Abstract
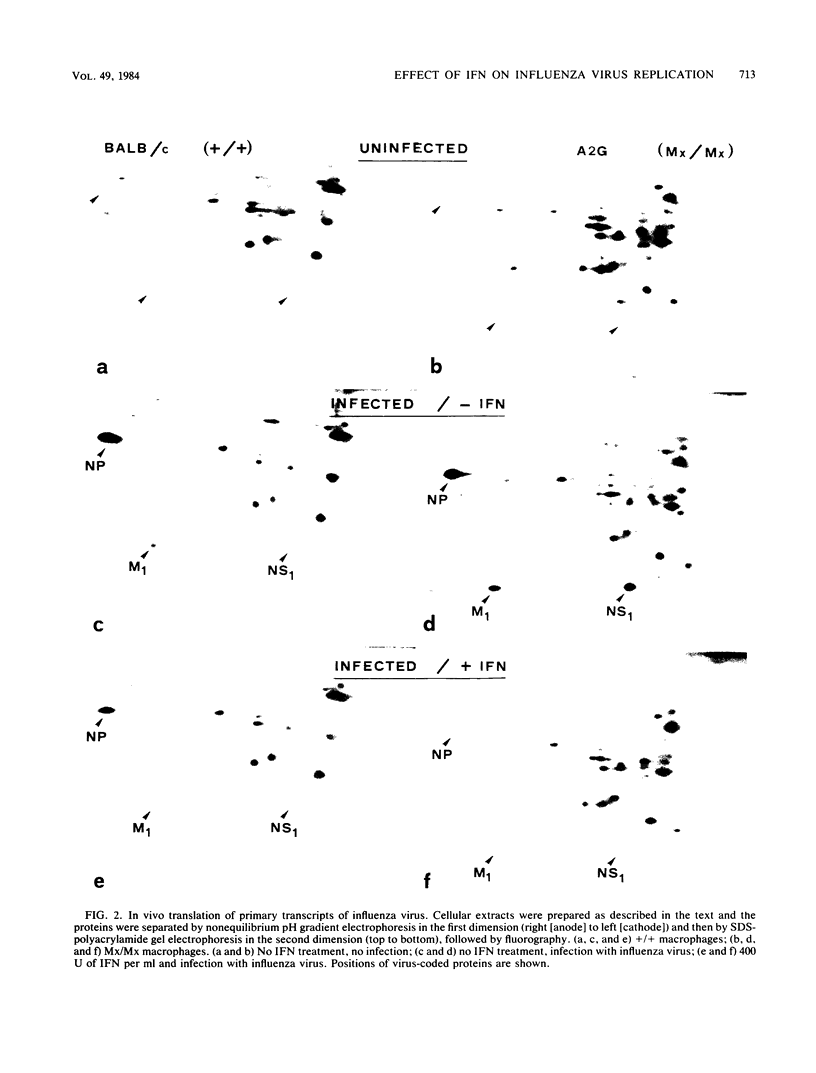
In mice, the combined action of alpha and beta interferons (IFNs) against influenza viruses is modulated by the host gene Mx. High concentrations of IFN fail to prevent efficiently the replication of influenza A virus in cultured macrophages lacking the gene Mx, whereas cultured macrophages carrying Mx develop strong antiviral activity even at low concentrations of IFN. Several steps in the replication cycle of influenza virus were compared in Mx/Mx and +/+ mouse macrophages treated with IFN-alpha + beta. Uncoating was not affected. A twofold reduction in the accumulation of primary transcripts was observed in IFN-treated macrophages at the highest concentration of IFN regardless of the genetic constitution of the host cell. No evidence was obtained for inhibition of influenza virus translation in macrophages which lacked Mx when treated with IFN-alpha + beta. In contrast, a marked shut-off of influenza virus polypeptide synthesis occurred in Mx-bearing macrophages treated with these IFNs, although the primary transcripts were active in directing the synthesis of viral polypeptides in a cell-free system. We concluded that a specific inhibitory mechanism for influenza virus translation was induced by IFN-alpha + beta in macrophages bearing the resistance gene Mx.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheiter H., Haller O., Lindenmann J. Host gene influence on interferon action in adult mouse hepatocytes: specificity for influenza virus. Virology. 1980 May;103(1):11–20. doi: 10.1016/0042-6822(80)90122-1. [DOI] [PubMed] [Google Scholar]
- Arnheiter H., Staeheli P. Expression of interferon dependent resistance to influenza virus in mouse embryo cells. Arch Virol. 1983;76(2):127–137. doi: 10.1007/BF01311696. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T., Wolstenholme A. J., Mahy B. W. Transcription and replication of influenza virus RNA. Virology. 1979 Oct 15;98(1):211–225. doi: 10.1016/0042-6822(79)90539-7. [DOI] [PubMed] [Google Scholar]
- Bean W. J., Jr, Simpson R. W. Primary transcription of the influenza virus genome in permissive cells. Virology. 1973 Dec;56(2):646–651. doi: 10.1016/0042-6822(73)90067-6. [DOI] [PubMed] [Google Scholar]
