Abstract
Previous research has suggested that perceivers spontaneously extract trait-specific information from the behaviour of others. However, little is known about whether perceivers spontaneously engage in the same depth of social-cognitive processing for all person information or reserve such processing specifically for information that conveys diagnostic clues about another person's dispositions. Moreover, a question remains as to whether the processing of such nondiagnostic information can be affected by perceivers’ explicit goal to consider another's dispositions or not. To examine processing of diagnostic and nondiagnostic social information as a function of perceivers’ explicit social-cognitive goals, participants underwent functional magnetic resonance imaging (fMRI) scanning while performing social (impression formation) or non-social orienting tasks using statements that conveyed either diagnostic or nondiagnostic information about the target's personality traits. Replicating two earlier studies, results identified a region of dorsal medial prefrontal cortex (mPFC) that was preferentially activated by impression formation. Interestingly, no difference between trait-diagnostic and nondiagnostic information was observed when participants had the explicit goal of forming an impression, but a substantial effect of diagnosticity emerged when task instructions oriented them away from considering the target as a social agent. These results suggest that trait-nondiagnostic information is not subject to spontaneous social-cognitive processing, but that such processing may nevertheless occur when perceivers have the explicit goal to use that information to form an impression of a target.
Recent research has consistently observed a distinct set of brain regions that preferentially activates during social-cognitive tasks that require thinking of another as a social agent. These regions—which include areas of the medial frontal cortex, superior temporal sulcus (STS), temporal poles and lateral parietal cortex (such as temporal–parietal junction)—have been implicated in a wide range of tasks that require participants to infer the mental states of others, such as understanding stories that require reference to another's beliefs (Fletcher et al., 1995; Gallagher et al., 2000; Saxe and Kanwisher, 2003; Saxe and Wexler, 2005) or feelings (Mitchell et al., 2005); speculating about another's knowledge (Goel et al., 1995) and competing or cooperating with a person in a computerized game (McCabe et al., 2001; Gallagher et al., 2002). For recent reviews, see Gallagher and Frith (2003) and Blakemore et al. (2004).
Recently, we reported a similar dissociation in dorsal aspects of the medial prefrontal cortex (mPFC) when perceivers considered less transient aspects of another person's mind, namely, their dispositional traits. Across two studies (Mitchell et al., 2004, 2005), we observed greater activation in dorsal mPFC when participants used experimentally provided statements to form an impression of a person (i.e. an inherently social judgement) than when they attended to the sequence in which statements were being presented (i.e. a relatively less social judgement). Importantly, this effect was obtained only when participants engaged in the social act of considering another's personality and does not extend to attempts to form impressions of inanimate objects (Mitchell et al., 2005).
In these earlier studies, stimulus statements described actions that were inherently diagnostic clues to a target's personality (e.g. ‘he played his music loud at the public picnic grounds’; ‘he finished the New York Times crossword puzzle in only 10 minutes’; ‘he went out of his way to meet someone of a different background’). Using exactly these kinds of stimuli, researchers have repeatedly demonstrated that perceivers make spontaneous and unintended use of such diagnostic information to infer the dispositional traits of others (for reviews, see Uleman et al., 1996, 2005). For example, after reading that John played his music loud in public, perceivers spontaneously infer that John is inconsiderate. Moreover, such spontaneous trait inferences seem to occur under a range of conditions in which perceivers are not explicitly oriented towards trying to understand the mind of another person (Uleman and Moskowitz, 1994; Todorov and Uleman, 2003). In other words, perceivers need not expressly intend to make meaning of another's internal mental states for the automatic inference of traits to occur.
However, many of the actions performed by a person in everyday life do not as readily communicate diagnostic information about his or her personality. Most often, we encounter people engaging in far more mundane behaviours: someone getting on the subway, ordering a cup of coffee at Starbucks, depositing a check at an ATM, etc. Relatively little is known about the kind of social processing perceivers perform when they come into contact with such everyday actions. Do perceivers spontaneously attempt to glean meaning from even such trait-impoverished behaviours as someone attempting to hail a cab or waiting for the walk signal at a busy intersection? Or does deep consideration of such mundane behaviours require particular processing goals, such as intentional, conscious attempts to form an impression of a target?
