Abstract
Personal (internal) and normative (external) impetuses for regulating racially biased behaviour are well-documented, yet the extent to which internally and externally driven regulatory processes arise from the same mechanism is unknown. Whereas the regulation of race bias according to internal cues has been associated with conflict-monitoring processes and activation of the dorsal anterior cingulate cortex (dACC), we proposed that responses regulated according to external cues to respond without prejudice involves mechanisms of error-perception, a process associated with rostral anterior cingulate cortex (rACC) activity. We recruited low-prejudice participants who reported high or low sensitivity to non-prejudiced norms, and participants completed a stereotype inhibition task in private or public while electroencephalography was recorded. Analysis of event-related potentials revealed that the error-related negativity component, linked to dACC activity, predicted behavioural control of bias across conditions, whereas the error-perception component, linked to rACC activity, predicted control only in public among participants sensitive to external pressures to respond without prejudice.
Keywords: prejudice, stereotyping, brain, internal motivation, external motivation, regulation, control, automatic, anterior cingulate, rostral cingulate, ERPs
Self-regulation has been a central theme of psychology since the field's inception (James, 1890), and questions of how we orchestrate intentional behaviour in the midst of countervailing impulses continue to drive much theoretical inquiry. Psychological scientists from diverse research traditions have examined mechanisms of behavioural regulation at multiple levels of analysis, ranging from cultural influences to neural transmission. In the social psychological literature, much research on self-regulatory processes has been conducted in the context of prejudice and stereotyping. Stereotypes of African Americans, for example, are pervasive in American culture, and for most Americans, these stereotypes come to mind automatically and may influence their responses towards black people (Devine, 1989). Despite strong automatic associations between African Americans and stereotypic traits, many white Americans are motivated to inhibit expressions of racial bias in the service of their egalitarian beliefs (Allport, 1954; Devine, 1989). Hence, for low-prejudice people, responding without prejudice constitutes a significant regulatory challenge. Laboratory research on regulating one's intergroup responses thus provides a powerful and ecologically valid context for examining mechanisms of self-regulation.
Classic conceptualizations of racial bias have emphasized the role of self-standards, such as one's personal attitudes and beliefs, in driving efforts to respond without prejudice (Devine et al., 1991; Monteith, 1993; Dovidio et al., 1996). These self-standards reflect internalized cues for self-regulation (Monteith and Devine, 1993), which provide chronic impetuses to detect and inhibit unintended stereotypic associations in order to respond in an unbiased manner (Devine, 1989). These cues constitute the chronic internal regulation of one's responses to African Americans that bring behaviour inline with one's beliefs. Because these regulatory cues reflect one's internalized attitudes and beliefs, they are activated across situations and tend to be stable predictors of behaviour (Ryan and Connell, 1989; Devine et al., 2002). Thus, for low-prejudice people, internal cues should predict the regulation of racial responses regardless of the presence or absence of external cues, such as normative pressures to respond without prejudice.
Since the passage of civil rights legislation in the 1950s, researchers have observed a rise in the American's normative standards of egalitarianism that proscribe expressions of intergroup bias, independent of personal beliefs (Crosby et al., 1980). These normative standards provide externally generated cues for response regulation. Because external cues correspond to the presence of peers and authority figures who would disapprove of prejudiced responses, they influence behaviour only in situations when such cues are present, as in public, but not in private, situations (Plant and Devine, 1998). Much of the research has demonstrated that the actual or anticipated presence of a potentially disapproving peer or authority figure leads to reduced expression of race bias in self-reports and behaviour (Blanchard et al., 1991; Monteith et al., 1996; Plant et al., 2003), although some research has also shown that the anxiety associated with such external pressures may interfere with successful inhibition of automatic stereotypes (Lambert et al., 2003).
Importantly, external social cues do not affect the behaviour of all people. Individuals vary considerably in the extent to which they are concerned with social pressures to respond without prejudice (Dunton and Fazio, 1997; Plant and Devine, 1998), and these external cues only affect the behaviour of people who report high sensitivity to normative egalitarian pressures (Plant et al., 2003). It is therefore critical to consider individual differences in sensitivity to normative egalitarian standards while examining the effects of public vs private response conditions on the regulation of race-biased behaviour. Taken together, the literature has demonstrated that the presence of social pressure enhances self-regulatory demands to respond without race bias, but only among individuals who are sensitive to such pressures.