- Caton A. J., Robertson J. S. Structure of the host-derived sequences present at the 5' ends of influenza virus mRNA. Nucleic Acids Res. 1980 Jun 25;8(12):2591–2603. doi: 10.1093/nar/8.12.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Content J., De Wit L., Horisberger M. A. Peptide mapping characterization of viral proteins generated in a cell-free coupled system for the transcription and translation of influenza virus mRNA. J Virol. 1978 Jun;26(3):817–821. doi: 10.1128/jvi.26.3.817-821.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., Chanock R. M., Lai C. J. Nonviral oligonucleotides at the 5' terminus of cytoplasmic influenza viral mRNA deduced from cloned complete genomic sequences. Cell. 1980 Sep;21(2):495–500. doi: 10.1016/0092-8674(80)90486-9. [DOI] [PubMed] [Google Scholar]
- Etkind P. R., Krug R. M. Influenza viral messenger RNA. Virology. 1974 Nov;62(1):38–45. doi: 10.1016/0042-6822(74)90301-8. [DOI] [PubMed] [Google Scholar]
- Friedman R. M. Antiviral activity of interferons. Bacteriol Rev. 1977 Sep;41(3):543–567. doi: 10.1128/br.41.3.543-567.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass S. E., McGeoch D., Barry R. D. Characterization of the mRNA of influenza virus. J Virol. 1975 Dec;16(6):1435–1443. doi: 10.1128/jvi.16.6.1435-1443.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O., Arnheiter H., Lindenmann J., Gresser I. Host gene influences sensitivity to interferon action selectively for influenza virus. Nature. 1980 Feb 14;283(5748):660–662. doi: 10.1038/283660a0. [DOI] [PubMed] [Google Scholar]
- Haller O. Inborn resistance of ice to orthomyxoviruses. Curr Top Microbiol Immunol. 1981;92:25–52. doi: 10.1007/978-3-642-68069-4_3. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Lomniczi B., Bellamy A. R., Skehel J. J. Transcription of the influenza virus genome. Virology. 1977 Dec;83(2):337–355. doi: 10.1016/0042-6822(77)90179-9. [DOI] [PubMed] [Google Scholar]
