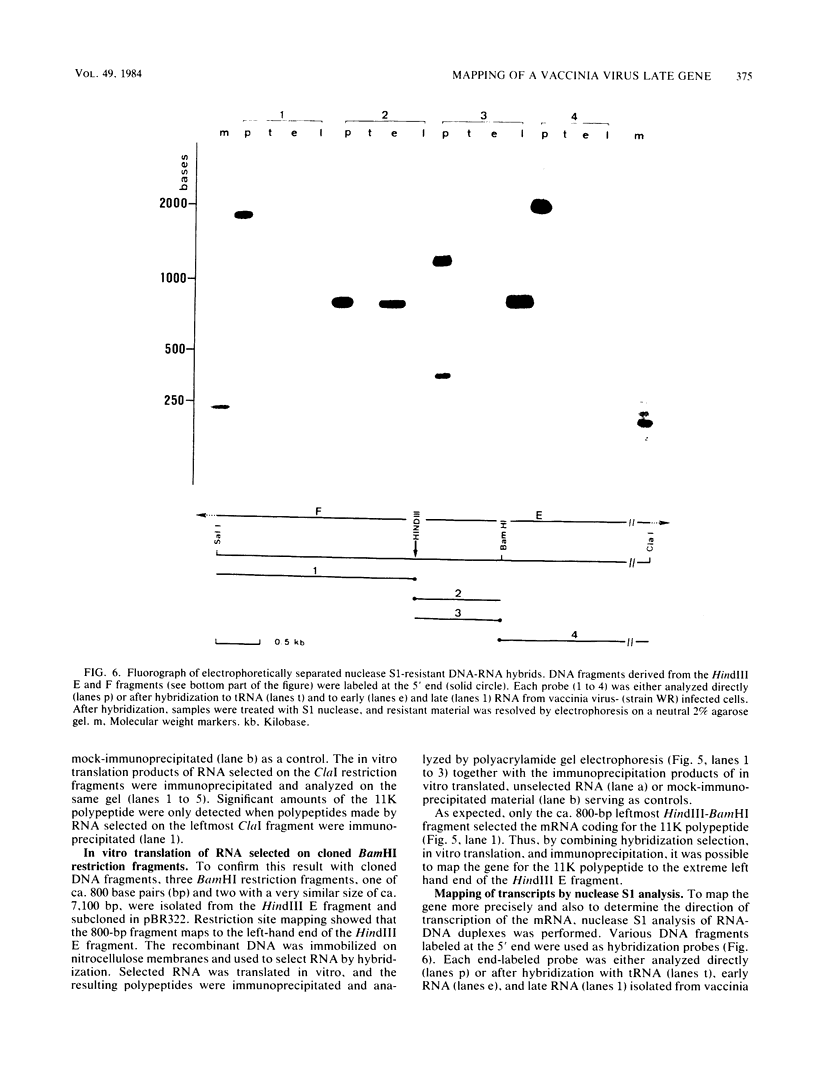
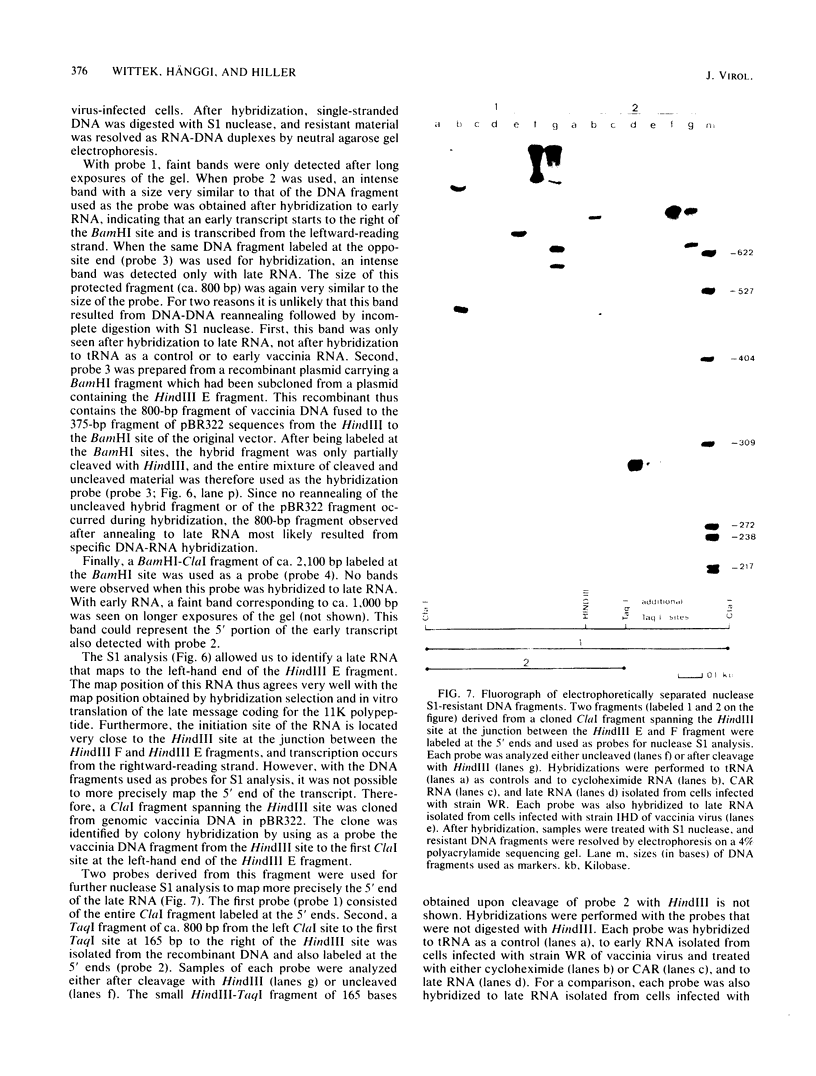
Cell-free translation of total RNA isolated from vaccinia virus-infected cells late in infection results in a complex mixture of polypeptides. A monospecific antibody directed against one of the major structural proteins of the virus particle immunoprecipitated a single polypeptide with a molecular weight of 11,000 (11K) from this mixture. Immunoprecipitation was therefore used to identify the structural polypeptide among the in vitro translation products of RNA purified by hybridization selection to restriction fragments of the vaccinia virus genome. This allowed us to map the mRNA coding for the 11K polypeptide to the extreme left-hand end of the HindIII E fragment. Detailed transcriptional mapping of this region of the genome by nuclease S1 analysis revealed the presence of a late RNA transcribed from the rightward-reading strand. Its 5' end mapped at ca. 130 base pairs to the left of the HindIII site at the junction between the HindIII F and E fragments. The map position of this RNA coincided precisely with the map position of the late message coding for the 11K polypeptide.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Boone R. F., Moss B. Sequence complexity and relative abundance of vaccinia virus mRNA's synthesized in vivo and in vitro. J Virol. 1978 Jun;26(3):554–569. doi: 10.1128/jvi.26.3.554-569.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Wittek R., Moss B. Hybridization selection and cell-free translation of mRNA's encoded within the inverted terminal repetition of the vaccinia virus genome. J Virol. 1981 Jan;37(1):284–294. doi: 10.1128/jvi.37.1.284-294.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFilippes F. M. Restriction enzyme mapping of vaccinia virus DNA. J Virol. 1982 Jul;43(1):136–149. doi: 10.1128/jvi.43.1.136-149.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Hiller G., Weber K. A phosphorylated basic vaccinia virion polypeptide of molecular weight 11,000 is exposed on the surface of mature particles and interacts with actin-containing cytoskeletal elements. J Virol. 1982 Nov;44(2):647–657. doi: 10.1128/jvi.44.2.647-657.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isle H. B., Venkatesan S., Moss B. Cell-free translation of early and late mRNAs selected by hybridization to cloned DNA fragments derived from the left 14 million to 72 million daltons of the vaccinia virus genome. Virology. 1981 Jul 15;112(1):306–317. doi: 10.1016/0042-6822(81)90636-x. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Mackett M., Archard L. C. Conservation and variation in Orthopoxvirus genome structure. J Gen Virol. 1979 Dec;45(3):683–701. doi: 10.1099/0022-1317-45-3-683. [DOI] [PubMed] [Google Scholar]
