Abstract
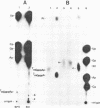
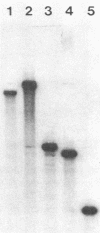
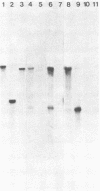
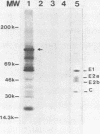
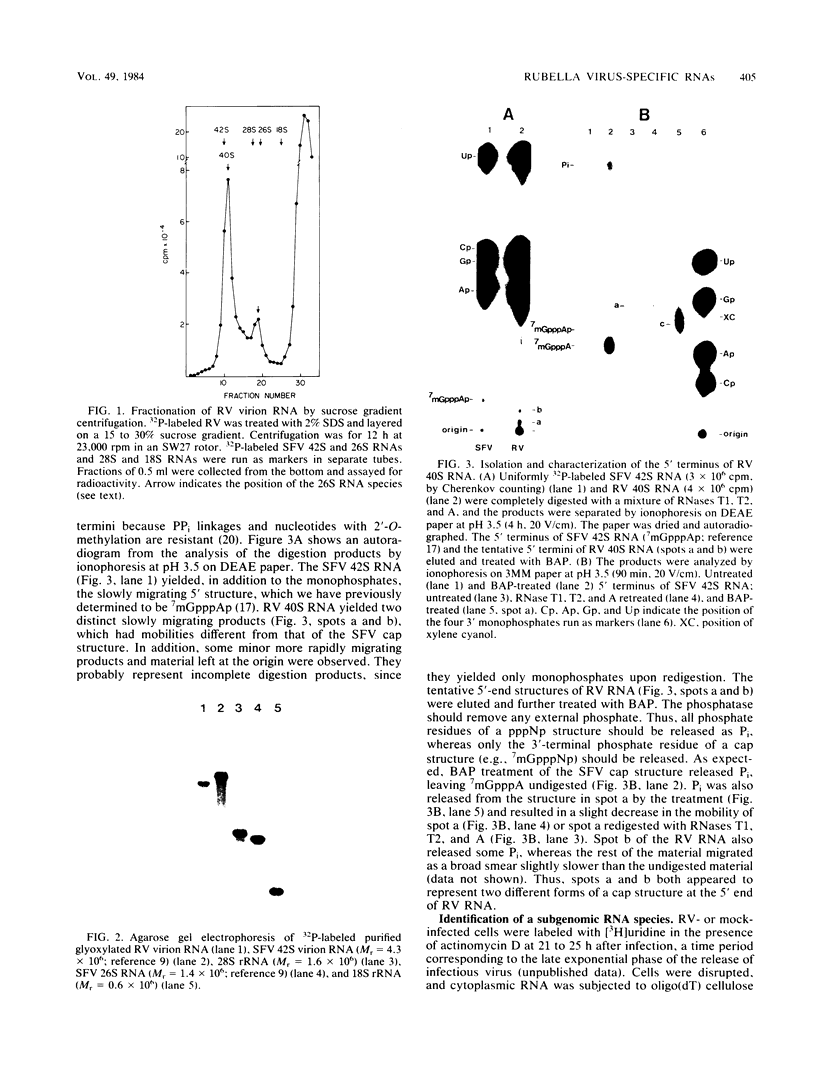
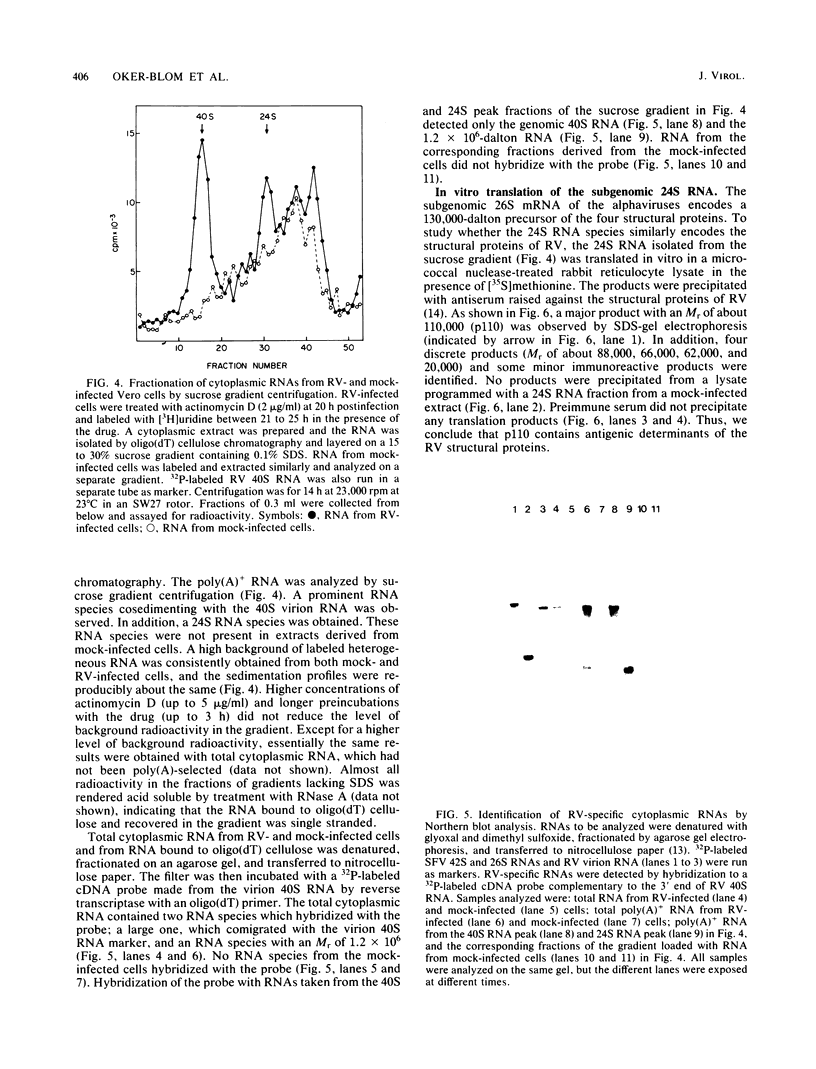
We have analyzed the structure of the rubella virus genome RNA and the virus-specific RNA species synthesized in B-Vero cells infected with rubella virus. A single-stranded, capped, and polyadenylated RNA species sedimenting at 40S in a sucrose gradient was released from purified virions treated with sodium dodecyl sulfate. This RNA species migrated with an Mr of about 3.8 X 10(6) in an agarose gel after denaturation with glyoxal and dimethyl sulfoxide. Infected cells labeled with [3H]uridine in the presence of actinomycin D contained, in addition to the 40S RNA, a single-stranded polyadenylated 24S RNA species as shown by sucrose gradient analysis. In a Northern blot analysis, this RNA hybridized to a cDNA probe derived from the 3' portion of the genomic 40S RNA. In vitro translation of the 24S RNA species yielded a 110,000-dalton polypeptide, in addition to some smaller products which were immunoprecipitated with an antiserum prepared against the structural proteins E1, E2a, E2b, and C. Since the sum of the molecular weights of the nonglycosylated envelope proteins and the capsid protein has been estimated to be about 116,000 (C. Oker-Blom et al., J. Virol. 46:964-973, 1983), these results suggest that the 24S RNA species represents a subgenomic mRNA coding for a precursor (p110) to the structural proteins of rubella virus. Thus, the strategy of gene expression of rubella virus appears to be similar to that of the alphaviruses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Expression of animal virus genomes. Bacteriol Rev. 1971 Sep;35(3):235–241. doi: 10.1128/br.35.3.235-241.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Hovi T., Vaheri A. Infectivity and some physicochemical characteristics of rubella virus ribonucleic acid. Virology. 1970 Sep;42(1):1–8. doi: 10.1016/0042-6822(70)90232-1. [DOI] [PubMed] [Google Scholar]
- Hovi T., Vaheri A. Rubella virus-specific ribonucleic acids in infected BHK21 cells. J Gen Virol. 1970 Jan;6(1):77–83. doi: 10.1099/0022-1317-6-1-77. [DOI] [PubMed] [Google Scholar]
