Abstract
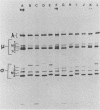
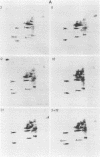
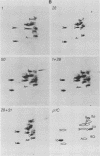
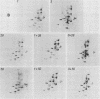
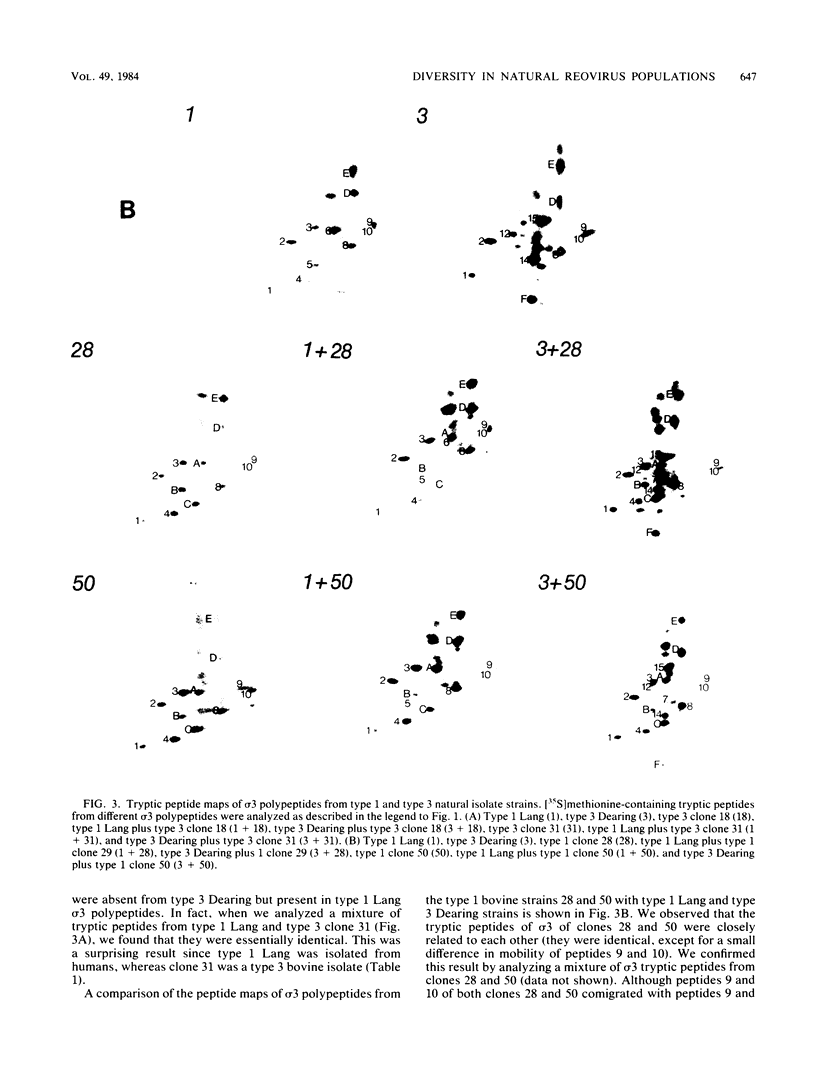
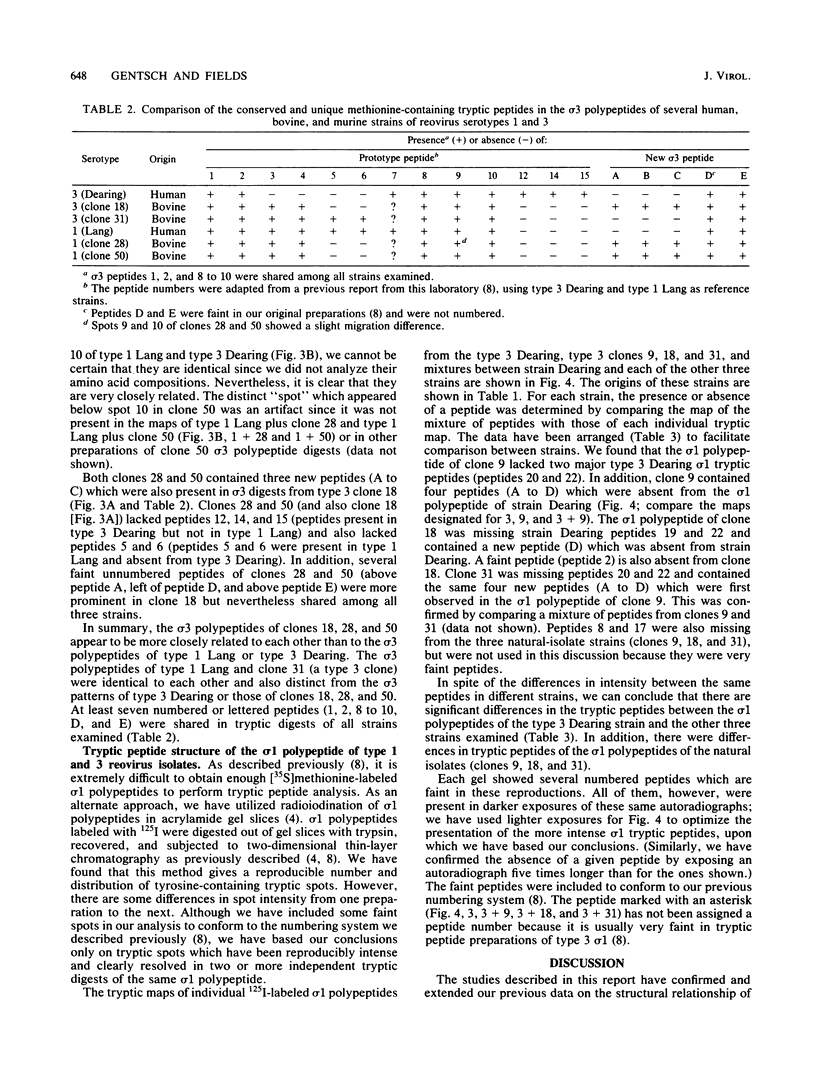
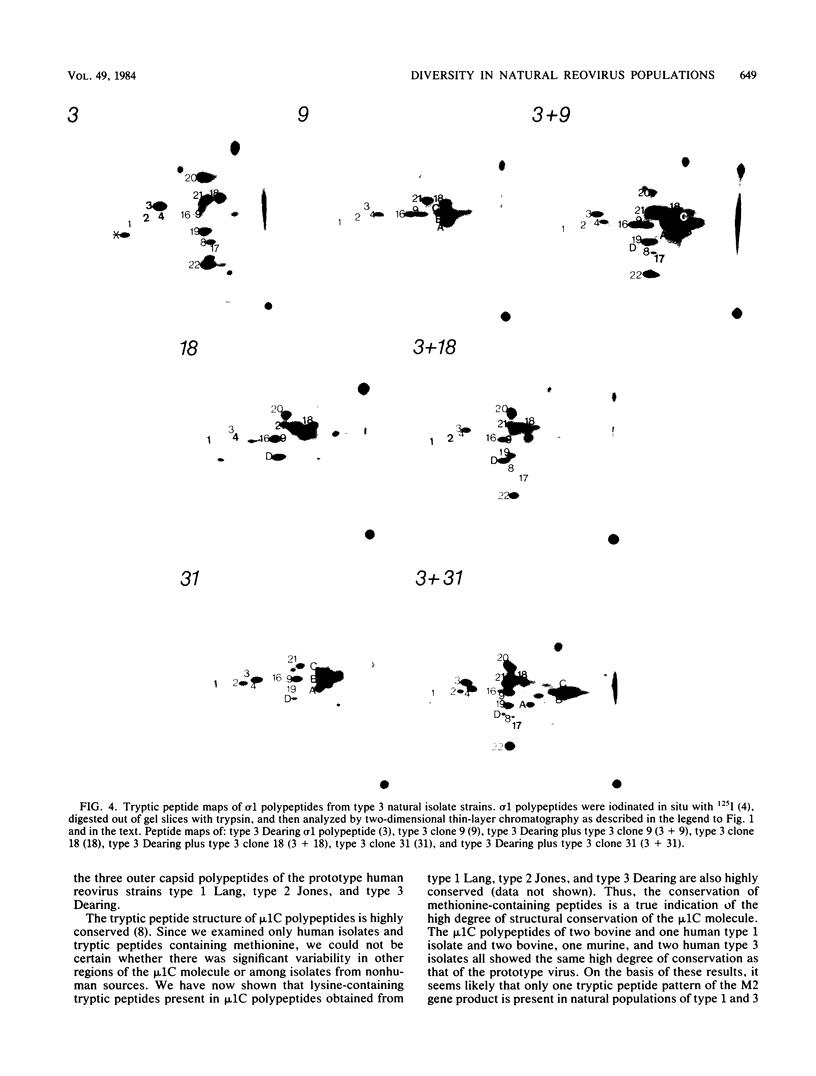
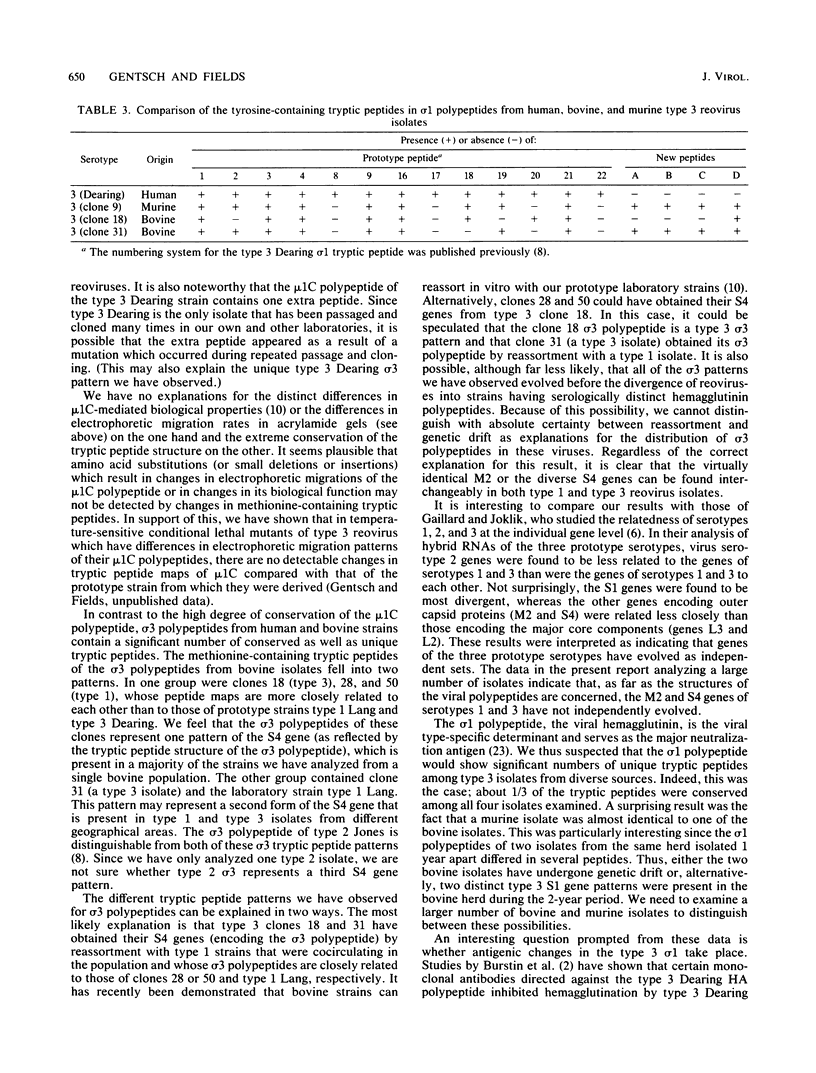
We have studied the structural relationships between the outer capsid polypeptides of eight murine, bovine, and human isolates of type 1 and 3 mammalian reoviruses. Our results show that the outer capsid polypeptides of reoviruses isolated from different mammalian species, in different years and different geographical areas, have both conserved and unique methionine-containing tryptic peptides. We found that tryptic peptides from mu 1C polypeptides of two human, one murine, and two bovine