- Moss B. Inhibition of HeLa cell protein synthesis by the vaccinia virion. J Virol. 1968 Oct;2(10):1028–1037. doi: 10.1128/jvi.2.10.1028-1037.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington T. H. Vaccinia virus polypeptide synthesis: sequential appearance and stability of pre- and post-replicative polypeptides. J Gen Virol. 1974 Dec;25(3):433–444. doi: 10.1099/0022-1317-25-3-433. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sarov I., Joklik W. K. Studies on the nature and location of the capsid polypeptides of vaccinia virions. Virology. 1972 Nov;50(2):579–592. doi: 10.1016/0042-6822(72)90409-6. [DOI] [PubMed] [Google Scholar]
- Schümperli D., Menna A., Schwendimann F., Wittek R., Wyler R. Symmetrical arrangement of the heterologous regions of rabbit poxvirus and vaccinia virus DNA. J Gen Virol. 1980 Apr;47(2):385–398. doi: 10.1099/0022-1317-47-2-385. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thayer R. E. An improved method for detecting foreign DNA in plasmids of Escherichia coli. Anal Biochem. 1979 Sep 15;98(1):60–63. doi: 10.1016/0003-2697(79)90705-x. [DOI] [PubMed] [Google Scholar]
- Venkatesan S., Baroudy B. M., Moss B. Distinctive nucleotide sequences adjacent to multiple initiation and termination sites of an early vaccinia virus gene. Cell. 1981 Sep;25(3):805–813. doi: 10.1016/0092-8674(81)90188-4. [DOI] [PubMed] [Google Scholar]
- Venkatesan S., Gershowitz A., Moss B. Complete nucleotide sequences of two adjacent early vaccinia virus genes located within the inverted terminal repetition. J Virol. 1982 Nov;44(2):637–646. doi: 10.1128/jvi.44.2.637-646.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. P., Moss B. Nucleotide sequence of the vaccinia virus thymidine kinase gene and the nature of spontaneous frameshift mutations. J Virol. 1983 May;46(2):530–537. doi: 10.1128/jvi.46.2.530-537.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Cooper J. A., Barbosa E., Moss B. Expression of the vaccinia virus genome: analysis and mapping of mRNAs encoded within the inverted terminal repetition. Cell. 1980 Sep;21(2):487–493. doi: 10.1016/0092-8674(80)90485-7. [DOI] [PubMed] [Google Scholar]
- Wittek R. Organization and expression of the poxvirus genome. Experientia. 1982 Mar 15;38(3):285–297. doi: 10.1007/BF01949349. [DOI] [PubMed] [Google Scholar]