Abstract
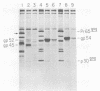
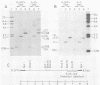
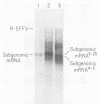
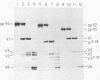
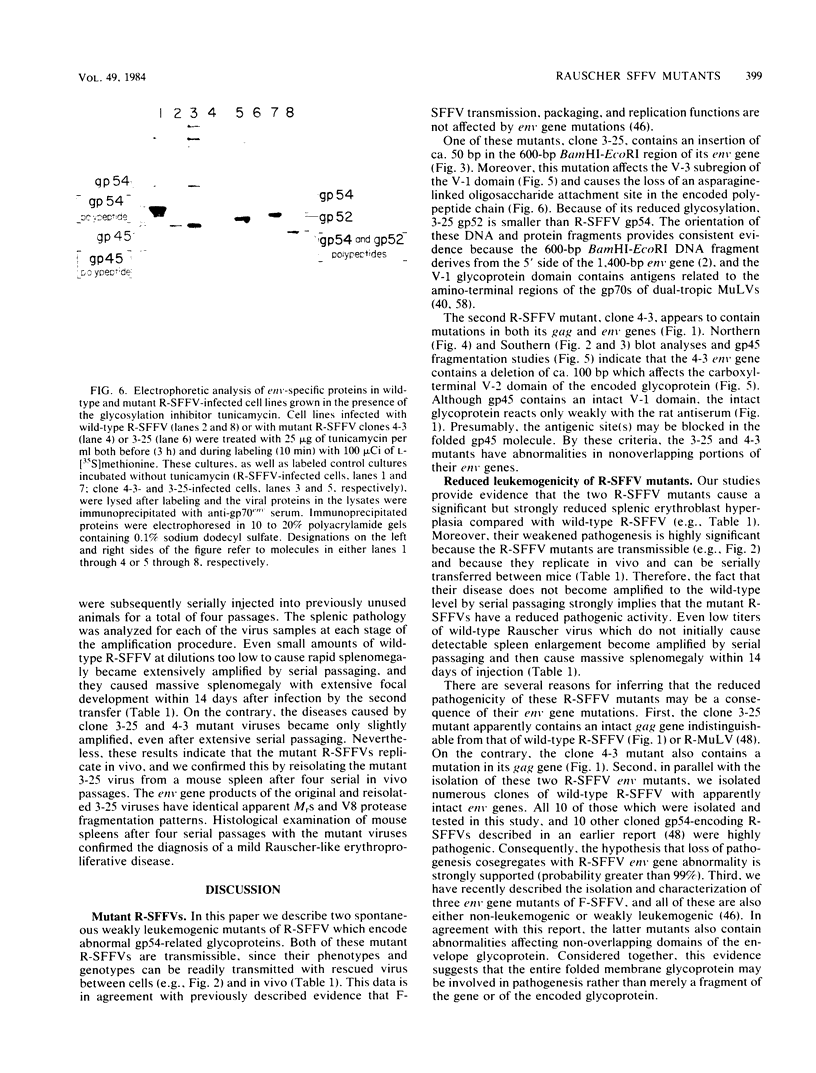
We isolated and characterized two spontaneous, weakly leukemogenic mutants of Rauscher spleen focus-forming virus (R-SFFV) that contain mutations in nonoverlapping regions of the membrane envelope (env) glycoprotein gene. As reported previously (M. Ruta and D. Kabat, J. Virol. 35:844-853, 1980), the replication-defective R-SFFV encodes a membrane glycoprotein with an apparent Mr of 54,000 (gp54) which is structurally and immunologically related to the membrane envelope glycoproteins of dual-tropic murine leukemia viruses. Mutant R-SFFV clones 3-25 and 4-3 encode abnormally sized gp54-related glycoproteins with apparent Mrs of 52,000 (gp52) and 45,000 (gp45), respectively. Northern and Southern blot analyses of the mutant R-SFFV nucleic acids indicated that an insertion has occurred in the 3-25 env gene and that a deletion has occurred in the 4-3 env gene. Furthermore, restriction endonuclease analyses and comparisons of the fragmentation patterns of the wild-type and mutant glycoproteins generated by partial proteolysis with Staphylococcus aureus V8 protease indicated that the mutations affect nonoverlapping domains of the envelope glycoprotein (amino-terminal fragment affected in 3-25 glycoprotein and carboxyl-terminal fragment affected in 4-3 glycoprotein). Glycosylation inhibition studies indicated that the reduced size of gp52 is caused at least partly by loss of an asparagine-linked oligosaccharide. In addition, these mutant viruses have dramatically reduced leukemogenicities compared with wild-type R-SFFV. We conclude that the gp54 structural gene is required for initiation or amplification of the splenic erythroblast hyperplasia which characterizes the preleukemic phase of Rauscher disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELRAD A. A., STEEVES R. A. ASSAY FOR FRIEND LEUKEMIA VIRUS: RAPID QUANTITATIVE METHOD BASED ON ENUMERATION OF MACROSCOPIC SPLEEN FOCI IN MICE. Virology. 1964 Nov;24:513–518. doi: 10.1016/0042-6822(64)90199-0. [DOI] [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Amanuma H., Katori A., Obata M., Sagata N., Ikawa Y. Complete nucleotide sequence of the gene for the specific glycoprotein (gp55) of Friend spleen focus-forming virus. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3913–3917. doi: 10.1073/pnas.80.13.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand R., Steeves R. How many types of erythroleukaemia are induced by retroviruses in mice? Nature. 