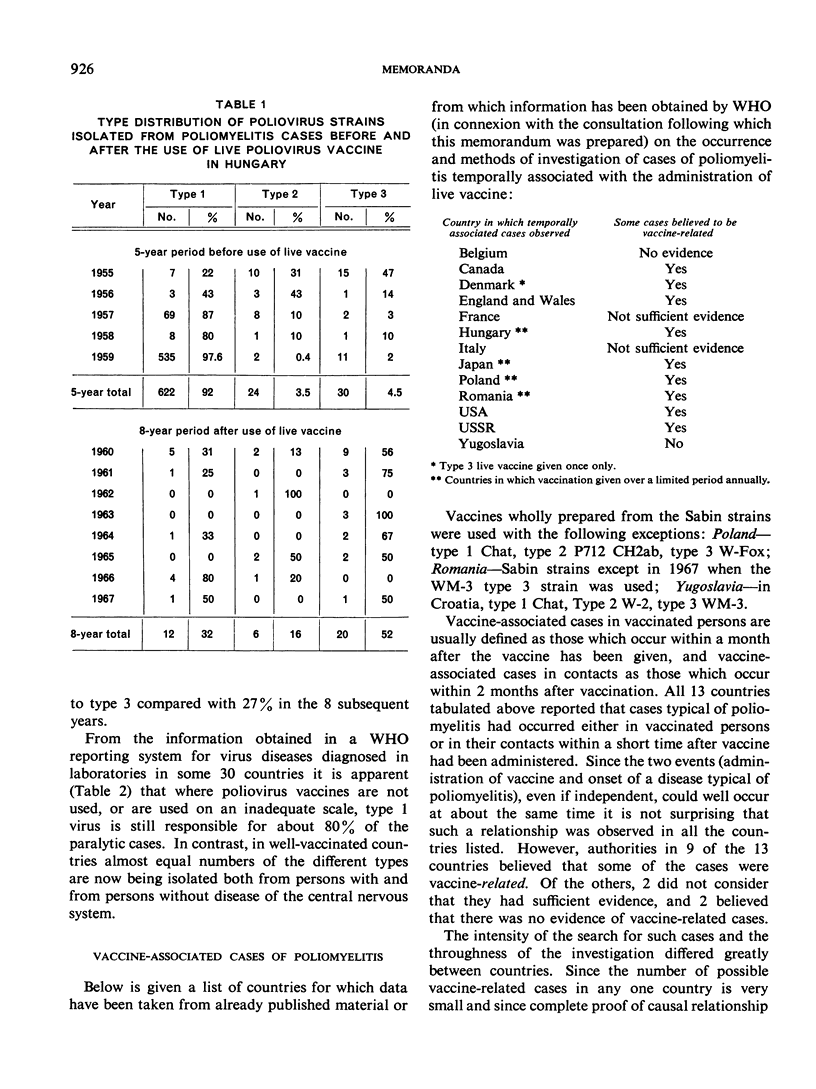
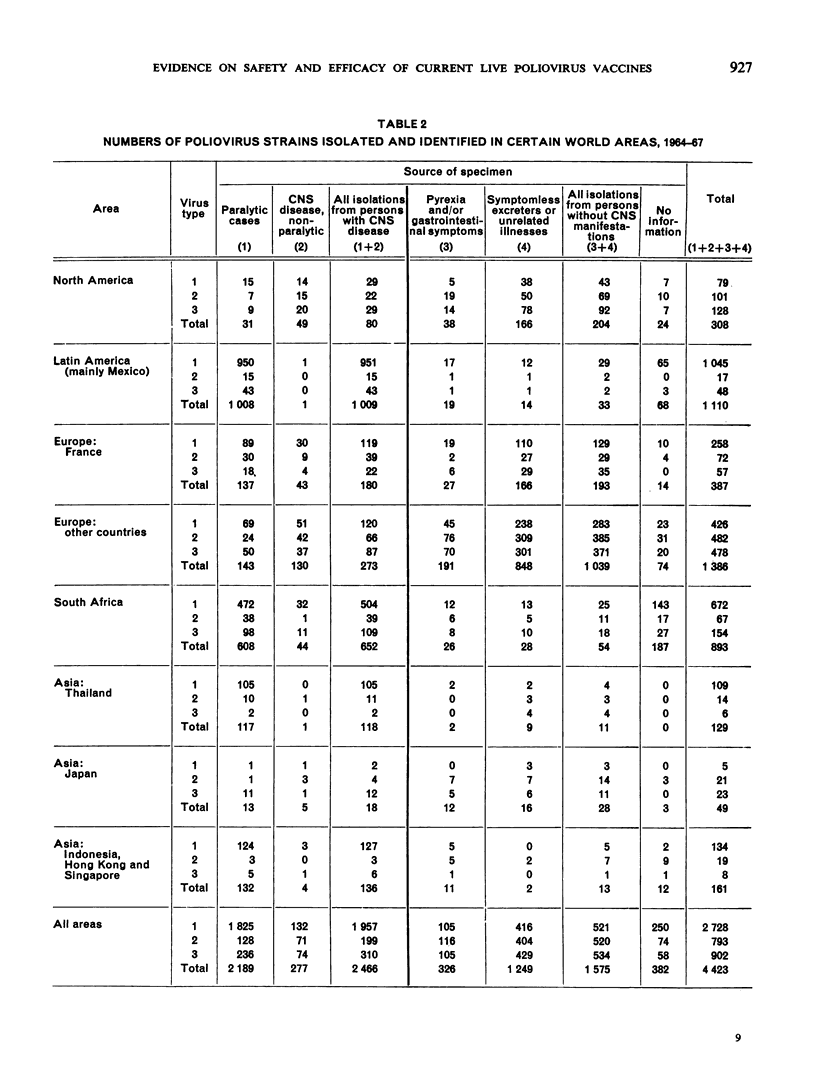
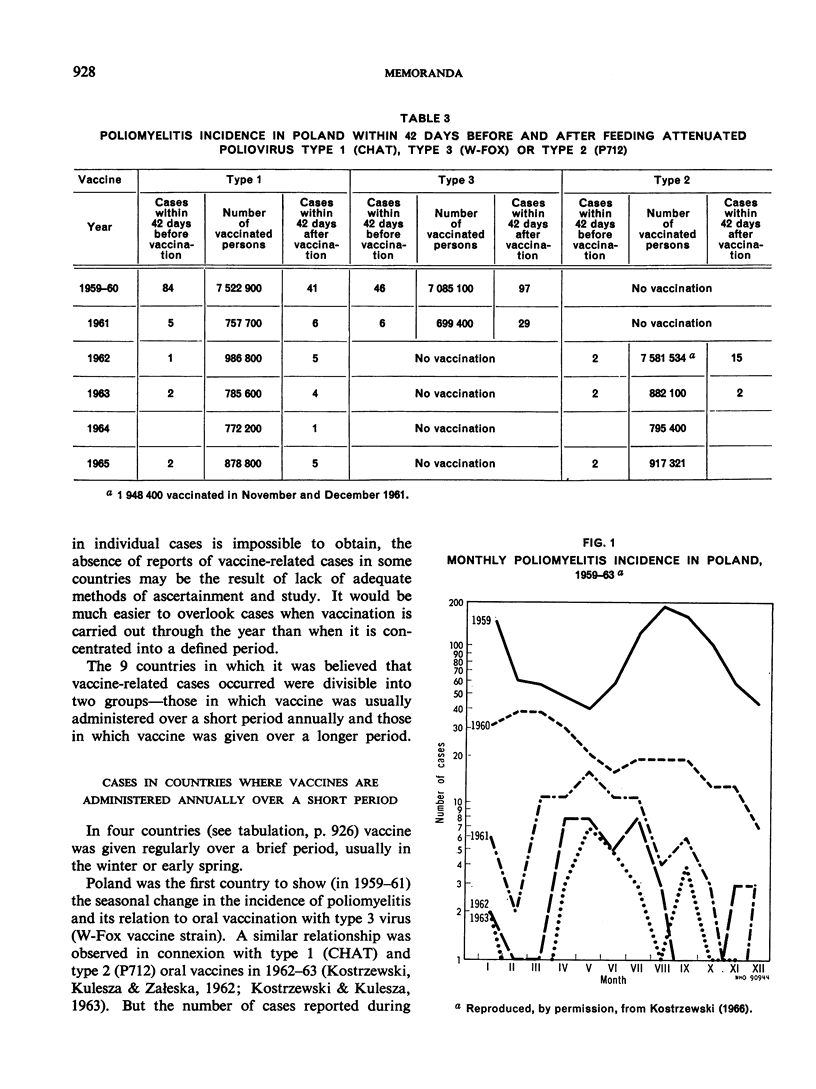
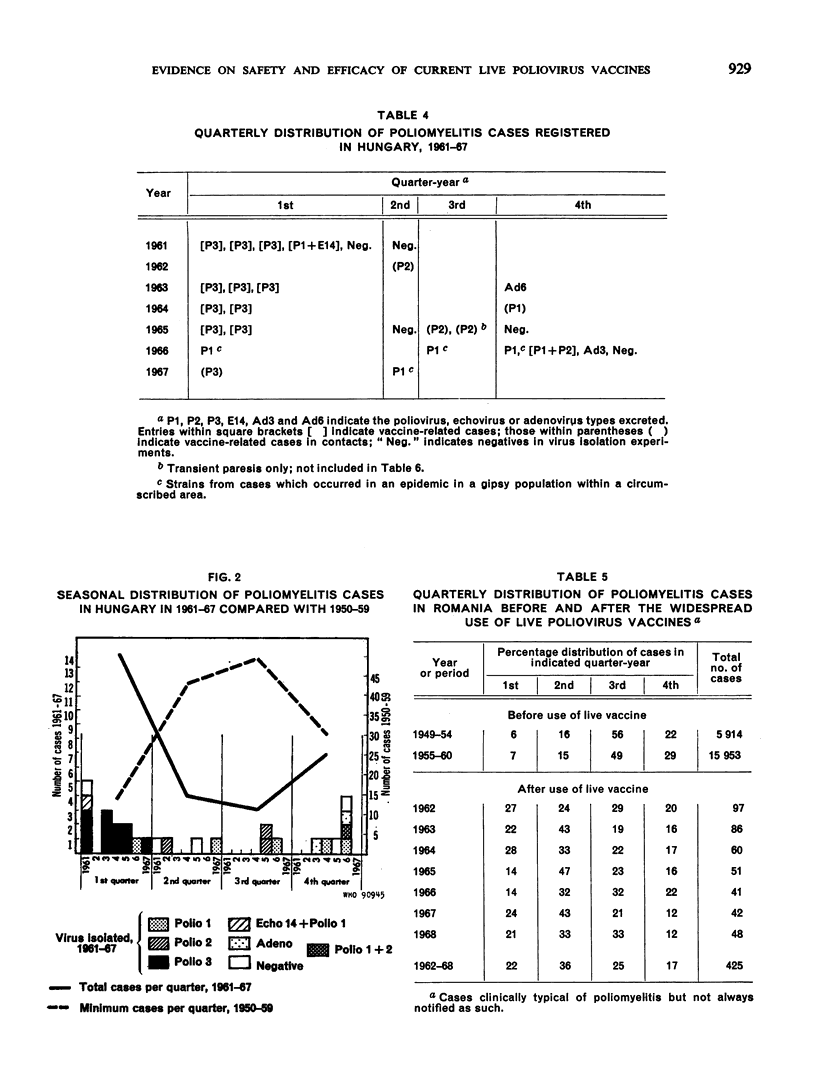
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benyesh-Melnick M., Melnick J. L., Rawls W. E., Wimberly I., Oro J. B., Ben-Porath E., Rennick V. Studies of the immunogenicity, communicability and genetic stability of oral poliovaccine administered during the winter. Am J Epidemiol. 1967 Jul;86(1):112–136. doi: 10.1093/oxfordjournals.aje.a120717. [DOI] [PubMed] [Google Scholar]
- Böttiger M. Rct-marker tests on isolates from WM3 and Sabin type 3 attenuated poliovirus vaccines. Arch Gesamte Virusforsch. 1968;25(3):299–307. doi: 10.1007/BF01556558. [DOI] [PubMed] [Google Scholar]
- DOMOK I., MOLNAR E. Enterovirus studies in connection with the 1959 poliomyelitis epidemic in Hungary. Acta Microbiol Acad Sci Hung. 1961;8:189–203. [PubMed] [Google Scholar]
- Deibel R., Macdonald D. Serodifferentiation of type 3 poliovirus strains isolated 1960-1965 from patients and healthy vaccinees. Characterization of strains associated with disease in vaccinated persons and household contacts. Am J Epidemiol. 1968 Mar;87(2):396–410. doi: 10.1093/oxfordjournals.aje.a120830. [DOI] [PubMed] [Google Scholar]