Despite much evidence for complementary internal and external motivations to respond without prejudice, research has not addressed whether regulation according to external cues relies on a different underlying mechanism than regulation according to internal cues. The extant literature concerning internal vs external cues for regulating race-biased responses is suggestive of different underlying processes, whereby externally driven forms of race-bias regulation likely involve an additional set of mechanisms beyond that of internally driven forms of regulation. Next, we describe a theoretical framework that identifies specific neurocognitive processes associated with response regulation.
NEUROCOGNITIVE MECHANISMS OF RESPONSE CONTROL
Neurocognitive models of response regulation suggest that the anterior cingulate cortex (ACC) plays a central role in monitoring ongoing response tendencies for competition and in signalling the need for enhanced control when such conflict occurs (Botvinick et al., 2001). Neuroimaging research has found that conflict between prepotent vs intentional response tendencies, elicited using cognitive control tasks such as the Stroop and Ericksen flankers tasks (Eriksen and Eriksen, 1974), activates dorsal regions of ACC (dACC) (Carter et al., 1998; Botvinick et al., 1999; Barch et al., 2001). As ACC activity increases, it engages regulatory mechanisms by signalling prefrontal cortical (PFC) activity associated with executive functions, such as selection for action, working memory and more deliberative processing (Gehring and Fencsik, 2001; Kerns et al., 2004).
Recent theoretical reviews suggest that dACC and the rostral subregions of the ACC (rACC) may support alternative aspects of conflict processing (Bush et al., 2000; Eisenberger and Lieberman, 2004). Although both (dACC/rACC) have strong anatomical connections to regions associated with regulating complex behaviours (Masterman and Cummings, 1997); the dACC has particularly strong connections with motor cortices (Bates and Goldman-Rakic, 1993; Luppino et al., 1993), whereas rACC has strong connections with regions of the orbital frontal cortex associated with outcome monitoring and processing complex external contingencies for reward and punishment (Rolls, 1996). In line with these anatomical distinctions, the dACC has been shown to reflect cognitive conflict independent of conscious awareness (Berns et al., 1997; Nieuwenhuis et al., 2001) and has been implicated in action monitoring processes regardless of whether or not the behaviour is relevant to social cognition. In contrast, the rACC activation has been associated with the perception of response errors, feeling states and the ability to process more complex external goal contingencies (Bush et al., 2000; Ullsperger and von Cramon, 2001; Garavan et al., 2003; Bishop et al., 2004). These findings are consistent with recent theorizing that more dorsal, posterior regions of the medial frontal cortex (MFC) are associated with the behavioural responses arising from internalized, self-monitoring processes, whereas more rostral, anterior regions are involved in the integration of action tendencies with expectancies of punishment or reward (Eisenberger and Lieberman, 2004; Ochsner et al., 2005; Amodio and Frith, 2006). These theoretical distinctions support the idea that dACC activity is involved in internally driven regulation of racial responses, independent of the situational context, whereas rACC activity should become important when successful regulation requires the consideration of external cues, such as when responding in the presence of an observer who would disapprove of one's race-biased behaviour.
Measuring conflict-related dACC and rACC activity using event-related potentials
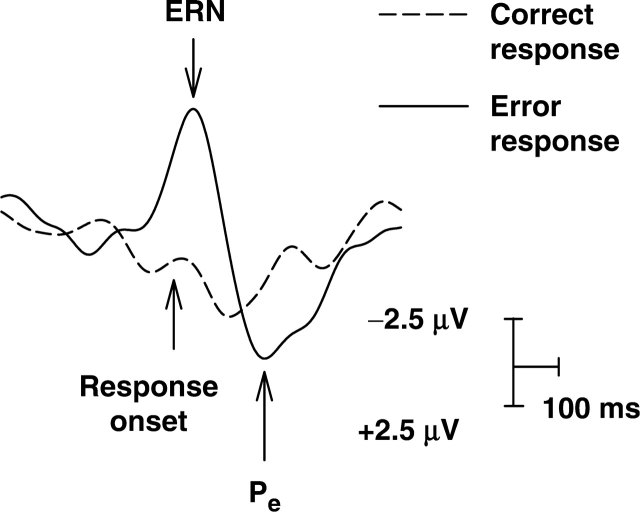
Rapid changes in ACC activity associated with self-regulatory conflict processing may be measured using event-related potentials (ERPs). ERPs reflect the firing of groups of neurons, measured using scalp electroencephalography (EEG), as an individual responds to a discrete event. By collecting EEG using a high sampling rate (e.g. 2500 Hz), ERPs can track real-time changes in brain activity as regulatory processes unfold. Two components of response-locked ERPs have been linked with conflict-related activity of the ACC: error-related negativity (ERN) and error-positivity (Pe); (Figure 1).