1980 Aug 7;286(5773):615–617. doi: 10.1038/286615a0. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Bestwick R., Ruta M., Kiessling A., Faust C., Linemeyer D., Scolnick E., Kabat D. Genetic structure of Rauscher spleen focus-forming virus. J Virol. 1983 Mar;45(3):1217–1222. doi: 10.1128/jvi.45.3.1217-1222.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bosselman R. A., van Straaten F., Van Beveren C., Verma I. M., Vogt M. Analysis of the env gene of a molecularly cloned and biologically active Moloney mink cell focus-forming proviral DNA. J Virol. 1982 Oct;44(1):19–31. doi: 10.1128/jvi.44.1.19-31.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Cloyd M. W., Linemeyer D. L., Lander M. R., Rands E., Lowy D. R. Cellular origin and role of mink cell focus-forming viruses in murine thymic lymphomas. Nature. 1982 Jan 7;295(5844):25–31. doi: 10.1038/295025a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cloyd M. W. Characterization of target cells for MCF viruses in AKR mice. Cell. 1983 Jan;32(1):217–225. doi: 10.1016/0092-8674(83)90512-3. [DOI] [PubMed] [Google Scholar]
- Cloyd M. W., Hartley J. W., Rowe W. P. Lymphomagenicity of recombinant mink cell focus-inducing murine leukemia viruses. J Exp Med. 1980 Mar 1;151(3):542–552. doi: 10.1084/jem.151.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. M., Neiman P. E. Two distinct candidate transforming genes of lymphoid leukosis virus-induced neoplasms. Nature. 1981 Aug 27;292(5826):857–858. doi: 10.1038/292857a0. [DOI] [PubMed] [Google Scholar]
- Dawson P. J., Rose W. M., Fieldsteel A. H. Lymphatic leukaemia in rats and mice inoculated with Friend virus. Br J Cancer. 1966 Mar;20(1):114–121. doi: 10.1038/bjc.1966.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresler S., Ruta M., Murray M. J., Kabat D. Glycoprotein encoded by the Friend spleen focus-forming virus. J Virol. 1979 May;30(2):564–575. doi: 10.1128/jvi.30.2.564-575.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Gautsch J. W., Jensen F. C., Lerner R. A., Hartley J. W., Rowe W. P. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4676–4680. doi: 10.1073/pnas.74.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. H., Dresler S., Kabat D. Synthesis and glycosylation of polyprotein precursors to the internal core proteins of Friend murine leukemia virus. J Virol. 1977 Dec;24(3):865–874. doi: 10.1128/jvi.24.3.865-874.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L., Nunn M., Duesberg P. H., Troxler D., Scolnick E. RNAs of defective and nondefective components of Friend anemia and polycythemia virus strains identified and compared. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):823–835. doi: 10.1101/sqb.1980.044.01.087. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Hart I. R. Biological diversity in metastatic neoplasms: origins and implications. Science. 1982 Sep 10;217(4564):998–1003. doi: 10.1126/science.7112116. [DOI] [PubMed] [Google Scholar]