These questions have been notoriously difficult to address using established experimental paradigms. In large part, this difficulty arises because the evidence that perceivers spontaneously process information about a target deeply enough to infer his or her traits has typically come from demonstrations that perceivers later make use of those inferences (e.g. in studies where a trait term serves as a useful memory cue for other aspects of the person). However, this approach has necessarily left open the question of whether the perceivers spontaneously engage in the same deep analysis of all behaviours or only those that intrinsically convey diagnostic trait information. However, extant research on the neural basis of social cognition now makes it possible to circumvent the constraints of behavioural methods through the use of neuroimaging. As discussed above, tasks that require an explicit consideration of another person's transient or dispositional mental states—such as forming an impression of another person—have been linked to a specific pattern of brain activity that has consistently implicated the medial frontal cortex in tasks that require perceivers to infer the mental characteristics of others. The ubiquity of medial frontal activation during such mentalizing tasks over the last decade suggests that modulation in this region can serve as a kind of neural indication that perceivers have engaged in elaborative social-cognitive processing. Accordingly, the presence or absence of increased medial frontal activity between tasks can be used as a marker of whether one task prompts greater social-cognitive processing than another.
We capitalized on these neural observations to examine the extent to which spontaneous social-cognitive processing accompanies the presentation of nondiagnostic information about a target. Participants in the current study were presented with a series of unfamiliar target individuals, each of whom was described by a series of trait-diagnostic and trait-nondiagnostic statements. As in our previous work, for some targets, participants were instructed to use the statements to form an impression of the target individual (impression formation task); for other targets, participants were instructed to encode the order in which statements were paired with a particular individual (sequencing task). In doing so, we asked two inter-related empirical questions. First, do perceivers automatically attempt to leverage all observed behaviours into inferences about a target or does such spontaneous social-cognitive processing only accompany inherently trait-diagnostic behaviours? Second, does the extent of social-cognitive processing of nondiagnostic information depend on a perceiver's goal when encountering another person? That is, if perceivers do not engage in deep social-cognitive processing of trait-nondiagnostic information in a spontaneous manner, can they do so more deliberately when they have the explicit goal to make sense of another person?
METHOD
Participants
Participants were 15 (11 female) right-handed, native English speakers with no history of neurological problems (mean age 19.6 years, range 18.4–22.9). Informed consent was obtained in a manner approved by the Committee for the Protection of Human Subjects at Dartmouth College.
Stimuli and behavioural procedure
Stimuli consisted of 360 statements that conveyed trait-diagnostic information about a person. Each of these statements described an action that had previously been normed to imply one of the 24 different personality traits (15 statements per trait). Half the traits were positive, such as considerate (‘he spent 2 hours showing his cousin how to set up his personal computer’) and motivated (‘he turned down three parties to study for organic chemistry’). The remaining half of the traits were the negative aspect of the same dimension, such as inconsiderate (‘he refused to loan his extra blanket to the other campers’) and lazy (‘he watched TV all day instead of looking for a job’). The 12 different personality dimensions were motivated–lazy, outgoing–introverted, funloving–boring, confident–unconfident, considerate–inconsiderate, cultured–uncultured, honest–dishonest, forgiving–unforgiving, cautious–reckless, intelligent–unintelligent, responsible–irresponsible and generous–stingy. In addition, the stimulus set included 60 trait-nondiagnostic statements that effectively conveyed no information that could be used to form an impression of a person. Examples of such trait-nondiagnostic statements included ‘he bought a new set of highlighters’; ‘he spent the Fourth of July at the beach’; ‘he opened his mail upon getting home’ and ‘he photocopied the article’.
