Abstract
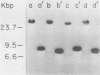
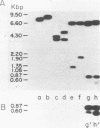
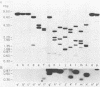
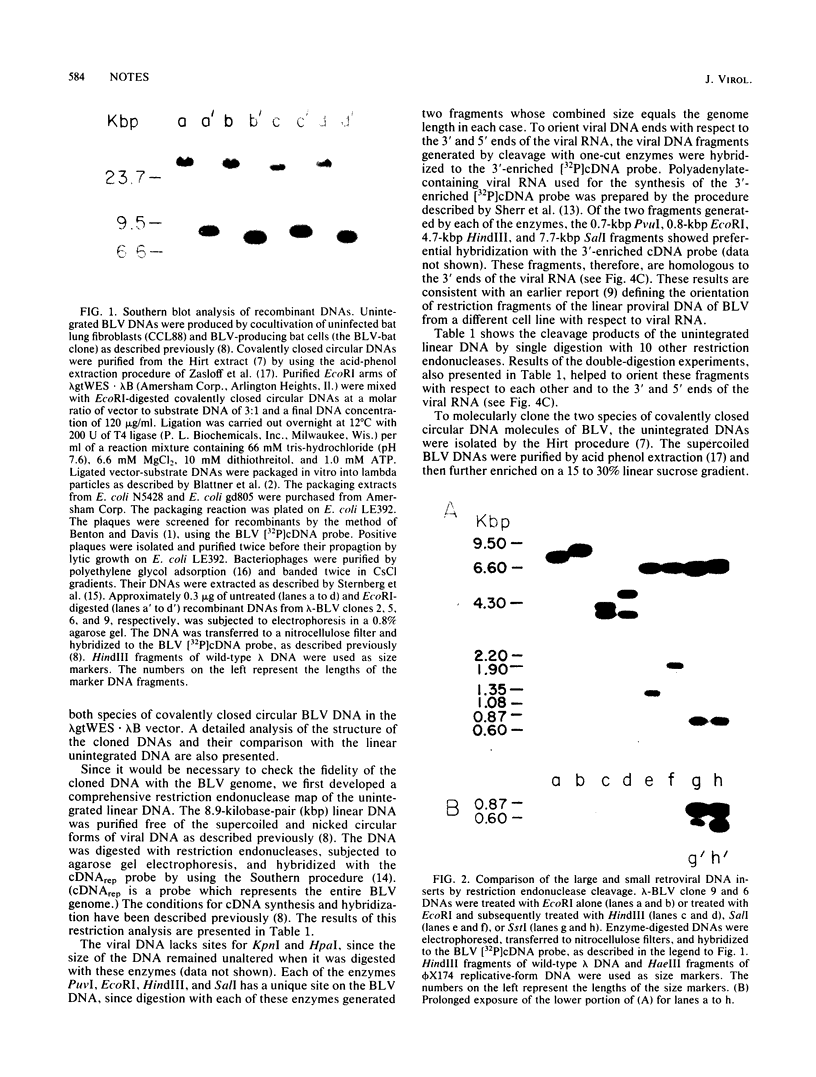
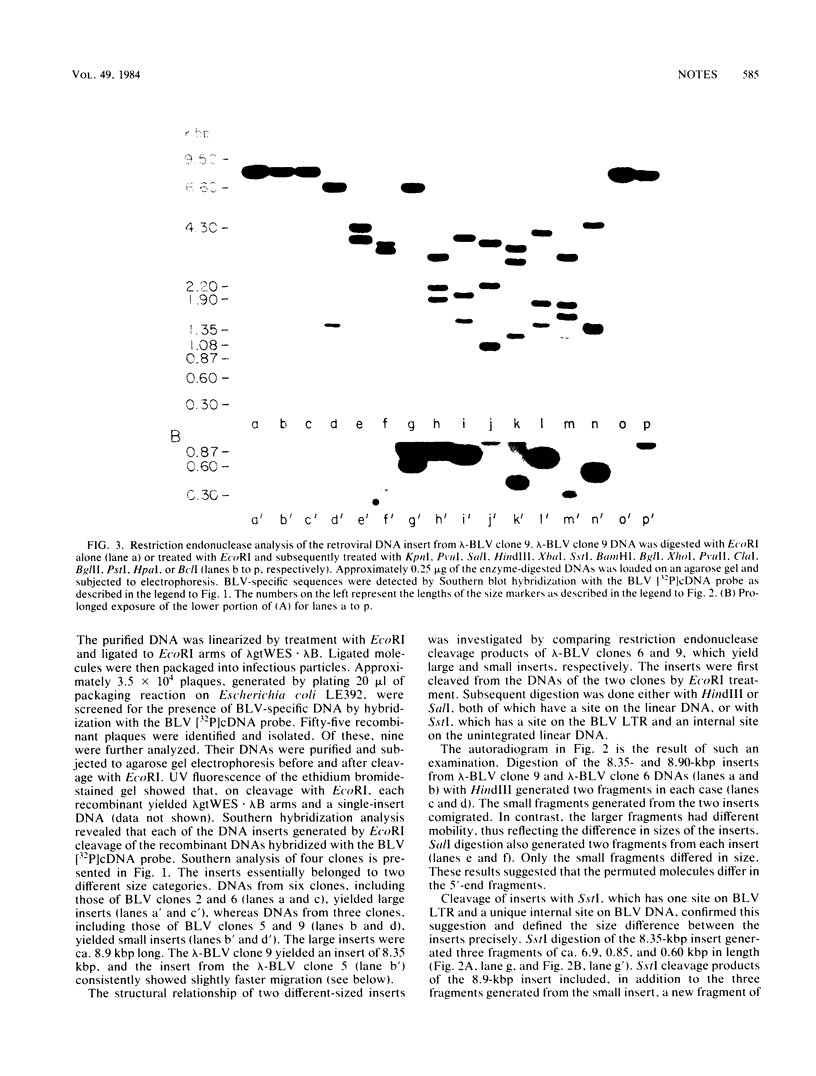
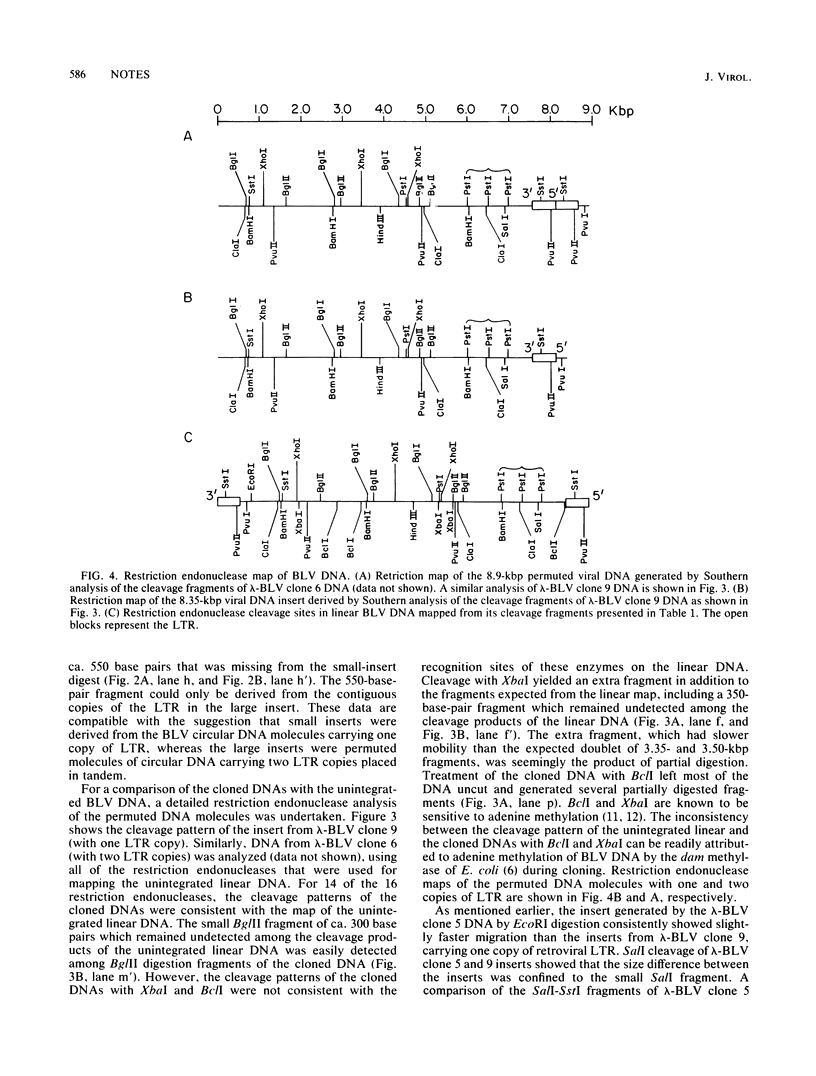
The two species of covalently closed circular DNA molecules of bovine leukemia virus were cloned in the lambda phage vector lambda gtWES X lambda B. Of the nine independent recombinant lambda-bovine leukemia virus clones that were analyzed, three were derived from the small and six were derived from the large circular molecules carrying, respectively, one and two copies of the long terminal repeat sequences. Comprehensive restriction endonuclease mapping of the unintegrated bovine leukemia virus and the cloned DNA molecules showed that eight of the nine clones carried viral information without any detectable deletions or insertions of more than ca. 50 base pairs. One of the nine clones, which carries a retroviral insert with one copy of the long terminal repeat, had a deletion of ca. 150 base pairs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Deschamps J., Kettmann R., Burny A. Experiments with cloned complete tumor-derived bovine leukemia virus information prove that the virus is totally exogenous to its target animal species. J Virol. 