Fig. 1.
Illustration of the ERN and Pe components elicited by errors on a behavioural task.
The ERN (also, Ne) has been shown to reflect activity of the caudal dACC (Dehaene et al., 1994; van Veen and Carter, 2002; see Luu et al., 2003 for additional potential sources). The ERN is a negative-polarity voltage deflection observed in scalp-recorded EEG at the frontocentral midline when participants make a response error on a cognitive conflict task (Falkenstein et al., 1991; Gerhing et al., 1993). The ERN typically peaks within 100 ms of an error response and is thought to reflect cognitive conflict between an intended response and the actual (erroneous) response being executed (Yeung et al., 2004). Several studies have demonstrated a relationship between ERN amplitude and behavioural regulation on conflict tasks, such that individuals with larger average ERN responses to errors exhibit patterns of greater response control across the task, as indicated by accuracy, post-error slowing and process-dissociation estimates of control (Gehring et al., 1993; Pailing et al., 2002; Amodio et al., 2004). Given the pattern of these findings, the ERN has been interpreted as serving a self-monitoring function (Luu et al., 2000).
The Pe component of the ERP has been shown to reflect the activity of the rACC (Keihl et al., 2001; van Veen and Carter, 2002; Herrmann et al., 2004). The Pe is a positive-polarity voltage deflection that peaks ∼100–200 ms following a response error, just after the ERN. Like the ERN, the Pe has been associated with behavioural control (Nieuwenhuis et al., 2001). Research by Nieuwenhuis et al. (2001) suggests that whereas the ERN reflects pre-conscious conflict processing associated with an impending error, the Pe reflects conscious evaluation of one's response error, that is, the subjective awareness of conflict between one's intended and actual behaviour. In Neiuwenhuis et al.'s (2001) study, participants completed an anti-saccade task, in which their eye gaze was tracked while they attempted to inhibit saccades to stimuli presented in different locations on a computer monitor. After each trial, participants reported whether they had made an erroneous saccade. Because saccades may occur automatically when a stimulus appears in the visual field, responses were difficult to inhibit, and participants made more unperceived errors (11.2%) than perceived errors (7.4%) on the task. Importantly, both perceived and unperceived errors resulted in similar ERN responses, but only perceived errors produced a significant Pe response. This finding is consistent with theory and research concerning rACC activity reviewed above and corroborates other research indicating that the Pe wave is an ERP indicator of rACC error-perception processes.
The role of ACC in regulating responses to race
Amodio et al. (2004) examined the role of conflict monitoring in the context of prejudice and stereotyping. In this study, participants’ EEG was recorded as they completed a sequential priming task designed to measure their ability to inhibit the influence of automatic racial stereotypes on their behaviour. In the task, called the weapons identification task (WIT), target pictures of guns and handtools were classified, via button-press, following brief presentations of black or white face primes on the computer monitor (Payne, 2001). Low-prejudice participants completed this task in private with no external cues to respond without bias. As in past work, black face primes facilitated correct responses to guns but interfered with responses to tools, as predicted given the strong cultural stereotype of African Americans as hostile and dangerous (Devine and Elliot, 1995; Payne, 2001). This pattern suggested that enhanced regulation was required to respond accurately on black–tool trials, whereby the pre-potent stereotype-consistent ‘gun’ response must be inhibited and replaced with the correct ‘tool’ response. Indeed, larger ERN amplitudes were observed for responses on black–tool trials, which required stereotype-inhibition, than on black–gun trials, which did not require inhibition. Furthermore, participants who had larger ERN responses on black–tool trials were more effective at inhibiting stereotypes from influencing their behaviour throughout the task, suggesting that people with more sensitive conflict-monitoring systems are generally more effective self-regulators.