During scanning, statements were paired with 16 faces (Caucasian males photographed against a blue background). Each trial consisted of a face-statement pair presented for 5500 ms. Each pair was accompanied by one of the two cues (Form Impression, Remember Order) that indicated, respectively, whether the impression formation or sequencing task was to be performed on that trial. In line with earlier behavioural (Hastie and Kumar, 1979; Hamilton et al., 1980, 1989; Srull and Wyer, 1989; Wyer et al., 1984) and neuroimaging (Mitchell et al., 2004, 2005) studies, for impression formation trials, participants were instructed to use the statement to generate an opinion about the person or object. Participants were told that, for these trials, their opinion about each target would later be measured. For sequencing trials, participants were instructed to encode the order in which statements were paired with each target. Participants were told that, for these trials, their memory for the sequences would later be tested. In actual fact, no such tests were administered.
Functional scanning took place over two separate runs. In each run, eight faces were each presented 15 times (60 impression formation and 60 sequencing trials). Across presentations, a given face was consistently associated with the same orienting task, although a different descriptive statement accompanied each presentation of a face. For each face, five trials were trait-diagnostic statements that suggested a single positive personality trait (e.g. considerate), five trials were trait-diagnostic statements that suggested a single negative trait (e.g. inconsiderate) and five trials were trait-nondiagnostic statements. No significant differences were observed between positive and negative statements and thus results were collapsed across statement valence. To optimize estimation of the event-related functional magnetic resonance imaging (fMRI) response, trials were intermixed in a pseudorandom order and separated by a variable interstimulus interval (500–9500 ms; Dale, 1999). During interstimulus intervals, participants passively viewed a fixation crosshair.
Immediately prior to each functional run, participants completed a brief practice session. Practice sessions comprised a random order of 20 impression formation and 20 sequencing trials, during which participants saw each of the eight faces that were to be presented during the subsequent run paired with five trait-diagnostic statements. These statements implied one of the same traits that were later associated with that same face. For example, if a face were later to be described by statements that implied the traits honesty and dishonesty, the five practice trials might all imply honesty. Whether the practice trials converged on the positive or negative trait was determined randomly, and thus for another participant the five practice trials might all imply dishonesty. No significant differences were observed between statements that implied the same trait as the one during practice and those that implied the opposite trait; accordingly, all analyses are reported collapsed across this factor.
Imaging procedure
Imaging was conducted using a 1.5 Tesla Siemens Sonata scanner. We first collected a high-resolution T1-weighted structural scan (MP-RAGE) followed by two functional runs of 440 volume acquisitions (25 axial slices; 5 mm thick; 1 mm skip). Functional scanning used a gradient-echo echo-planar pulse sequence (TR = 2 s; TE = 40 ms; 3.75 × 3.75 in-plane resolution). Stimuli were projected onto a screen at the end of the magnet bore that participants viewed by way of a mirror mounted on the head coil. Stimulus presentation was controlled by PsyScope software (Cohen et al., 1993).
The fMRI data were preprocessed and analysed using SPM99 (Wellcome Department of Cognitive Neurology, London, UK). First, functional data were time-corrected for differences in acquisition time between slices for each whole-brain volume and realigned to correct for head movement. Data were then transformed into a standard anatomical space (3 mm isotropic voxels) based on the ICBM 152 brain template (Montreal Neurological Institute), which approximates Talairach and Tournoux atlas space. Normalized data were then spatially smoothed [8 mm full-width-at-half-maximum (FWHM)] using a Gaussian kernel.
Statistical analyses were performed using the general linear model in which the event-related design was modelled using a canonical haemodynamic response function, its temporal derivative and covariates of no interest (a session mean and a linear trend). Comparisons of interest were implemented as linear contrasts using a random-effects model. A voxel-based statistical threshold of P < 0.005 was used for all comparisons; regions of interest (ROIs) were required to exceed 100 contiguous voxels in extent (providing an α-level of P < 0.05, corrected) for all contrasts. Statistical comparisons between conditions were conducted using analysis of variance (ANOVA) procedures on the parameter estimates associated with each trial type.
