Abstract
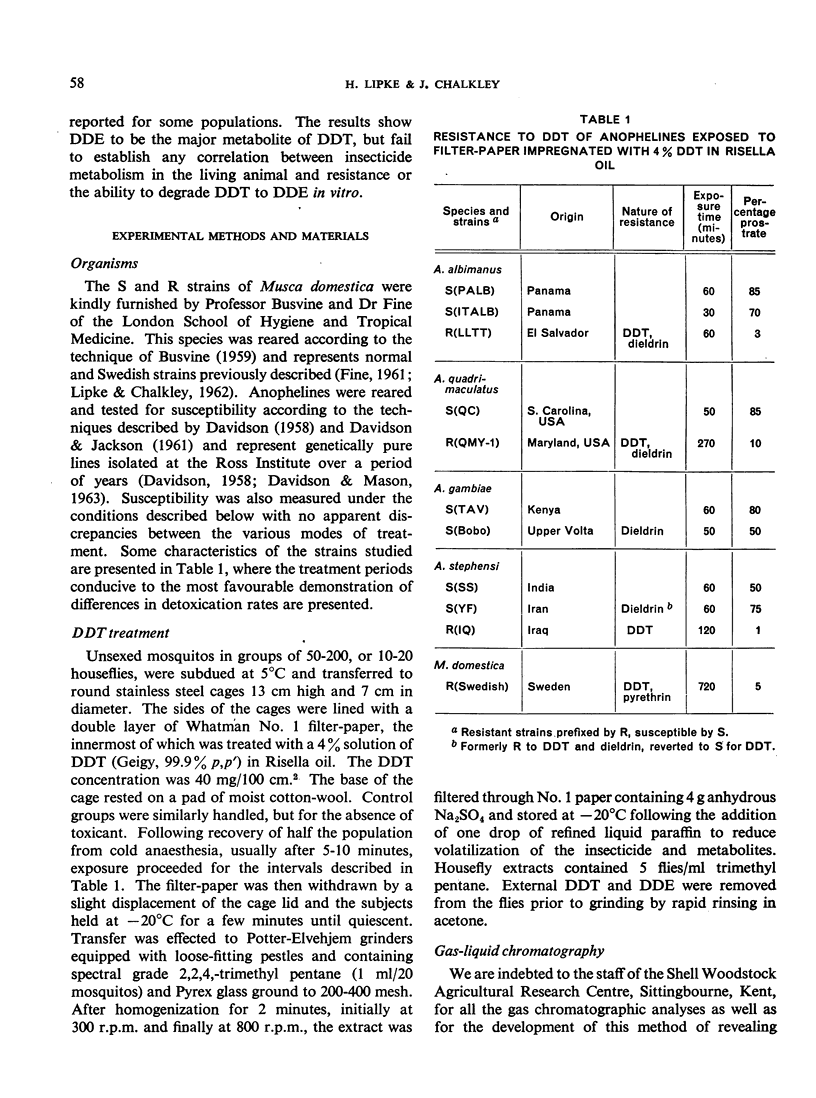
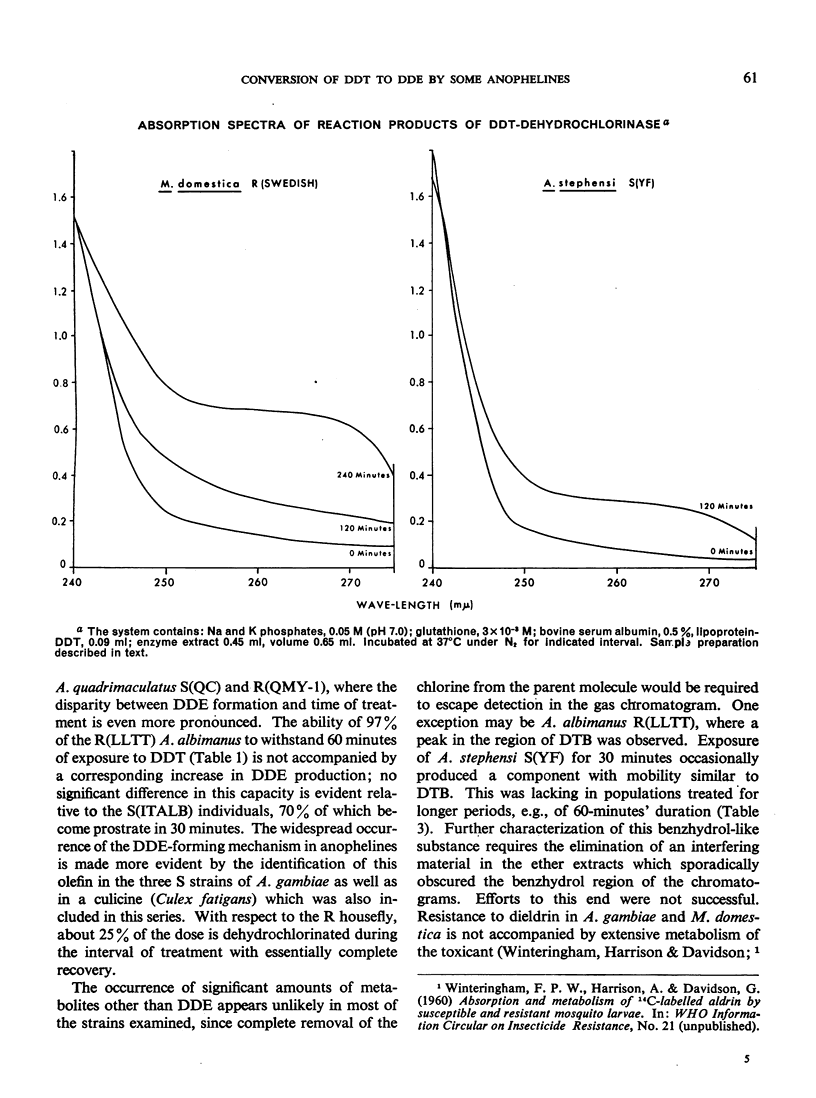
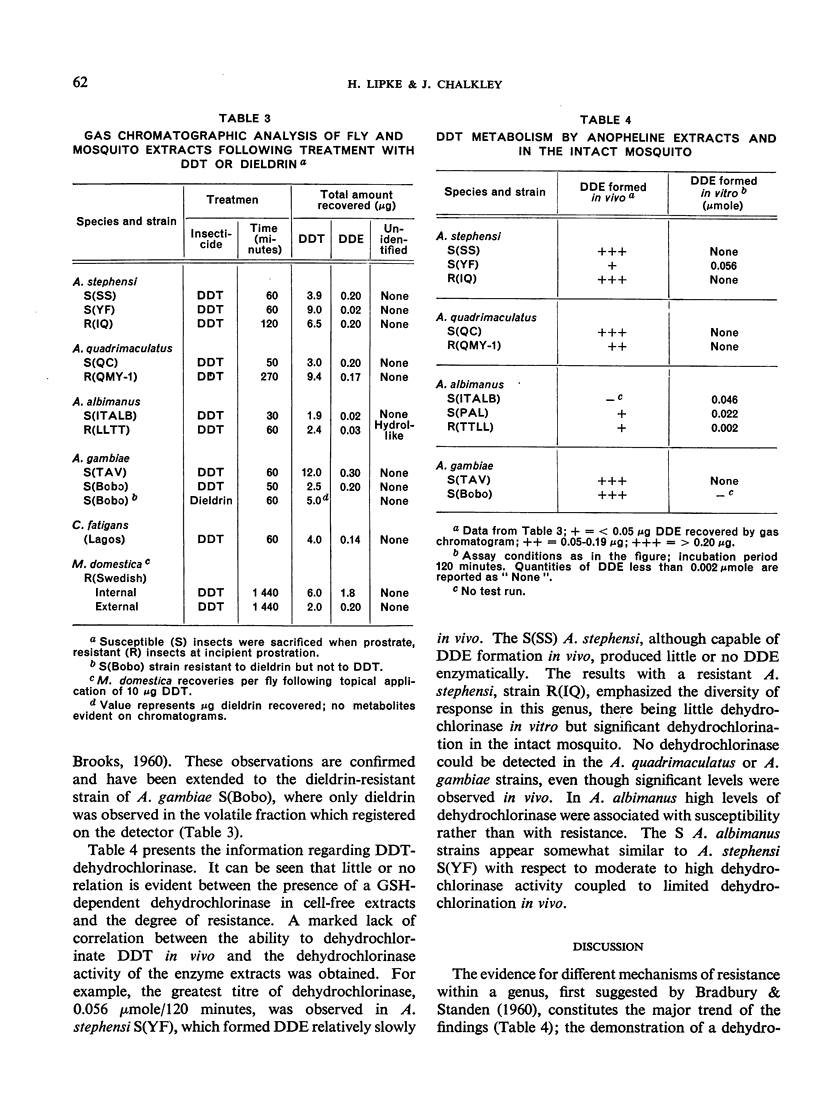
The metabolism of DDT has been followed in pure lines of laboratory-reared resistant and susceptible anophelines using gas-liquid chromatography. Relatively large amounts of DDE were formed in vivo by susceptible strains of Anopheles stephensi, A. quadrimaculatus and A. gambiae, and by resistant strains of A. stephensi and A. quadrimaculatus. Resistant and susceptible A. albimanus showed no difference in the rate of DDE production. Volatile metabolites of DDT other than DDE were not observed in most of the strains chromatographed. A procedure for the measurement of DDT-dehydrochlorinase in small numbers of mosquitos was devised based on conditions developed for the glutathione-dependent DDT-dehydrochlorinase present in resistant houseflies. This method has shown the enzyme to occur in highest titre in DDT-susceptible A. albimanus and A. stephensi, indicating little or no correlation with resistance to DDT. Major differences in dehydro-chlorination rates and in patterns of resistance between anopheline species have been observed. In A. stephensi, these differences extend to the level of strains.