- FURESZ J., ARMSTRONG R. E., YAROSH W., NAGLER F. P. GENETIC MARKERS OF POLIOVIRUS STRAINS ISOLATED FROM PARALYTIC PATIENTS PRIOR TO AND AFTER SABIN VACCINATION PROGRAMS. I. STUDIES ON TYPE 1 STRAINS. Am J Hyg. 1964 Jul;80:45–54. doi: 10.1093/oxfordjournals.aje.a120457. [DOI] [PubMed] [Google Scholar]
- Furesz J., Armstrong R. E., Moreau P., Yarosh W., Nagler F. P. Antigenic studies on Sabin types 1 & 3 poliovaccine virus during 1 to 7 passages in the human intestinal tract. Am J Epidemiol. 1966 May;83(3):501–508. doi: 10.1093/oxfordjournals.aje.a120601. [DOI] [PubMed] [Google Scholar]
- GELFAND H. M., NAKANO J. H., COLE J. T. Serodifferentiation of poliovirus strains for studies of oral vaccine. Public Health Rep. 1962 Nov;77:941–946. [PMC free article] [PubMed] [Google Scholar]
- GSELL O., WIESMANN E. [Poliomyelitis antibody development in adults after vaccination with Lederle triple vaccine of Sabin strains]. Schweiz Med Wochenschr. 1962 Apr 21;92:475–476. [PubMed] [Google Scholar]
- Janda Z., Adam E., Vonka V. Properties of a new type 3 attenuated poliovirus. VI. Alimentary tract resistance in children fed previously with type 3 Sabin vaccine to reinfection with homologous and heterologous type 3 attenuated poliovirus. Arch Gesamte Virusforsch. 1967;20(1):87–98. [PubMed] [Google Scholar]
- LYNG J., BENTZON M. W. INTERNATIONAL STANDARDS FOR ANTI-POLIOVIRUS SERA TYPES 1, 2 AND 3. Bull World Health Organ. 1963;29:711–720. [PMC free article] [PubMed] [Google Scholar]