RESULTS
Differences between orienting tasks
We first conducted a whole-brain, random-effects analysis contrasting impression formation > sequencing, regardless of statement diagnosticity. Replicating our earlier findings (Mitchell et al., 2004, 2005), impression formation trials were associated with reliably greater activation (compared with sequencing trials) in a single location: dorsal mPFC (Table 1). This region was distributed as a fairly extensive arc (comprising 140 voxels) along the medial banks of the superior frontal gyrus bilaterally. No other brain regions were identified by this contrast.
Table 1.
Coordinates of peak activations and number of voxels for regions obtained from comparisons between orienting tasks (P < 0.05, corrected)
| Anatomical label | X | Y | Z | Max. t | Voxels |
|---|---|---|---|---|---|
| Impression formation > sequencing | |||||
| Dorsal mPFC | −12 | 24 | 63 | 6.10 | 140 |
| −12 | 39 | 51 | 5.65 | ||
| −9 | 33 | 60 | 4.85 | ||
| Sequencing > impression formation | |||||
| Post-central gyrus | 51 | −45 | 54 | 5.72 | 156 |
| 57 | −48 | 42 | 5.29 | ||
| Superior frontal gyrus | 27 | 3 | 54 | 4.41 | 119 |
Note: t-tests reflect the statistical difference between the two conditions, as computed by SPM99. Coordinates refer to the Montreal Neurological Institute stereotaxic space. For each region, a number of individual, local peak activations are reported along with the overall number of voxels in the region.
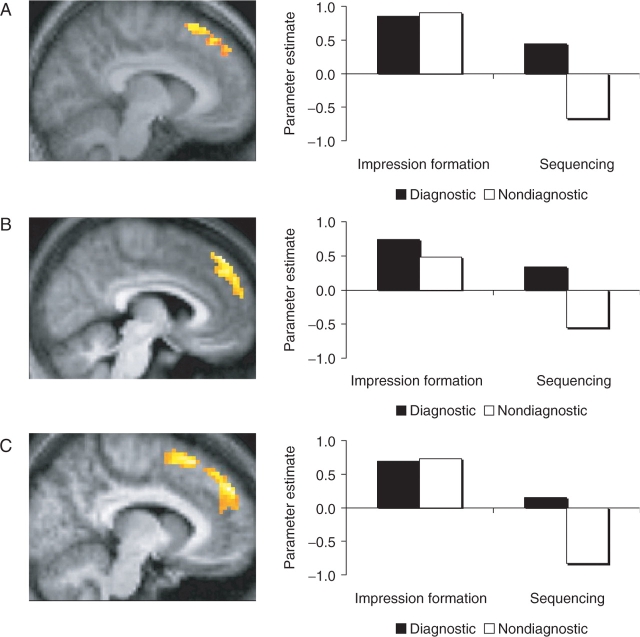
Subsequently, we examined the pattern of responses across all trials in this dorsal mPFC region. The parameter estimates associated with each trial type were entered into a two-way ANOVA. The pattern of activity in this region demonstrated a significant main effect of statement diagnosticity, F(1,14) = 17.38, P < 0.001, such that greater overall activation was observed for trait-diagnostic than nondiagnostic statements. Moreover, as displayed in Figure 1A, these main effects were qualified by a significant two-way interaction of orienting task and statement diagnosticity, F(1,14) = 6.97, P < 0.02, suggesting that the difference between impression formation and sequencing varied as a function of statement diagnosticity. Whereas the sequencing task was associated with significantly greater activity for diagnostic than nondiagnostic statements, t(14) = 4.04, P < 0.002, the impression formation task was associated with nearly identical levels of activity for the two types of statements, t(14) = 0.21, P = 0.84. Likewise, whereas the difference between orienting tasks was highly significant for nondiagnostic statements, t(14) = 4.40, P < 0.001, only a marginal difference between impression formation and sequencing was obtained for diagnostic statements, t(14) = 1.92, P < 0.08, two-tailed. Lastly, whereas impression formation trials were associated with significant activation above baseline regardless of diagnosticity (both P-values <0.005), the sequencing task was associated with only a marginally significant activation for diagnostic statements and a marginally significant deactivation for nondiagnostic statements (both P- values <0.07, two-tailed).1
Fig. 1.