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRIDGES R. G., HARRISON A., WINTERINGHAM F. P. Separation of the isomers of benzene hexachloride by reversed-phase paperpartition chromatography. Nature. 1956 Jan 14;177(4498):86–86. doi: 10.1038/177086a0. [DOI] [PubMed] [Google Scholar]
- BROOKS G. T. Mechanisms of resistance of the adult housefly (Musca domestica) to 'cyclodiene' insecticides. Nature. 1960 Apr 2;186:96–98. doi: 10.1038/186096a0. [DOI] [PubMed] [Google Scholar]
- DAVIDSON G., JACKSON C. E. DDT-resistance in Anopheles stephensi. Bull World Health Organ. 1961;25:209–217. [PMC free article] [PubMed] [Google Scholar]
- DAVIDSON G., MASON G. F. Genetics of mosquitoes. Annu Rev Entomol. 1963;8:177–196. doi: 10.1146/annurev.en.08.010163.001141. [DOI] [PubMed] [Google Scholar]
- DINAMARCA M. L., AGOSIN M., NEGHME A. The metabolic fate of C-14-DDT in Triatoma infestans. Exp Parasitol. 1962 Feb;12:61–72. doi: 10.1016/0014-4894(62)90070-x. [DOI] [PubMed] [Google Scholar]