type 3 isolates and one human and two bovine type 1 reoviruses are highly conserved. Our data show that only one tryptic peptide pattern of the mu 1C polypeptide (encoded by the M2 gene) was present in reoviruses isolated from the three different mammalian species. The mu 1C polypeptide of the type 3 Dearing strain contained one tryptic peptide not found in any other reovirus isolate examined. In marked contrast to the mu 1C polypeptides, the sigma 3 polypeptides (encoded by the S4 gene) of three type 1 and three type 3 isolates were divided into two patterns based on significant differences in their tryptic peptides. In addition, at least seven tryptic peptides were conserved among the sigma 3 polypeptides of all virus strains examined. The sigma 3 polypeptide of the type 3 Dearing strain was distinguishable from the sigma 3 polypeptides of all other strains examined. The one mu 1C and two sigma 3 tryptic peptide patterns were found to occur interchangeably in isolates of type 1 or type 3. About 1/3 of the tyrosine-containing tryptic peptides of sigma 1 polypeptides of four type 3 isolates examined were conserved. Comparison of peptide differences in sigma 1 polypeptides of these isolates showed that each had one or more unique tryptic peptides, suggesting that the S1 genes coding for these polypeptides had undergone genetic drift or, alternatively, that there are at least two tryptic peptide patterns present among the sigma 1 polypeptides of these isolates. Our results suggest that genetic drift and reassortment are the most likely explanation for the extensive genetic diversity found in natural populations of mammalian reoviruses.
