Abstract
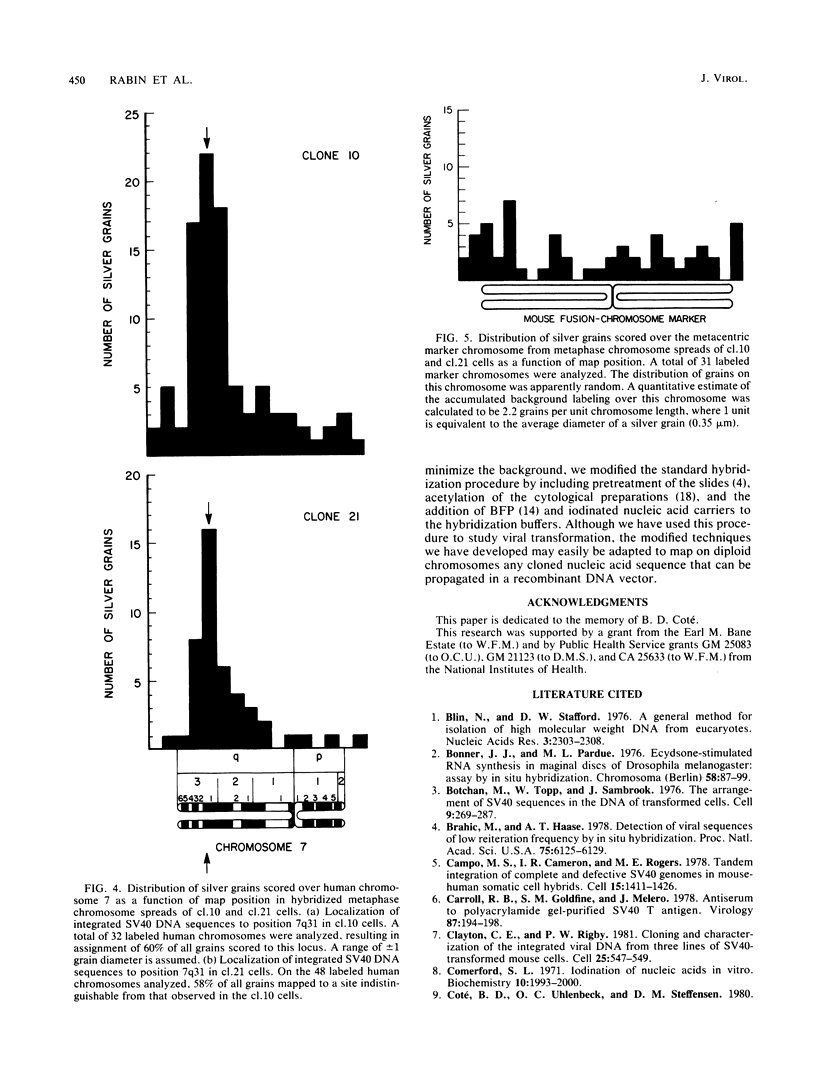
Simian virus 40 DNA sequences, integrated on human chromosome 7 in two transformed human-mouse hybrid somatic cell lines, were mapped to a specific chromosomal locus by in situ hybridization. To detect the integrated viral DNA by in situ hybridization, we increased the sensitivity of the technique by using as a probe 125I-labeled simian virus 40 cRNA (2 X 10(9) to 3 X 10(9) dpm/micrograms) prepared by in vitro transcription of simian virus 40 DNA with high-specific-radioactivity [125I]CTP. Although the viral nucleic acid was shown by blot hybridization in the two cell lines to be integrated in different restriction fragments, it was shown by in situ hybridization in the two cell lines to map to the same position, q31, on the long arm of human chromosome 7. The viral DNA integration sites were localized with a precision of +/- 1 silver grain diameter, equivalent to about 6.2 X 10(7) nucleotide pairs in the human genome. The procedures we describe may be adapted for localization of any gene on diploid chromosomes that can be cloned in a recombinant DNA vector.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J. J., Pardue M. L. Ecdysone-stimulated RNA synthesis in imaginal discs of Drosophila melanogaster. Assay by in situ hybridization. Chromosoma. 1976 Oct 12;58(1):87–99. doi: 10.1007/BF00293443. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Brahic M., Haase A. T. Detection of viral sequences of low reiteration frequency by in situ hybridization. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6125–6129. doi: 10.1073/pnas.75.12.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo M. S., Cameron I. R., Rogers M. E. Tandem integration of complete and defective SV40 genomes in mouse-human somatic cell hybrids. Cell. 1978 Dec;15(4):1411–1426. doi: 10.1016/0092-8674(78)90065-x. [DOI] [PubMed] [Google Scholar]
- Carroll R. B., Goldfine S. M., Melero J. A. Antiserum to polyacrylamide gel-purified simian virus 40 T antigen. Virology. 1978 Jun 1;87(1):194–198. doi: 10.1016/0042-6822(78)90171-x. [DOI] [PubMed] [Google Scholar]
- Clayton C. E., Rigby P. W. Cloning and characterization of the integrated viral DNA from three lines of SV40-transformed mouse cells. Cell. 1981 Aug;25(2):547–559. doi: 10.1016/0092-8674(81)90073-8. [DOI] [PubMed] [Google Scholar]
- Croce C. M. Integration of oncogenic viruses in mammalian cells. Int Rev Cytol. 1981;71:1–17. doi: 10.1016/s0074-7696(08)61180-8. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Knowles B. B., Koprowski H. Preferential retention of the human chromosome C-7 in human-(thymidine kinase deficient) mouse hybrid cells. Exp Cell Res. 1973 Dec;82(2):457–461. doi: 10.1016/0014-4827(73)90366-2. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Martin E., Livingston D. C., Ward D. C. Direct covalent mercuration of nucleotides and polynucleotides. Biochemistry. 1975 Jun 3;14(11):2447–2457. doi: 10.1021/bi00682a027. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Ward D. C., Livingston D. C., Martin E. Conversion of covalently mercurated nucleic acids to tritiated and halogenated derivatives. Nucleic Acids Res. 1975 Jun;2(6):915–930. doi: 10.1093/nar/2.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Gerhard D. S., Kawasaki E. S., Bancroft F. C., Szabo P. Localization of a unique gene by direct hybridization in situ. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3755–3759. doi: 10.1073/pnas.78.6.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Addison W. R., Gillam I. C., Grigliatti T. A., Tener G. M. Hybridization of tRNAs of Drosophila melanogaster to the region of the 5S RNA genes of the polytene chromosomes. Chromosoma. 