- Herz C., Stavnezer E., Krug R., Gurney T., Jr Influenza virus, an RNA virus, synthesizes its messenger RNA in the nucleus of infected cells. Cell. 1981 Nov;26(3 Pt 1):391–400. doi: 10.1016/0092-8674(81)90208-7. [DOI] [PubMed] [Google Scholar]
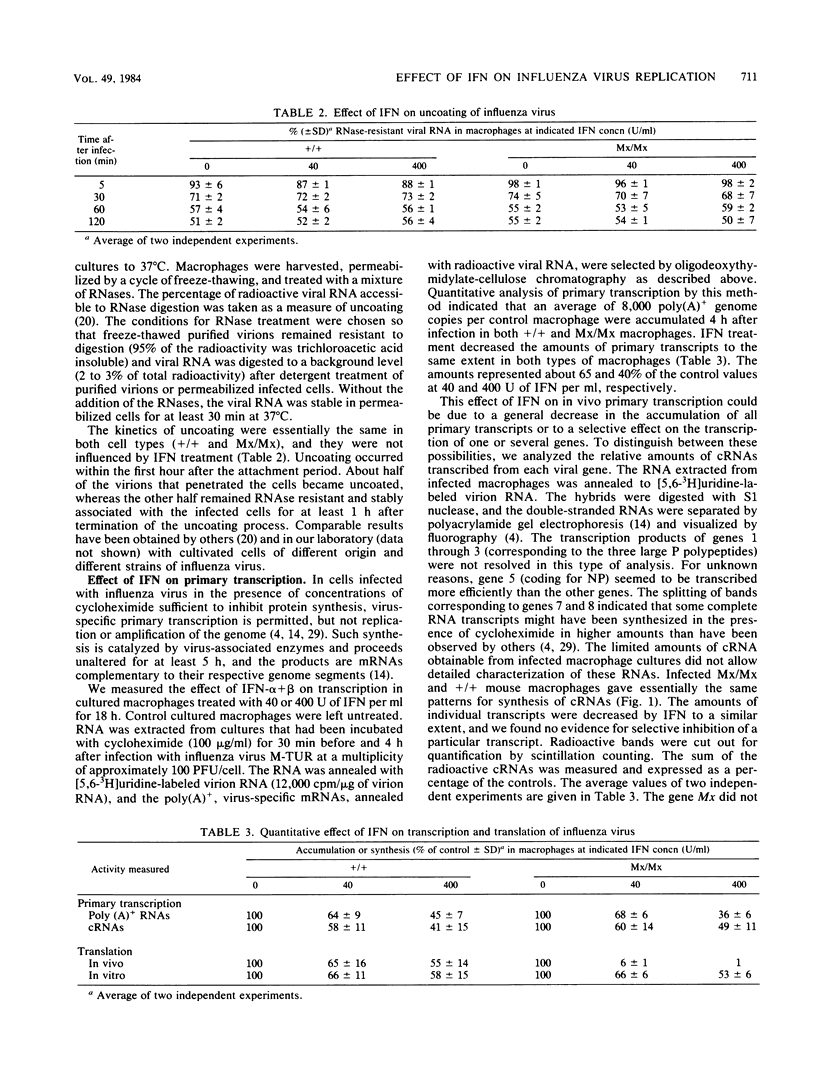
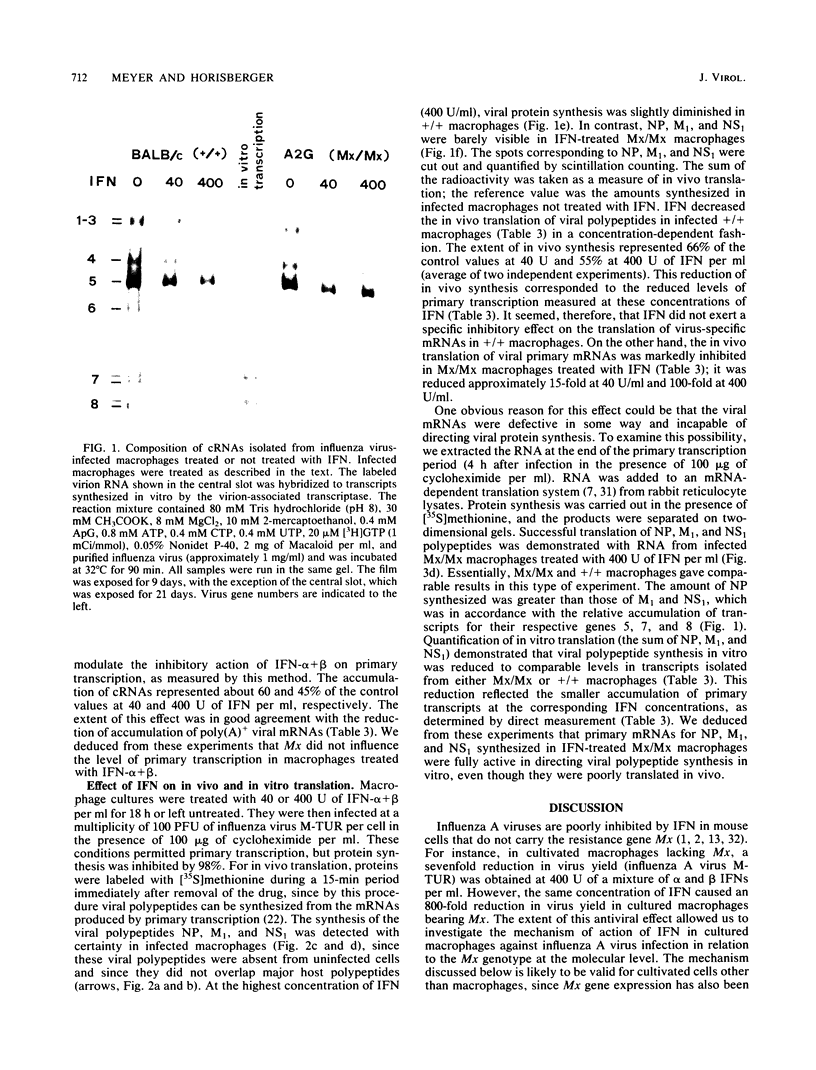
- Horisberger M. A., Haller O., Arnheiter H. Interferon-dependent genetic resistance to influenza virus in mice: virus replication in macrophages is inhibited at an early step. J Gen Virol. 1980 Sep;50(1):205–210. doi: 10.1099/0022-1317-50-1-205. [DOI] [PubMed] [Google Scholar]
- Horisberger M. A., Staeheli P., Haller O. Interferon induces a unique protein in mouse cells bearing a gene for resistance to influenza virus. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1910–1914. doi: 10.1073/pnas.80.7.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M. A. The large P proteins of influenza A viruses are composed of one acidic and two basic polypeptides. Virology. 1980 Nov;107(1):302–305. doi: 10.1016/0042-6822(80)90296-2. [DOI] [PubMed] [Google Scholar]
- Koff W. C., Knight V. Inhibition of influenza virus uncoating by rimantadine hydrochloride. J Virol. 1979 Jul;31(1):261–263. doi: 10.1128/jvi.31.1.261-263.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R. M., Broni B. A., Bouloy M. Are the 5' ends of influenza viral mRNAs synthesized in vivo donated by host mRNAs? Cell. 1979 Oct;18(2):329–334. doi: 10.1016/0092-8674(79)90052-7. [DOI] [PubMed] [Google Scholar]
- LINDENMANN J., LANE C. A., HOBSON D. THE RESISTANCE OF A2G MICE TO MYXOVIRUSES. J Immunol. 1963 Jun;90:942–951. [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Identification of a second protein (M2) encoded by RNA segment 7 of influenza virus. Virology. 1981 Jul 30;112(2):729–737. doi: 10.1016/0042-6822(81)90317-2. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Synthesis of influenza virus proteins in infected cells: translation of viral polypeptides, including three P polypeptides, from RNA produced by primary transcription. Virology. 1976 Oct 15;74(2):504–519. doi: 10.1016/0042-6822(76)90356-1. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J. Spliced and unspliced messenger RNAs synthesized from cloned influenza virus M DNA in an SV40 vector: expression of the influenza virus membrane protein (M1). Virology. 1982 Dec;123(2):237–256. doi: 10.1016/0042-6822(82)90258-6. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology. 1971 Dec;46(3):830–843. doi: 10.1016/0042-6822(71)90084-5. [DOI] [PubMed] [Google Scholar]