Amodio et al.'s (2004) findings suggested that the conflict-related dACC activity was associated with internally driven forms of behavioural regulation. However, their study did not address externally driven forms of race-bias control, nor did it examine the extent to which error-perception and the rACC were involved in response control. The present research was designed to extend the theoretical contributions of Amodio et al. (2004) and the previous research on error-perception processes to test the hypothesis that racial responses regulated according to external cues involve the unique contribution of rACC activity, as assessed by the Pe.
STUDY OVERVIEW
We hypothesized that conflict monitoring processes are critical for internally motivated regulation, such that ERN amplitudes would be associated with stereotype inhibition in both the presence and absence of external social pressure. In contrast, we proposed that error-perception processes are particularly important for regulating responses according to external cues, such that Pe amplitudes would additionally be associated with stereotype inhibition in the presence of external pressure, specifically among participants reporting high sensitivity to social pressures to respond without prejudice. To test these hypotheses, we measured participants’ neural activity while they completed the WIT in the presence or absence of external social pressure (i.e. in public or private). Past findings that individuals differ in their sensitivity to normative egalitarian pressures suggest that only highly externally motivated participants should perceive the public response condition as an external cue for self-regulation. For this reason, we examined the effects of public vs private response condition as a function of participants’ self-reported external motivation to respond without prejudice (Plant and Devine, 1998).
Following the past work (Payne, 2001; Amodio et al., 2004), our primary dependant measure was response accuracy on trials requiring inhibition of automatic stereotypes (i.e. black–tool trials), as compared with accuracy on trials not requiring inhibition (i.e. black–gun trials). Whereas much of the previous research has documented enhanced amplitudes of the ERN, and to a lesser extent the Pe, in response to conflict, the present research focused on the association between conflict-related ERN and Pe amplitudes and behavioural regulation in response to internal vs external cues. Hence, we predicted that (i) ERN amplitude would predict stereotype inhibition across conditions and participant groups, whereas (ii) Pe amplitude would predict stereotype inhibition only in the public condition among the participants who reported being sensitive to egalitarian normative pressures.
METHOD
Participants
A total of 66 right-handed undergraduate students (32 female and 34 male) participated for extra course credit. The participants were selected from a sample that had completed the Internal and External Motivation to Respond Without Prejudice scales in an earlier mass-testing session (Plant and Devine, 1998). The validity of these scales has been established in several studies (e.g. Plant and Devine, 2001; Devine et al., 2002; Amodio et al., 2003; Plant et al., 2003; see Plant and Devine, 1998, for a description of psychometric properties). The internal motivation scale (α = 0.81) includes items such as ‘I attempt to act in non-prejudiced ways toward Black people because it is personally important to me’. The external motivation scale (α = 0.80), which provided an index of participants’ sensitivity to external pressures, includes items such as ‘I attempt to appear non-prejudiced toward Black people in order to avoid disapproval from others’. Agreement with each item was rated from 1 (strongly disagree) to 9 (strongly agree). We recruited participants with high internal motivation (M = 7.15, significantly above the scale midpoint, t(65) = 11.26, P < 0.001) who reported external motivations in the upper (M = 6.54) or lower (M = 2.50) thirds of the sample distribution. Internal motivation scores did not differ as a function of external motivation (high vs low) × response condition (private vs public), Fs < 1, and scores on the internal and external motivation scales were uncorrelated, r(64) = −0.02, ns, consistent with previous findings (Plant and Devine, 1998, 2001; Amodio et al., 2003; Plant et al., 2003).
Procedure
After providing informed consent, participants were prepared for physiological recording and given instructions for the WIT. All participants were told that certain responses could reveal an influence of race bias on their responses (i.e. errors in black–tool trials) and that they should pay attention to the types of errors they made. In the private condition, participants were told that their responses would remain confidential such that they should not be concerned with external pressures to appear non-prejudiced. In the public condition, participants were told that the experimenter would be paying attention to their responses to determine whether they showed signs of racial prejudice.
After completing the WIT, participants rated the extent to which various trait attributes described African Americans as a group. Participants in the private condition made these ratings privately on a sheet of paper and then placed the sheet in a confidential envelope. Participants in the public condition reported their ratings aloud to the experimenter, who then wrote the responses on the rating form. This measure served as a check of the public vs private manipulation.
At the conclusion of the session, the experimenter probed participants for suspicion and then explained the hypotheses. No participant expressed suspicion about the response condition manipulation or guessed the experimental hypotheses. Each session took ∼2.5 h.