- MILLER D. L., GALBRAITH N. S. SURVEILLANCE OF THE SAFETY OF ORAL POLIOMYELITIS VACCINE IN ENGLAND AND WALES 1962-4. Br Med J. 1965 Aug 28;2(5460):504–509. doi: 10.1136/bmj.2.5460.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGLER F. P. RECENT EXPERIENCE WITH ORAL POLIOVIRUS VACCINE (SABIN) IN CANADA. Can J Public Health. 1963 Nov;54:509–514. [PubMed] [Google Scholar]
- Nakano J. H., Gelfand H. M., Cole J. T. Antigenic segregation of type 3 poliovirus isolates related and unrelated to Sabin's vaccine strain with the use of modified Wecker and McBride techniques. Am J Epidemiol. 1966 Jan;83(1):130–145. doi: 10.1093/oxfordjournals.aje.a120561. [DOI] [PubMed] [Google Scholar]
- OZAKI Y., DIWAN A. R., TAKIZAWA M., MELNICK J. L. CHROMATOGRAPHY OF POLIOVIRUS ON CALCIUM PHOSPHATE AND ITS APPLICATION TO THE IDENTIFICATION OF VACCINE PROGENY STRAINS. J Bacteriol. 1965 Mar;89:603–610. doi: 10.1128/jb.89.3.603-610.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLOTKIN S. A., NORTON T. W., COHEN B. J., KOPROWSKI H. A type 3 attenuated poliovirus genetically stable after human intestinal passage. Proc Soc Exp Biol Med. 1961 Aug-Sep;107:829–834. doi: 10.3181/00379727-107-26769. [DOI] [PubMed] [Google Scholar]