- FINE B. C. Pattern of pyrethrin-resistance in houseflies. Nature. 1961 Aug 26;191:884–885. doi: 10.1038/191884a0. [DOI] [PubMed] [Google Scholar]
- GARMS R. [Insecticide resistance of Anopheles]. Z Tropenmed Parasitol. 1960 Oct;11:353–388. [PubMed] [Google Scholar]
- GARTRELL F. E., LUDVIK G. F. The role of insecticides in the TVA malaria control program. Am J Trop Med Hyg. 1954 Sep;3(5):817–820. doi: 10.4269/ajtmh.1954.3.817. [DOI] [PubMed] [Google Scholar]
- LIPKE H., KEARNS C. W. DDT dehydrochlorinase. I. Isolation, chemical properties, and spectrophotometric assay. J Biol Chem. 1959 Aug;234(8):2123–2128. [PubMed] [Google Scholar]
- Lipke H., Chalkley J. Glutathione, oxidized and reduced, in some dipterans treated with 1,1,1-trichloro-2,2-di-(p-chlorophenyl)ethane. Biochem J. 1962 Oct;85(1):104–109. doi: 10.1042/bj0850104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAPIDO H. Recent experiments on possible resistance to DDT by Anopheles albimanus in Panama. Bull World Health Organ. 1954;11(4-5):885–889. [PMC free article] [PubMed] [Google Scholar]