Full text
PDF

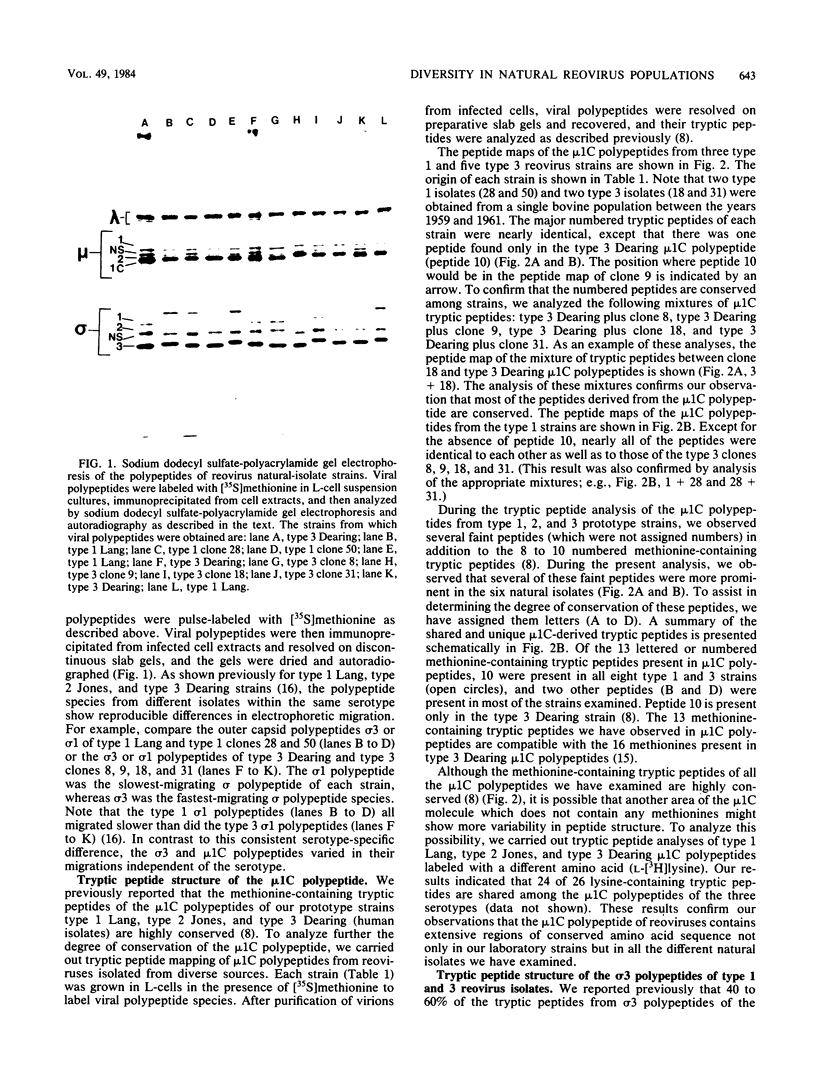
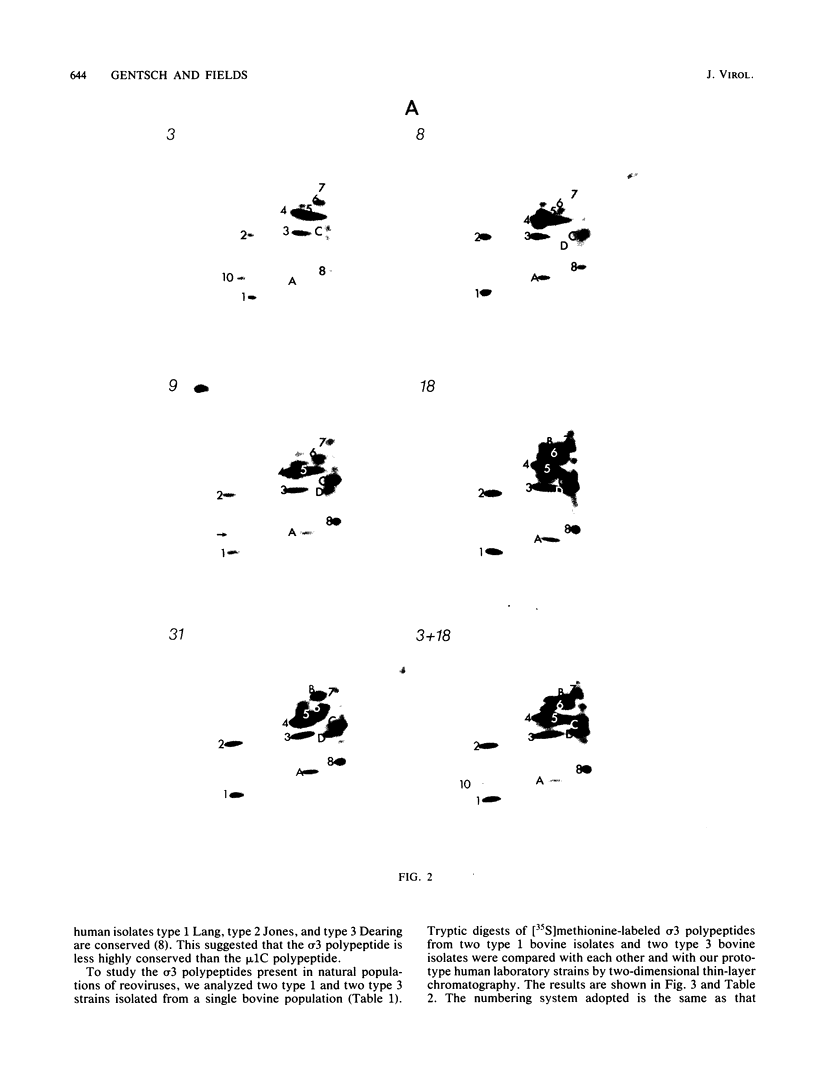
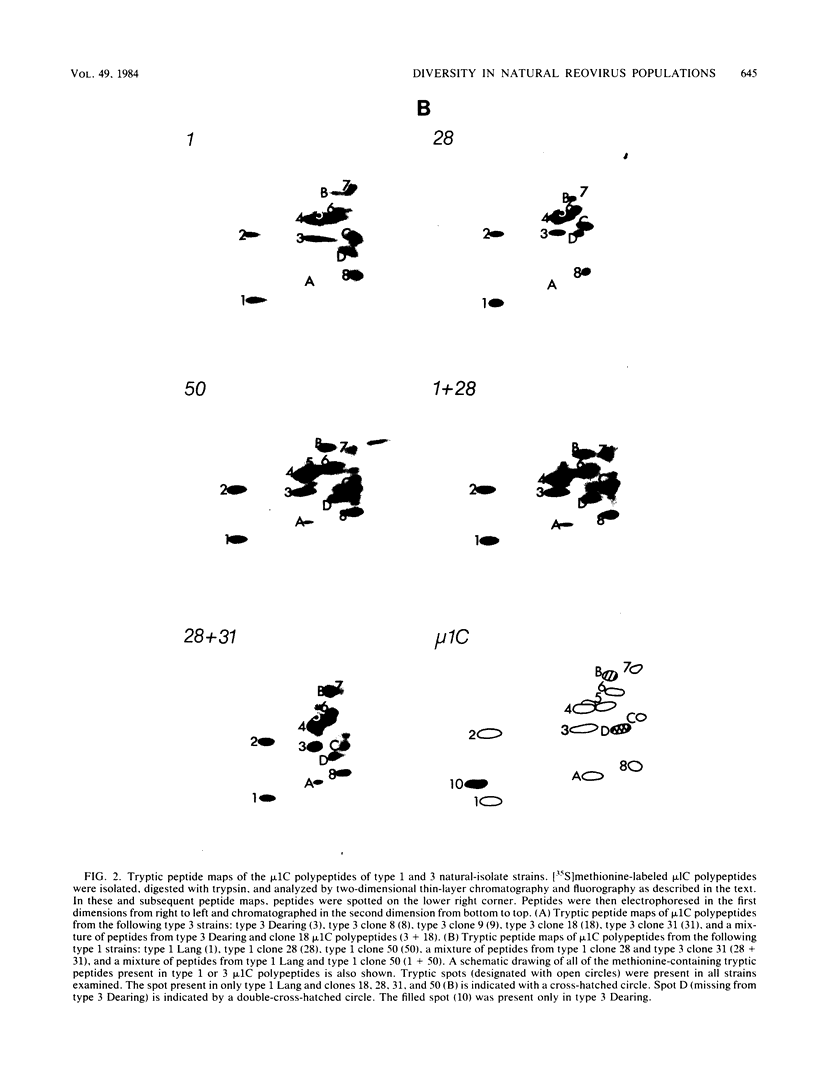
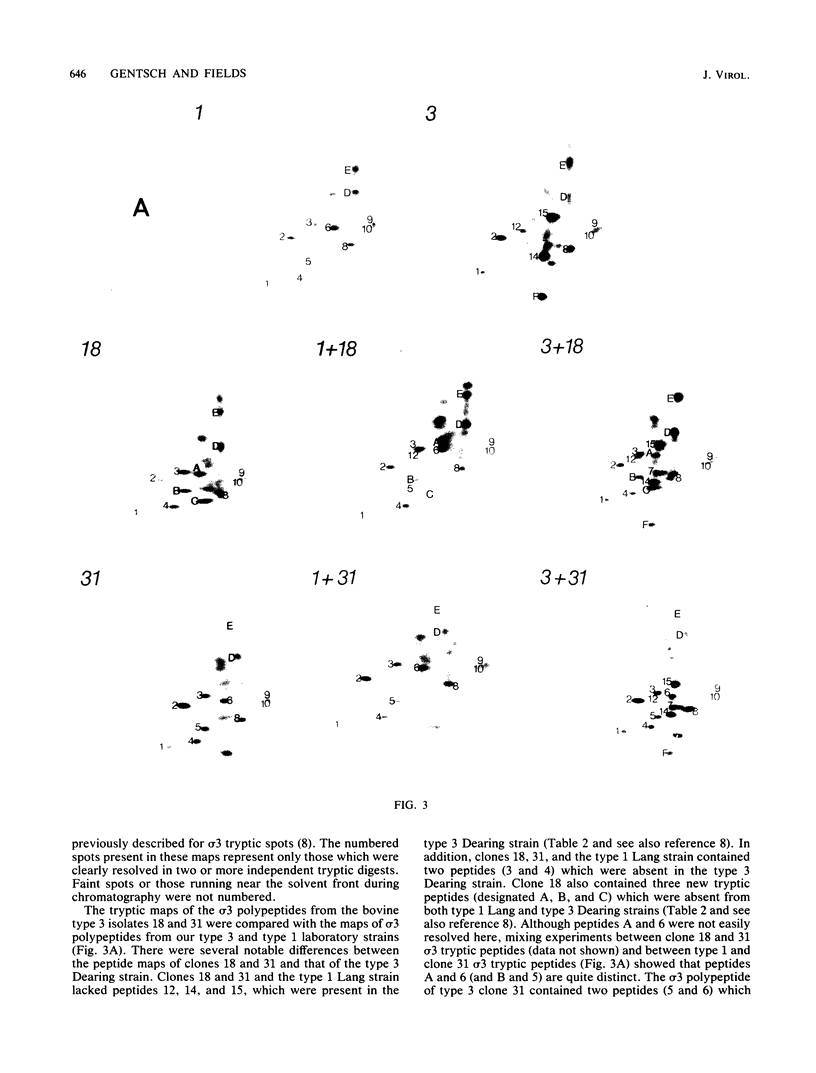

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burstin S. J., Spriggs D. R., Fields B. N. Evidence for functional domains on the reovirus type 3 hemagglutinin. Virology. 1982 Feb;117(1):146–155. doi: 10.1016/0042-6822(82)90514-1. [DOI] [PubMed] [Google Scholar]
- Cross R. K., Fields B. N. Reovirus-specific polypeptides: analysis using discontinuous gel electrophoresis. J Virol. 1976 Jul;19(1):162–173. doi: 10.1128/jvi.19.1.162-173.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Fields B. N., Joklik W. K. Isolation and preliminary genetic and biochemical characterization of temperature-sensitive mutants of reovirus. Virology. 1969 Mar;37(3):335–342. doi: 10.1016/0042-6822(69)90217-7. [DOI] [PubMed] [Google Scholar]
- Gaillard R. K., Jr, Joklik W. K. Quantitation of the relatedness of reovirus serotypes 1, 2, and 3 at the gene level. Virology. 1982 Nov;123(1):152–164. doi: 10.1016/0042-6822(82)90302-6. [DOI] [PubMed] [Google Scholar]