Materials
Weapons identification task
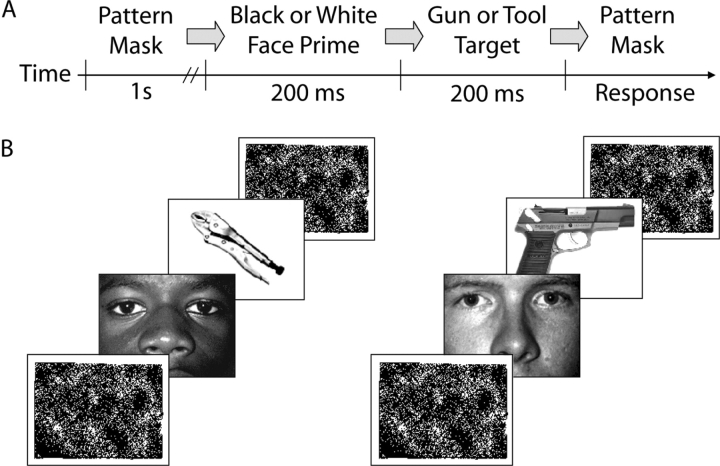
The stimuli and instructions used in the WIT were identical to those used by Amodio et al. (2004), originally adapted from Payne (2001). Each trial began with a pattern mask (1 s), followed by a black or white male face prime (200 ms), a gun or tool target (200 ms) and then a second pattern mask, which remained on the screen until a response was made (Figure 2). Stimuli included pictures of two white faces and two black faces, cropped to show only facial features, four handguns and four handtools. The participants were encouraged to classify targets as guns or tools via button-press within 500 ms of the target presentation. A ‘Too Slow!’ message appeared following responses that exceeded this deadline. Intertrial intervals ranged from 2.5 to 4 s. Prime-target pairs were equally probable and randomly ordered. Stimuli and physiological event markers were presented using DMDX software (Forster and Forster, 2003). The task included 26 practice trials and two blocks of 144 critical trials. Accuracy feedback was given during practice trials but not during critical trials. However, as in other response inhibition tasks, participants could easily detect when they made an error.1
Fig. 2.
Schematic of weapons identification task, adapted from Payne (2001), illustrating the timecourse of events (A) and example trial stimuli (B).
Correct response latencies occurring between 250 and 1500 ms were natural-log transformed and averaged within trial type for analysis (mean latencies are presented in raw millisecond). Error rates were computed by dividing total errors by total trials within each trial type.
EEG recording and processing
EEG was recorded from eight tin electrodes embedded in a stretch-lycra cap (ElectroCap, Eaton, OH, USA) corresponding to frontal (F3, F4), parietal (P3, P4) and midline (Fz, Fcz, Cz, Pz) scalp regions, based on the 10–20 international system (Jasper, 1958). EEG was referenced to the left earlobe (signal was also collected from the right earlobe for offline average-ears re-referencing), with a ground electrode placed on the forehead. All electrode impedances were below 5000 Ω. EEG signal was collected from a DC coupling, low-pass filtered at 100 Hz and digitized at 2500 Hz (Neuroscan Synamps, Sterling, VA), and stored to a computer hard drive.
ERN and Pe derivation
Offline, EEG signal was visually scored for artifact, submitted to a regression-based blink correction procedure (Semlitsch et al., 1986), and passed through a 1–15 Hz band-pass digital filter. An 800 ms epoch of EEG signal, centred on response, was selected for each artifact-free trial. Epochs associated with response latencies below 250 ms and above 1500 ms were excluded because they likely reflected impulsive errors or inattentiveness to the task (Amodio et al., 2004). Baseline correction procedures subtracted the average voltage occurring from 400 to 50 ms pre-response within each epoch from the entire epoch (because the average ERN began to rise at ∼50 ms prior to response). Waveforms derived from correct and incorrect trials were then averaged for each trial type. Preliminary analyses showed that, on average, the ERN wave peaked at 40 ms post-response, and the Pe peaked at 158 ms post-response. ERN amplitude was scored as the peak negative voltage obtained from the frontocentral (Fcz) electrode occurring between 50 ms pre- and 150 ms post-response (Amodio et al., 2004). The Pe amplitude was scored as the peak positive voltage between 100 and 200 ms post-response at Fcz.2 The average number of individual EEG epochs comprising each ERN/Pe score was as follows: 14.92 (black–tool), 11.59 (black–gun), 13.15 (white–tool) and 16.55 (white–gun), and the minimum number of epochs for any ERN or Pe score was 5.