- Fieldsteel A. H., Dawson P. J., Kurahara C. Induction of lymphatic leukaemia in BALB/c mice from the original isolate of Rauscher virus. Br J Cancer. 1969 Dec;23(4):806–813. doi: 10.1038/bjc.1969.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M., Patch V. Cell-surface antigens associated with dualtropic and thymotropic murine leukemia viruses inducing thymic and nonthymic lymphomas. J Exp Med. 1980 Jun 1;151(6):1321–1333. doi: 10.1084/jem.151.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins W. D., Troxler D. Polycythemia- and anemia-inducing erythroleukemia viruses exhibit differential erythroid transforming effects in vitro. Cell. 1980 Dec;22(3):693–699. doi: 10.1016/0092-8674(80)90545-0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kabat D., Ruta M., Murray M. J., Polonoff E. Immunoselection of mutants deficient in cell surface glycoproteins encoded by murine erythroleukemia viruses. Proc Natl Acad Sci U S A. 1980 Jan;77(1):57–61. doi: 10.1073/pnas.77.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol. 1977 Jan;21(1):375–385. doi: 10.1128/jvi.21.1.375-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Horak I., Ihle J. N. Mechanisms in T cell leukemogenesis. II. T cell responses of preleukemic BALB/c mice to Moloney leukemia virus antigens. J Immunol. 1981 Feb;126(2):715–722. [PubMed] [Google Scholar]
- Linemeyer D. L., Menke J. G., Ruscetti S. K., Evans L. H., Scolnick E. M. Envelope gene sequences which encode the gp52 protein of spleen focus-forming virus are required for the induction of erythroid cell proliferation. J Virol. 1982 Jul;43(1):223–233. doi: 10.1128/jvi.43.1.223-233.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linemeyer D. L., Ruscetti S. K., Menke J. G., Scolnick E. M. Recovery of biologically active spleen focus-forming virus from molecularly cloned spleen focus-forming virus-pBR322 circular DNA by cotransfection with infectious type C retroviral DNA. J Virol. 1980 Sep;35(3):710–721. doi: 10.1128/jvi.35.3.710-721.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linemeyer D. L., Ruscetti S. K., Scolnick E. M., Evans L. H., Duesberg P. H. Biological activity of the spleen focus-forming virus is encoded by a molecularly cloned subgenomic fragment of spleen focus-forming virus DNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1401–1405. doi: 10.1073/pnas.78.3.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- METCALF D., FURTH J., BUFFETT R. F. Pathogenesis of mouse leukemia caused by Friend virus. Cancer Res. 1959 Jan;19(1):52–58. [PubMed] [Google Scholar]