An extensive region of dorsal mPFC was obtained from the contrast of impression formation > sequencing and is displayed on a sagittal (x = −6) slice of subjects’ mean normalized brain (Panel A). Analysis of the parameter estimates associated with trial types revealed main effects of both orienting task (impression formation vs sequencing) and statement diagnosticity (diagnostic vs nondiagnostic), as well as a significant two-way interaction. Specifically, for trials encountered as part of the impression formation task (left set of bars), no difference was observed between diagnostic and nondiagnostic statements. In contrast, for trials encountered as part of the sequencing task (right set of bars), a significant effect of diagnosticity was observed. Qualitatively similar results were obtained in regions-of-interest defined in earlier research on the neural basis of impression formation: Mitchell et al. (2004; Panel B) and Mitchell et al. (2005; Panel C).
To examine differences among trial types in the exact regions previously associated with impression formation, we also interrogated the same dorsal mPFC regions reported in our earlier work (Mitchell et al., 2004, 2005). Qualitatively similar effects were obtained in these regions, although the effects were numerically weaker in the region obtained in our first study than our second one. Specifically, we observed significant main effects of both orienting task and diagnosticity from the dorsal mPFC region defined from our first study (Figure 1B; both P- values <0.02) as well as the region defined from our second study (Figure 1C; both P- values <0.01). However, the two-way interaction of orienting task × diagnosticity reached statistical significance only for the region defined from the second study (P < 0.01).
The opposite contrast, sequencing > impression formation, produced differences in post-central gyrus and superior frontal gyrus (Table 1). These two regions corresponded closely (peak activations within a few voxels) to the activations observed for the identical contrast in both of our earlier studies (Mitchell et al., 2004, 2005).
Differences between trait-diagnostic and nondiagnostic statements
We next examined the overall pattern of brain activation during the processing of diagnostic social information by contrasting diagnostic > nondiagnostic statements. This contrast revealed four regions of activation: dorsal mPFC; a right-lateralized region that extended from the right STS to the temporal pole; an extensive region along the entire length of the left STS and bilateral occipital cortex (Table 2).
Table 2.
Coordinates of peak activations of regions obtained from the direct contrast of diagnostic > nondiagnostic statements (P < 0.05, corrected)
| Anatomical label | X | Y | Z | Max. t | Voxels |
|---|---|---|---|---|---|
| Dorsal mPFC | 12 | 36 | 57 | 4.87 | 155 |
| −3 | 48 | 48 | 4.75 | ||
| −9 | 36 | 54 | 4.53 | ||
| Right STS | 51 | 15 | −27 | 5.03 | 133 |
| 60 | −6 | −30 | 4.60 | ||
| 51 | −12 | −30 | 4.13 | ||
| Left STS† | −54 | −42 | 0 | 8.13 | 618 |
| −57 | −54 | 21 | 6.58 | ||
| −60 | −12 | −18 | 5.95 | ||
| Occipital cortex† | 18 | −99 | −6 | 8.08 | 558 |
| −15 | −96 | −9 | 7.85 | ||
| −21 | −102 | −9 | 7.53 |
†Regions for which the additional activity associated with diagnostic statements corresponded to differences associated with statement length.
However, post-hoc analyses indicated that, on average, trait-diagnostic statements were longer than trait-nondiagnostic statements (M = 59.8 and 33.9 characters, respectively). To examine whether statement length accounted for the activations in the four regions that were more engaged for diagnostic than nondiagnostic statements, we conducted two additional analyses. First, based on a median split, we divided diagnostic statements into those that were relatively long (M = 73.0 characters) and relatively short (M = 48.8 characters), and compared the response for these trial types in all the four regions. A significant main effect of statement length was observed in both left STS and occipital cortex [both F(1,14) values >17.20, both P-values <0.001] but was not observed in either dorsal mPFC or right STS/temporal pole (both F values <0.30, both P-values >0.28). Second, to identify regions that displayed a continuous relation between statement length and BOLD signal, we included statement length as a parametric modulator separately for both diagnostic and nondiagnostic statements. The parameter estimates associated with the modulator variable of statement length were significantly different from zero in both left STS and occipital cortex (both P-values <0.01), indicating that a significant linear relation existed between BOLD response and statement length in these two regions. No such relation was observed in either dorsal mPFC or right STS/temporal pole (both P-values > 0.80), confirming the results of the median-split analysis and demonstrating that activity in these regions was insensitive to the length of statements.