- RATHBUN M. L., BROAD R. H., FONT W., MILHAM S., AMES W. R. Mass immunization with Sabin and Cox oral poliomyelitis vaccines. Experience in Monroe and Tompkins Counties, New York, 1960. N Y State J Med. 1962 Jun 1;62:1767–1775. [PubMed] [Google Scholar]
- SABIN A. B., MICHAELS R. H., SPIGLAND I., PELON W., RHIM J. S., WEHR R. E. Community-wide use of oral poliovirus vaccine. Efectiveness of the Cincinnati program. Am J Dis Child. 1961 May;101:546–567. doi: 10.1001/archpedi.1961.04020060004002. [DOI] [PubMed] [Google Scholar]
- SABIN A. B. Present position of immunization against poliomyelitis with live virus vaccines. Br Med J. 1959 Mar 14;1(5123):663–680. doi: 10.1136/bmj.1.5123.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin A. B. Oral poliovirus vaccine. History of its development and prospects for eradication of poliomyelitis. JAMA. 1965 Nov 22;194(8):872–876. doi: 10.1001/jama.194.8.872. [DOI] [PubMed] [Google Scholar]
- Vonka V., Janda Z., Simon J., Adam E., Stárek M. A new type 3 attenuated poliovirus for possible use in oral poliovirus vaccine. Prog Med Virol. 1967;9:204–255. [PubMed] [Google Scholar]
- WOODS W. A., WEISS R. A., ROBBINS F. C. Comparison of neutralization rate and agar diffusion methods for intratypic sero-differentiation of polioviruses. Proc Soc Exp Biol Med. 1962 Nov;111:401–404. doi: 10.3181/00379727-111-27805. [DOI] [PubMed] [Google Scholar]