1981;82(3):385–397. doi: 10.1007/BF00285764. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Gillam I. C., Delaney A. D., Tener G. M. Acetylation of chromosome squashes of Drosophila melanogaster decreases the background in autoradiographs from hybridization with [125I]-labeled RNA. J Histochem Cytochem. 1978 Aug;26(8):677–679. doi: 10.1177/26.8.99471. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hwang S. P., Kucherlapati R. Localization and organization of integrated simian virus 40 sequences in a human cell line. Virology. 1980 Aug;105(1):196–204. doi: 10.1016/0042-6822(80)90167-1. [DOI] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Structure of integrated simian virus 40 DNA in transformed mouse cells. J Mol Biol. 1980 Dec 5;144(2):163–182. doi: 10.1016/0022-2836(80)90031-5. [DOI] [PubMed] [Google Scholar]
- Kucherlapati R., Hwang S. P., Shimizu N., McDougall J. K., Botchan M. R. Another chromosomal assignment for a simian virus 40 integration site in human cells. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4460–4464. doi: 10.1073/pnas.75.9.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Lavi S., Winocour E. Acquisition of sequences homologous to host deoxyribonucleic acid by closed circular simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):309–316. doi: 10.1128/jvi.9.2.309-316.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel W. F. Initial steps in the large-scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. Arch Biochem Biophys. 1974 Jul;163(1):172–177. doi: 10.1016/0003-9861(74)90466-4. [DOI] [PubMed] [Google Scholar]
- McDougall J. K., Gallimore P. H., Dunn A. R., Webb T. P., Kucherlapati R. S., Nichols E. A., Ruddle F. H. Mapping viral integration sites in somatic cell hybrids. Cytogenet Cell Genet. 1976;16(1-5):206–210. doi: 10.1159/000130592. [DOI] [PubMed] [Google Scholar]
- Mougneau E., Birg F., Rassoulzadegan M., Cuzin F. Integration sites and sequence arrangement of SV40 DNA in a homogeneous series of transformed rat fibroblast lines. Cell. 1980 Dec;22(3):917–927. doi: 10.1016/0092-8674(80)90569-3. [DOI] [PubMed] [Google Scholar]
- Prensky W., Steffensen D. M., Hughes W. L. The use of iodinated RNA for gene localization. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1860–1864. doi: 10.1073/pnas.70.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes G., Chamberlin M. J. Ribonucleic acid chain elongation by Escherichia coli ribonucleic acid polymerase. I. Isolation of ternary complexes and the kinetics of elongation. J Biol Chem. 1974 Oct 25;249(20):6675–6683. [PubMed] [Google Scholar]
- Sager R., Anisowicz A., Howell N. Genomic rearrangements in a mouse cell line containing integrated SV40 DNA. Cell. 1981 Jan;23(1):41–50. doi: 10.1016/0092-8674(81)90268-3. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Botchan M., Hu S. L., Mitchison T., Stringer J. Integration of viral DNA sequences in cells transformed by adenovirus 2 or SV40. Proc R Soc Lond B Biol Sci. 1980 Nov 19;210(1180):423–435. doi: 10.1098/rspb.1980.0144. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T., Croce C. M. Selective induction of murine oncornavirus gene expression in somatic cell hybrids between mouse peritoneal macrophages and SV-40-transformed human cells. Virology. 1976 Apr;70(2):545–549. doi: 10.1016/0042-6822(76)90296-8. [DOI] [PubMed] [Google Scholar]
- Szabo P., Elder R., Steffensen D. M., Uhlenbeck O. C. Quantitative in situ hybridization of ribosomal RNA species to polytene chromosomes of Drosophila melanogaster. J Mol Biol. 1977 Sep 25;115(3):539–563. doi: 10.1016/0022-2836(77)90170-x. [DOI] [PubMed] [Google Scholar]