- Käriäinen L., Söderlund H. Structure and replication of alpha-viruses. Curr Top Microbiol Immunol. 1978;82:15–69. doi: 10.1007/978-3-642-46388-4_2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oker-Blom C., Kalkkinen N., Käriäinen L., Pettersson R. F. Rubella virus contains one capsid protein and three envelope glycoproteins, E1, E2a, and E2b. J Virol. 1983 Jun;46(3):964–973. doi: 10.1128/jvi.46.3.964-973.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pettersson R. F., Hewlett M. J., Baltimore D., Coffin J. M. The genome of Uukuniemi virus consists of three unique RNA segments. Cell. 1977 May;11(1):51–63. doi: 10.1016/0092-8674(77)90316-6. [DOI] [PubMed] [Google Scholar]
- Pettersson R. F., Söderlund H., Käriäinen L. The nucleotide sequences of the 5'-terminal T1 oligonucleotides of Semliki-Forest-virus 42-S and 26-S RNAs are different. Eur J Biochem. 1980 Apr;105(3):435–443. doi: 10.1111/j.1432-1033.1980.tb04518.x. [DOI] [PubMed] [Google Scholar]
- Porterfield J. S., Casals J., Chumakov M. P., Gaidamovich S. Y., Hannoun C., Holmes I. H., Horzinek M. C., Mussgay M., Oker-Blom N., Russell P. K. Togaviridae. Intervirology. 1978;9(3):129–148. doi: 10.1159/000148930. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Haseltine W. A., Baltimore D. 5'-terminus of Moloney murine leukemia virus 35s RNA is m7G5' ppp5' GmpCp. J Virol. 1976 Oct;20(1):324–329. doi: 10.1128/jvi.20.1.324-329.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K. Heterogneeous 5'-terminal structures occur on vesicular stomatitis virus mRNAs. J Biol Chem. 1975 Oct 25;250(20):8098–8104. [PubMed] [Google Scholar]
- Sedwick W. D., Sokol F. Nucleic acid of rubella virus and its replication in hamster kidney cells. J Virol. 1970 Apr;5(4):478–489. doi: 10.1128/jvi.5.4.478-489.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund H., Keränen S., Lehtovaara P., Palva I., Pettersson R. F., Käriäinen L. Structural complexity of defective-interfering RNAs of Semliki Forest virus as revealed by analysis of complementary DNA. Nucleic Acids Res. 1981 Jul 24;9(14):3403–3417. doi: 10.1093/nar/9.14.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxham M. N., Wolinsky J. S. Immunochemical identification of rubella virus hemagglutinin. Virology. 1983 Apr 15;126(1):194–203. doi: 10.1016/0042-6822(83)90471-3. [DOI] [PubMed] [Google Scholar]
- Wong K. T., Robinson W. S., Merigan T. C. Synthesis of viral-specific ribonucleic Acid in rubella virus-infected cells. J Virol. 1969 Dec;4(6):901–903. doi: 10.1128/jvi.4.6.901-903.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]