Because this analysis suggested that the differences between diagnostic and nondiagnostic statements in left STS and occipital cortex were due to the greater length of diagnostic statements, further analysis focused specifically on the pattern of responses in dorsal mPFC and right STS/temporal pole. A qualitatively similar pattern of results was obtained in the dorsal mPFC region identified from the contrast of diagnostic > nondiagnostic as for the one obtained from the contrast of impression formation > sequencing reported above, although the statistical reliability of the differences among conditions was somewhat weaker in the region when defined in this way.
A somewhat different pattern was observed in right STS/temporal pole. Although ROI analyses revealed a significant main effect of orienting task, F(1,14) = 6.78, P < 0.03, the two-way interaction of orienting task and diagnosticity failed to reach significance in this region, F(1,14) = 2.39, P = 0.14. Further analysis demonstrated that, unlike dorsal mPFC, the difference between diagnostic and nondiagnostic statements was significant both for sequencing trials, t(14) = 4.51, P < 0.0005, as well as impression formation trials, t(14) = 2.14, P < 0.05. However, like dorsal mPFC, the difference between orienting tasks was significant for nondiagnostic, t(14) = 2.33, P < 0.04, but not diagnostic statements, t(14) = 0.92, P = 0.37.
The reverse contrast of nondiagnostic > diagnostic revealed activations in several brain regions that included bilateral regions of parietal cortex comprising intraparietal sulcus and angular gyrus, bilateral insula, left inferior frontal gyrus, right middle frontal gyrus, anterior cingulate and a right-lateralized region of inferior occipital cortex.
DISCUSSION
The current study used functional neuroimaging to examine the way in which perceivers spontaneously process person information that fails to convey meaningful clues about the nature of that person's mind. We observed three main findings. First, we replicated our earlier observations that an extensive region of dorsal mPFC was differentially activated when participants engaged in impression formation than when they processed the same stimuli as part of a non-social task (Mitchell et al., 2004, 2005). Second, overall activity in dorsal mPFC and right STS/temporal pole was greater for statements that conveyed trait-diagnostic information than statements that were inherently trait-nondiagnostic.
Finally, further interrogation of the pattern of results in dorsal mPFC suggested that when participants explicitly attempted to form impressions of targets, activity in dorsal mPFC did not significantly differentiate between diagnostic and nondiagnostic statements. However, when participants were oriented away from the social-cognitive aspects of the stimuli during the sequencing task, dorsal mPFC failed to activate over baseline for nondiagnostic information and was significantly less activated for nondiagnostic than for diagnostic information. The interaction between orienting task and statement diagnosticity suggests the twin observations that trait-nondiagnostic information is not subject to spontaneous social-cognitive processing, but that such processing may occur when perceivers have the explicit goal to use that information to form an impression of a target. This interpretation is consistent with earlier formulations regarding the spontaneous nature of trait inferences, which have suggested that perceivers will automatically make use of trait-diagnostic information about another person (Winter et al., 1985; Uleman et al., 1996, 2005) and further suggests that perceivers may need to be explicitly directed to engage in deep social-cognitive processing when information does not inherently imply such traits.
Although our earlier work on impression formation has consistently observed differential activation in a single brain region—dorsal mPFC—the results of the current study also revealed modulation in the right STS extending into the temporal pole. The STS region (including both the STS and surrounding gyri) has previously been implicated in social cognition through its activation in a wide range of experimental situations that include the perception of biological motion (for a review, see Allison et al., 2000). These results have generally been interpreted to suggest that the STS responds preferentially to the perception of meaningful social stimuli, especially those conveyed visually. Consistent with this notion, trait-diagnostic statements also convey uniquely useful information about the mind of another person (like meaningful hand movements or shifts in eye gaze), whereas trait-nondiagnostic statements provide little basis for such social inferences. Interestingly, although the STS has typically been linked most strongly to visual perception of social behaviour, the current results suggest that the contributions of this area may extend to situations in which social meaning is implied through verbal stimuli. Interestingly, Harris et al. (2005) have recently observed a similar dissociation in a more posterior region of STS, which was preferentially engaged when participants read information that conveyed the idiosyncratic (and hence, socially diagnostic) aspects of another person's personality.