- Lin B. C., Lai C. J. The influenza virus nucleoprotein synthesized from cloned DNA in a simian virus 40 vector is detected in the nucleus. J Virol. 1983 Jan;45(1):434–438. doi: 10.1128/jvi.45.1.434-438.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmann J., Deuel E., Fanconi S., Haller O. Inborn resistance of mice to myxoviruses: macrophages express phenotype in vitro. J Exp Med. 1978 Feb 1;147(2):531–540. doi: 10.1084/jem.147.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark G. E., Taylor J. M., Broni B., Krug R. M. Nuclear accumulation of influenza viral RNA transcripts and the effects of cycloheximide, actinomycin D, and alpha-amanitin. J Virol. 1979 Feb;29(2):744–752. doi: 10.1128/jvi.29.2.744-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Repik P., Flamand A., Bishop D. H. Effect of interferon upon the primary and secondary transcription of vesicular stomatitis and influenza viruses. J Virol. 1974 Nov;14(5):1169–1178. doi: 10.1128/jvi.14.5.1169-1178.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. S., Schubert M., Lazzarini R. A. Polyadenylation sites for influenza virus mRNA. J Virol. 1981 Apr;38(1):157–163. doi: 10.1128/jvi.38.1.157-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Hay A. J. Nucleotide sequences at the 5' termini of influenza virus RNAs and their transcripts. Nucleic Acids Res. 1978 Apr;5(4):1207–1219. doi: 10.1093/nar/5.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J. Polypeptide synthesis in influenza virus-infected cells. Virology. 1972 Jul;49(1):23–36. doi: 10.1016/s0042-6822(72)80004-7. [DOI] [PubMed] [Google Scholar]
- Tovey M. G., Begon-Lours J., Gresser I. A method for the large scale production of potent interferon preparations. Proc Soc Exp Biol Med. 1974 Jul;146(3):809–815. doi: 10.3181/00379727-146-38196. [DOI] [PubMed] [Google Scholar]