- Gentsch J. R., Bishop D. H. Small viral RNA segment of bunyaviruses codes for viral nucleocapsid protein. J Virol. 1978 Oct;28(1):417–419. doi: 10.1128/jvi.28.1.417-419.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch J. R., Fields B. N. Tryptic peptide analysis of outer capsid polypeptides of mammalian reovirus serotypes 1, 2, and 3. J Virol. 1981 Apr;38(1):208–218. doi: 10.1128/jvi.38.1.208-218.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdy D. B., Rosen L., Fields B. N. Polymorphism of the migration of double-stranded RNA genome segments of reovirus isolates from humans, cattle, and mice. J Virol. 1979 Jul;31(1):104–111. doi: 10.1128/jvi.31.1.104-111.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdy D. B., Rubin D. H., Fields B. N. Molecular basis of reovirus neurovirulence: role of the M2 gene in avirulence. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1298–1302. doi: 10.1073/pnas.79.4.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCrae M. A., Joklik W. K. The nature of the polypeptide encoded by each of the 10 double-stranded RNA segments of reovirus type 3. Virology. 1978 Sep;89(2):578–593. doi: 10.1016/0042-6822(78)90199-x. [DOI] [PubMed] [Google Scholar]
- Mustoe T. A., Ramig R. F., Sharpe A. H., Fields B. N. Genetics of reovirus: identification of the ds RNA segments encoding the polypeptides of the mu and sigma size classes. Virology. 1978 Sep;89(2):594–604. doi: 10.1016/0042-6822(78)90200-3. [DOI] [PubMed] [Google Scholar]
- Pett D. M., Vanaman T. C., Joklik W. K. Studies on the amino and carboxyl terminal amino acid sequences of reovirus capsid polypeptides. Virology. 1973 Mar;52(1):174–186. doi: 10.1016/0042-6822(73)90407-8. [DOI] [PubMed] [Google Scholar]
- RAMOS-ALVAREZ M., SABIN A. B. Enteropathogenic viruses and bacteria; role in summer diarrheal diseases of infancy and early childhood. J Am Med Assoc. 1958 May 10;167(2):147–156. doi: 10.1001/jama.1958.02990190001001. [DOI] [PubMed] [Google Scholar]
- ROSEN L. Serologic grouping of reoviruses by hemagglutination-inhibition. Am J Hyg. 1960 Mar;71:242–249. doi: 10.1093/oxfordjournals.aje.a120107. [DOI] [PubMed] [Google Scholar]
- Ramig R. F., Cross R. K., Fields B. N. Genome RNAs and polypeptides of reovirus serotypes 1, 2, and 3. J Virol. 1977 Jun;22(3):726–733. doi: 10.1128/jvi.22.3.726-733.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe A. H., Ramig R. F., Mustoe T. A., Fields B. N. A genetic map of reovirus. 1. Correlation of genome RNAs between serotypes 1, 2, and 3. Virology. 1978 Jan;84(1):63–74. doi: 10.1016/0042-6822(78)90218-0. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Spriggs D. R., Fields B. N. Attenuated reovirus type 3 strains generated by selection of haemagglutinin antigenic variants. Nature. 1982 May 6;297(5861):68–70. doi: 10.1038/297068a0. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Fields B. N. Neutralization of reovirus: the gene responsible for the neutralization antigen. J Exp Med. 1977 Nov 1;146(5):1305–1310. doi: 10.1084/jem.146.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Ramig R. F., Mustoe T. A., Fields B. N. Identification of the gene coding for the hemagglutinin of reovirus. Virology. 1978 May 15;86(2):581–584. doi: 10.1016/0042-6822(78)90099-5. [DOI] [PubMed] [Google Scholar]