1981 Nov;40(2):605–609. doi: 10.1128/jvi.40.2.605-609.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer J. F. Bovine lymphosarcoma. Adv Vet Sci Comp Med. 1980;24:1–68. [PubMed] [Google Scholar]
- Hattman S., Brooks J. E., Masurekar M. Sequence specificity of the P1 modification methylase (M.Eco P1) and the DNA methylase (M.Eco dam) controlled by the Escherichia coli dam gene. J Mol Biol. 1978 Dec 15;126(3):367–380. doi: 10.1016/0022-2836(78)90046-3. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kashmiri S. V., Mehdi R., Ferrer J. F. Detection, purification, and characterization of two species of covalently closed circular proviral DNA molecules of bovine leukemia virus. J Virol. 1983 Mar;45(3):1172–1176. doi: 10.1128/jvi.45.3.1172-1176.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Couez D., Burny A. Restriction endonuclease mapping of linear unintegrated proviral DNA of bovine leukemia virus. J Virol. 1981 Apr;38(1):27–33. doi: 10.1128/jvi.38.1.27-33.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Deschamps J., Cleuter Y., Couez D., Burny A., Marbaix G. Leukemogenesis by bovine leukemia virus: proviral DNA integration and lack of RNA expression of viral long terminal repeat and 3' proximate cellular sequences. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2465–2469. doi: 10.1073/pnas.79.8.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M. The effect of site specific methylation on restriction endonuclease cleavage (update). Nucleic Acids Res. 1983 Jan 11;11(1):r169–r173. doi: 10.1093/nar/11.1.235-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Donner L., Turek L. P. Restriction endonuclease mapping of unintegrated proviral DNA of Snyder-Theilen feline sarcoma virus: localization of sarcoma-specific sequences. J Virol. 1979 Dec;32(3):860–875. doi: 10.1128/jvi.32.3.860-875.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Tiemeier D., Enquist L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene. 1977 May;1(3-4):255–280. doi: 10.1016/0378-1119(77)90049-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- Zasloff M., Ginder G. D., Felsenfeld G. A new method for the purification and identification of covalently closed circular DNA molcules. Nucleic Acids Res. 1978 Apr;5(4):1139–1152. doi: 10.1093/nar/5.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]