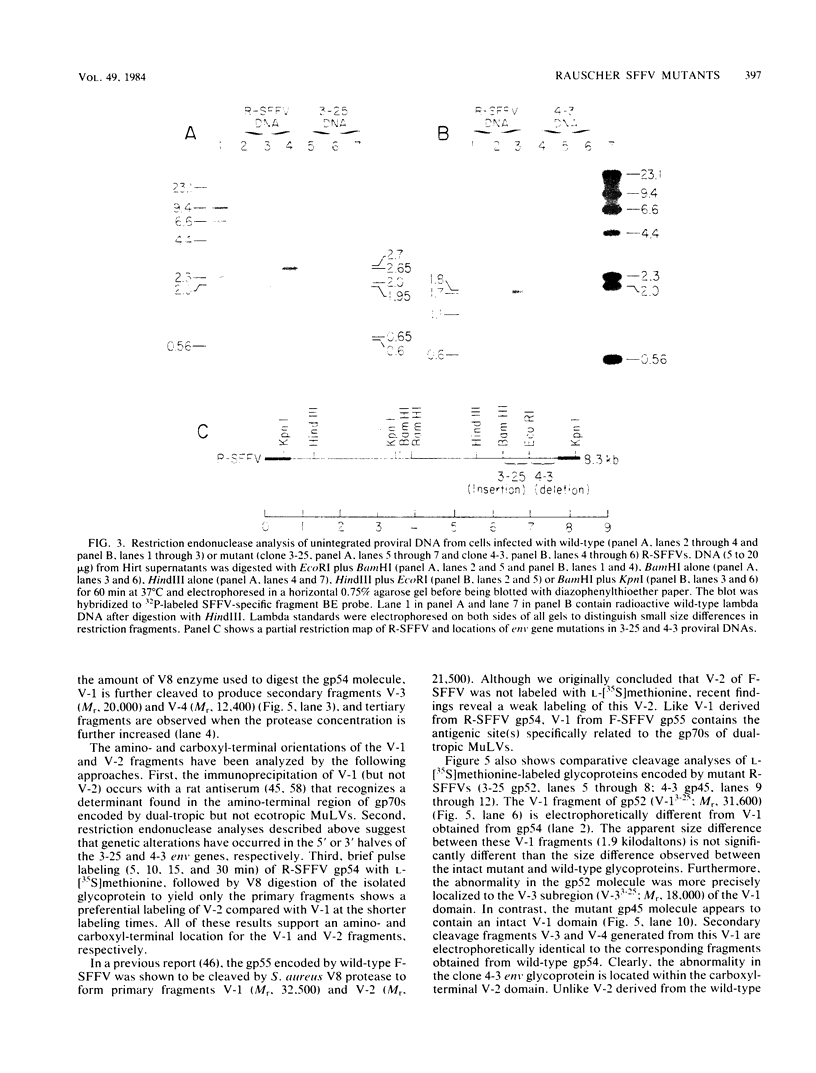
- Machida C. A., Kabat D. Role of partial proteolysis in processing murine leukemia virus membrane envelope glycoproteins to the cell surface. A viral mutant with uncleaved glycoprotein. J Biol Chem. 1982 Dec 10;257(23):14018–14022. [PubMed] [Google Scholar]
- Mager D., Mak T. W., Bernstein A. Friend leukaemia virus-transformed cells, unlike normal stem cells, form spleen colonies in Sl/sld mice. Nature. 1980 Dec 11;288(5791):592–594. doi: 10.1038/288592a0. [DOI] [PubMed] [Google Scholar]
- McGrath M. S., Weissman I. L. AKR leukemogenesis: identification and biological significance of thymic lymphoma receptors for AKR retroviruses. Cell. 1979 May;17(1):65–75. doi: 10.1016/0092-8674(79)90295-2. [DOI] [PubMed] [Google Scholar]
- Murray M. J., Kabat D. Genetic and sialylation sources of heterogeneity of the murine leukemia virus membrane envelope glycoproteins gp69/71. J Biol Chem. 1979 Feb 25;254(4):1340–1348. [PubMed] [Google Scholar]
- O'Farrell P. H., Kutter E., Nakanishi M. A restriction map of the bacteriophage T4 genome. Mol Gen Genet. 1980;179(2):421–435. doi: 10.1007/BF00425473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter A., Honnen W. J., Tung J. S., O'Donnell P. V., Hämmerling U. Structural domains of endogenous murine leukemia virus gp70s containing specific antigenic determinants defined by monoclonal antibodies. Virology. 1982 Jan 30;116(2):499–516. doi: 10.1016/0042-6822(82)90143-x. [DOI] [PubMed] [Google Scholar]
- Polonoff E., Machida C. A., Kabat D. Glycosylation and intracellular transport of membrane glycoproteins encoded by murine leukemia viruses. Inhibition by amino acid analogues and by tunicamycin. J Biol Chem. 1982 Dec 10;257(23):14023–14028. [PubMed] [Google Scholar]
- ROWE W. P., BRODSKY I. A graded-response assay for the Friend leukemia virus. J Natl Cancer Inst. 1959 Dec;23:1239–1248. [PubMed] [Google Scholar]
- Reddy E. P., Dunn C. Y., Aaronson S. A. Different lymphoid cell targets by transformation by replication-competent Moloney and Rauscher mouse leukemia viruses. Cell. 1980 Mar;19(3):663–669. doi: 10.1016/s0092-8674(80)80043-2. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruscetti S. K., Linemeyer D., Feild J., Troxler D., Scolnick E. M. Characterization of a protein found in cells infected with the spleen focus-forming virus that shares immunological cross-reactivity with the gp70 found in mink cell focus-inducing virus particles. J Virol. 1979 Jun;30(3):787–798. doi: 10.1128/jvi.30.3.787-798.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