Greater activation for diagnostic than nondiagnostic information extended from STS into the temporal poles, a region previously identified with social-cognitive processing. Although the nature of the contributions made by temporal poles to social cognition remain somewhat mysterious, some researchers have suggested that this region plays an important role in accessing knowledge in the form of schemas and scripts (Gallagher and Frith, 2003). That this region was preferentially engaged in the current study by diagnostic information is highly consistent with this view of temporal pole activity; indeed, the very reason that some statements convey trait-diagnostic information must be that they activate a pre-defined schema that communicates the social meaning of particular behaviours.
We note that what has been designated here as ‘nondiagnostic’ social information may not always fail to provide diagnostic clues about another person's mind. Situated in an appropriate context, even the most mundane actions may reveal important aspects of another's mental states. For example, learning that someone ‘opened his mail upon getting home’ may not, in and of itself, communicate much about either his mental states or his dispositional traits, unless one also knows that the individual has been awaiting a letter about medical school admissions. In other words, having an appropriate contextual backdrop may imbue otherwise nondiagnostic information with important social-cognitive meaning. Whether the ability for context to alter the diagnostic value of person information is reflected in changes in the brain regions we have reported here—in particular, dorsal mPFC and right STS/temporal pole—poses an interesting empirical question for future research.
Lastly, we highlight the value of having adopted a functional neuroimaging approach to a question that, although of theoretical interest within social psychology, could not easily be addressed using traditional behavioural measures. In suggesting that the nondiagnostic person information can be subjected to the same depth of processing as highly diagnostic information—but only when perceivers have adopted the explicit goal of forming impressions of another person—this study helps establish the limits to the spontaneity of our social inferences without the need to rely on behavioural indices of such processing (which has been particularly difficult for nondiagnostic information). We are especially heartened by the fact that research into the neural basis of social cognition has now progressed to the point where extant neuroimaging findings can be used both to formulate specific hypotheses of psychological interest and, in turn, to provide a useful means for subjecting such hypotheses to empirical inquiry.
Acknowledgments
We thank J. Uleman for providing most of the trait-diagnostic stimulus statements, T. Laroche for assistance with data collection, and A. Jenkins, L. Davachi, and J. Zaki for helpful discussions. J.P.M. was supported by a post-doctoral National Research Service Award (NRSA). C.N.M. was supported by a Royal Society-Wolfson Fellowship.
Footnotes
1 Although the dorsal mPFC region observed in the current study was generally associated with activations above baseline, earlier work has observed deactivations in a very similar region (Mitchell et al., 2002). Although impression formation was associated with similar activations above baseline in our earlier work (Mitchell et al., 2004, 2005), little is currently understood about the conditions under which modulations in the medial frontal cortex appear as activations vs deactivations (for an in-depth discussion of issues regarding deactivations in medial frontal cortex, see Gusnard et al., 2001; Gusnard and Raichle, 2001).