Stereotype questionnaire
Participants rated the extent to which each of 35 trait words described African Americans on a scale ranging from 1 (uncharacteristic) to 7 (characteristic). This list included a subset of words most strongly associated with the African American stereotype (Devine and Elliot, 1995): lazy, poor, athletic, promiscuous, intelligent (reverse-scored), and ratings of these words were averaged to form a stereotype index (α = 0.60). Ratings for a subset of words not typically associated with the African American stereotype (kind, loyal, healthy, straightforward) were averaged to form a filler index (α = 0.63). A 2 (response condition: private vs public) × 2 (external motivation: high vs low) analysis of variance (ANOVA) produced a marginal interaction, F(1, 61) = 3.12, P = 0.08. Simple effects revealed that high external motivation participants reported less endorsement of stereotypes in public (M = 3.18, s.d. = 0.66) than in private (M = 3.77, s.d. = 0.96), t(37) = 2.43, P = 0.03. In contrast, low-external-motivation subjects’ reported endorsement did not vary as a function of response condition, t < 1. No effects were obtained for filler ratings. These analyses suggested that the manipulation was successful.
RESULTS
Behavioural analyses
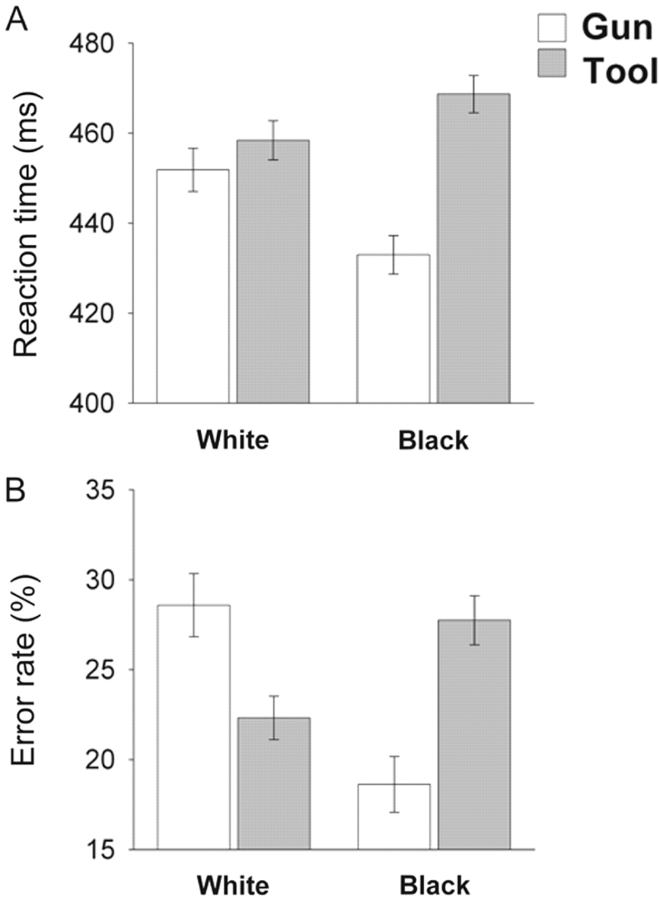
A 2 (prime: black vs white face) × 2 (target: gun vs tool) ANOVA of response latencies produced the expected interaction (Figure 3, panel A), F(1, 65) = 28.70, P < 0.001, such that black faces facilitated responses to guns and interfered with responses to tools, relative to white faces, ts > 3.47, Ps < 0.001. This effect was not moderated by response condition or level of external motivation. A 2 (prime: black vs white face) × 2 (target: gun vs tool) ANOVA of error rates produced a significant interaction, F(1, 65) = 20.01, P < 0.001 (Figure 3, panel B), indicating more erroneous classifications of tools than guns when they followed black face primes, t(65) = 4.93, P < 0.001. Conversely, participants misclassified guns more than tools when they followed white faces, t(65) = 2.39, P = 0.02. This interaction was not moderated by response condition or external motivation.3
Fig. 3.
Reaction times for correct responses (A) and error rates (B) on the weapons identification task as a function of trial type.