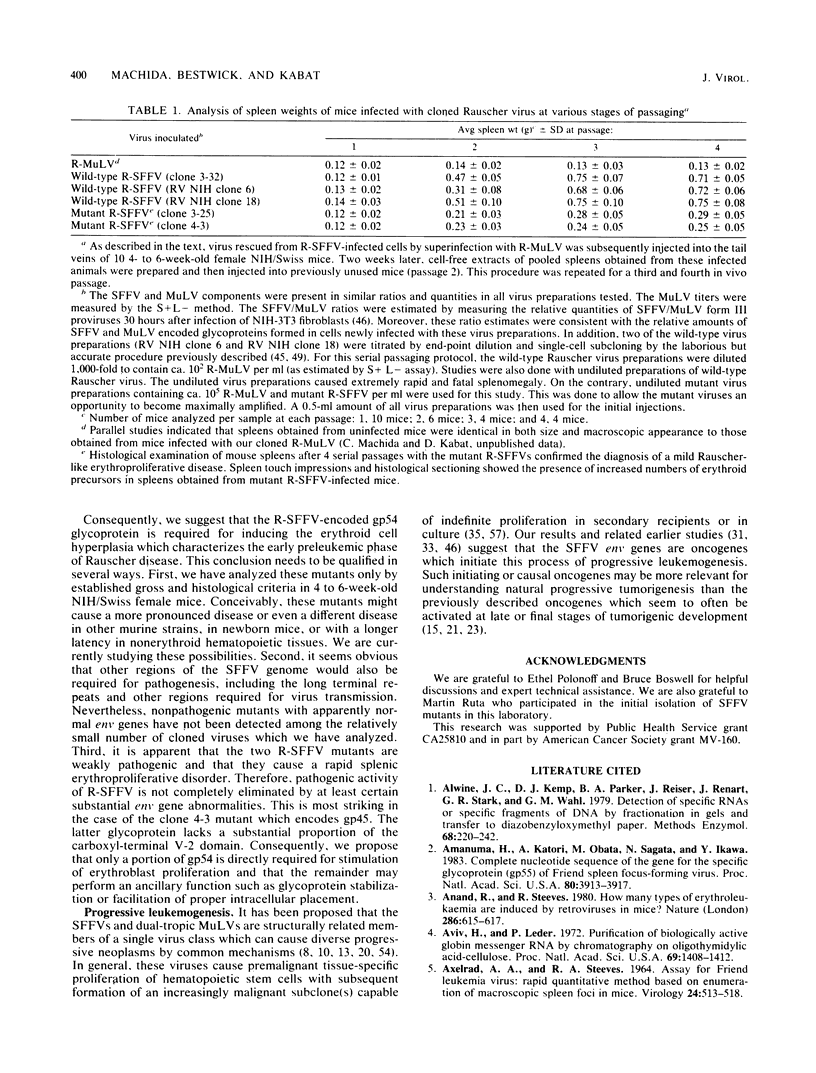
- Ruta M., Bestwick R., Machida C., Kabat D. Loss of leukemogenicity caused by mutations in the membrane glycoprotein structural gene of Friend spleen focus-forming virus. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4704–4708. doi: 10.1073/pnas.80.15.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruta M., Clarke S., Boswell B., Kabat D. Heterogeneous metabolism and subcellular localization of a potentially leukemogenic membrane glycoprotein encoded by Friend erythroleukemia virus. Isolation of viral and cellular processing mutants. J Biol Chem. 1982 Jan 10;257(1):126–134. [PubMed] [Google Scholar]
- Ruta M., Murray M. J., Webb M. C., Kabat D. A murine leukemia virus mutant with a temperature-sensitive defect in membrane glycoprotein synthesis. Cell. 1979 Jan;16(1):77–88. doi: 10.1016/0092-8674(79)90189-2. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Gallimore P. H., Flint S. J. Mapping of adenovirus 2 RNA sequences in lytically infected cells and transformed cell lines. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):457–474. doi: 10.1101/sqb.1974.039.01.058. [DOI] [PubMed] [Google Scholar]
- Shields A., Witte W. N., Rothenberg E., Baltimore D. High frequency of aberrant expression of Moloney murine leukemia virus in clonal infections. Cell. 1978 Jul;14(3):601–609. doi: 10.1016/0092-8674(78)90245-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas C. Y., Coffin J. M. Genetic alterations of RNA leukemia viruses associated with the development of spontaneous thymic leukemia in AKR/J mice. J Virol. 1982 Aug;43(2):416–426. doi: 10.1128/jvi.43.2.416-426.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling F., Moreau-Gachelin F., Tambourin P. Emergence of tumorigenic cells during the course of Friend virus leukemias. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3614–3618. doi: 10.1073/pnas.78.6.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff L., Koller R., Ruscetti S. Monoclonal antibody to spleen focus-forming virus-encoded gp52 provides a probe for the amino-terminal region of retroviral envelope proteins that confers dual tropism and xenotropism. J Virol. 1982 Aug;43(2):472–481. doi: 10.1128/jvi.43.2.472-481.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski C. C., Waksal S. D., Tempelis L. D., Khiroya R. H., Schwartz R. S. Surface phenotypes in T-cell leukaemia are determined by oncogenic retroviruses. Nature. 1980 Dec 4;288(5790):489–491. doi: 10.1038/288489a0. [DOI] [PubMed] [Google Scholar]