REFERENCES
- Allison T, Puce A, McCarthy G. Social perception from visual cues: Role of the STS region. Trends in Cognitive Science. 2000;7:267–78. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Winston J, Frith U. Social cognitive neuroscience: where are we heading? Trends in Cognitive Science. 2004;8(5):216–22. doi: 10.1016/j.tics.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Cohen J, MacWhinney B, Flatt M, Provost J. PsyScope: a new graphic interactive environment for designing psychology experiments. Behavioral Research Methods, Instruments, and Computers. 1993;25:257–71. [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–14. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Happe F, Frith U, et al. Other minds in the brain: a functional imaging study of ‘theory of mind’ in story comprehension. Cognition. 1995;57(2):109–28. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends in Cognitive Science. 2003;7(2):77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happé F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: An fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Jack AI, Roepstorff A, Frith CD. Imaging the intentional stance in a competitive game. NeuroImage. 2002;16(3 Pt 1):814–21. doi: 10.1006/nimg.2002.1117. [DOI] [PubMed] [Google Scholar]
- Goel V, Grafman J, Sadato N, Hallett M. Modeling other minds. Neuroreport. 1995;6(13):1741–746. doi: 10.1097/00001756-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences USA. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hamilton DL, Driscoll DM, Worth LT. Cognitive organization of impressions: effects of incongruency in complex representations. Journal of Personality and Social Psychology. 1989;57:925–39. doi: 10.1037//0022-3514.57.6.925. [DOI] [PubMed] [Google Scholar]
- Hamilton DL, Katz LB, Leirer VO. Cognitive representation of personality impressions: organizational processes in first impression formation. Journal of Personality & Social Psychology. 1980;39(1-Sup-6):1050–63. [Google Scholar]
- Harris LT, Todorov A, Fiske ST. Attributions on the brain: neuro-imaging dispositional inferences, beyond theory of mind. Neuroimage. 2005;28(4):763–69. doi: 10.1016/j.neuroimage.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Hastie R, Kumar PA. Person memory: personality traits as organizing principles in memory for behaviors. Journal of Personality & Social Psychology. 1979;37(1):25–38. [Google Scholar]
- McCabe K, Houser D, Ryan L, Smith V, Trouard T. A functional imaging study of cooperation in two-person reciprocal exchange. Proceedings of the National Academy of Sciences USA. 2001;98(20):11832–5. doi: 10.1073/pnas.211415698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17(8):1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Heatherton TF, Macrae CN. Distinct neural systems subserve person and object knowledge. Proceedings of the National Academy of Sciences, USA. 2002;99:15238–43. doi: 10.1073/pnas.232395699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Encoding specific effects of social cognition on the neural correlates of subsequent memory. Journal of Neuroscience. 2004;24(21):4912–17. doi: 10.1523/JNEUROSCI.0481-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Forming impressions of people versus inanimate objects: Social-cognitive processing in the medial prefrontal cortex. NeuroImage. 2005;26:251–57. doi: 10.1016/j.neuroimage.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people: fMRI investigations of theory of mind. Neuroimage. 2003;19:1835–42. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. 2005;43(10):1391–99. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Srull TK, Wyer RS. Person memory and judgment. Psychological Review. 1989;96:58–83. doi: 10.1037/0033-295x.96.1.58. [DOI] [PubMed] [Google Scholar]
- Todorov A, Uleman JS. The efficiency of binding spontaneous trait inferences to actors' faces. Journal of Experimental Social Psychology. 2003;39:549–62. [Google Scholar]
- Uleman JS, Blader S, Todorov A. Implicit impressions. In: Hassin R, Uleman JS, Bargh JA, editors. The New Unconscious. New York: Oxford University Press; 2005. pp. 362–92. [Google Scholar]
- Uleman JS, Moskowitz GB. Unintended effects of goals on unintended inferences. Journal of Personality and Social Psychology. 1994;66(3):490–501. doi: 10.1037//0022-3514.66.3.490. [DOI] [PubMed] [Google Scholar]
- Uleman JS, Newman LS, Moskowitz GB. People as flexible interpreters: evidence and issues from spontaneous trait inference. Advances in Experimental Social Psychology. 1996;28:211–79. [Google Scholar]
- Winter L, Uleman JS, Cunniff C. How automatic are social judgments? Journal of Personality and Social Psychology. 1985;49:904–17. doi: 10.1037//0022-3514.49.4.904. [DOI] [PubMed] [Google Scholar]
- Wyer RS, Bodenhausen GV, Srull TK. The cognitive representation of persons and groups and its effect on recall and recognition memory. Journal of Experimental Social Psychology. 1984;20:445–69. [Google Scholar]