This set of behavioural results demonstrated that (i) black face primes activated a pre-potent stereotype-consistent response tendency and (ii) correctly responding ‘tool’ following a black face presented a greater regulatory challenge than responding ‘gun’ following a black face, presumably due to the automatic stereotypic association between African Americans and weapons. This pattern replicated previous findings (e.g. Payne, 2001; Amodio et al., 2004).
Task-related ERN and Pe effects
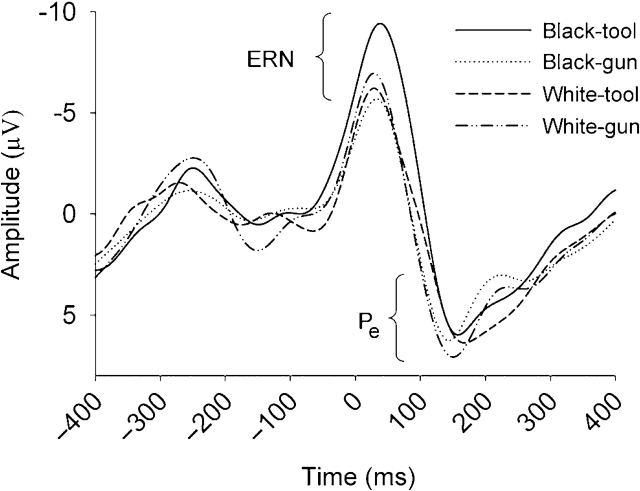
An initial analysis confirmed that ERN amplitudes on error trials were larger (more negative; M = −9.07, s.d. = 3.88) than amplitudes on correct trials (M = −0.18, s.d. = 2.37), t(65) = 18.65, P < 0.001. A 2 (prime: black vs white face) × 2 (target: gun vs tool) × 2 (response condition: public vs private) × 2 (external motivation: high vs low) ANOVA of ERN amplitudes on error trials produced significant main effects for prime, F(1, 62) = 7.46, P < 0.01, and target F(1, 62) = 8.36, P < 0.01. These effects were qualified by a prime × target interaction, F(1, 62) = 15.03, P < 0.001, such that ERN amplitude for black–tool trials was larger than that of any other trial type, ts > 4.0, Ps < .001 (Figure 4). This pattern of ERN results replicated Amodio et al. (2004). Moreover, this pattern was not moderated by response condition, external motivation, or their interaction.
Fig. 4.
Error-related negativity (ERN) and error-positivity (Pe) waveforms associated with each trial type of the weapons identification task. Zero represents the time of response.
The average Pe amplitude on error trials (M = 8.63, s.d. = 3.61) was marginally larger than on correct trials (M = 7.77, s.d. = 3.32), t(65) = 1.70, P = 0.09. We did not expect the Pe amplitude to vary as a function of condition, however, because (i) our task was designed so that participants could be aware of their errors (as opposed to Neuiwenhuis et al., 2001) and (ii) all participants were internally motivated to respond without prejudice and thus were expected to monitor their responses. Indeed, a 2 (prime: black vs white face) × 2 (target: gun vs tool) × 2 (response condition: public vs private) × 2 (external motivation: high vs low) ANOVA indicated that Pe amplitudes did not vary as a function of response condition, external motivation or their interaction.
Differential contributions of ERN and Pe to response regulation
Our primary theoretical question is concerned with the contributions of conflict-monitoring and error-perception processes to response regulation in private vs public conditions, as a function of participants’ degree of external motivation. ERN and Pe amplitudes associated with black–tool trials represented our indices of conflict-monitoring and error-perception, respectively. The primary outcome variable was response accuracy on black–tool trials, which required the inhibition of automatic stereotypes and thus constituted high-conflict trials. Additional analyses examined effects on response accuracy for black–gun responses, which did not require the inhibition of stereotypes and thus constituted low-conflict trials.4
ERN effects
We predicted that the ERN amplitude would predict response accuracy on black–tool trials, regardless of response condition or external motivation. In line with this prediction, the correlation between the ERN amplitude and accuracy for black–tool trials was significant, r(64) = −0.35, P < 0.005. ERN amplitude was not associated with accuracy on black–gun trials, r(64) = 0.01, P = 0.91. When black–gun accuracy was covaried in a partial correlation analysis, ERN continued to predict black–tool errors, r(63) = −0.35, P < 0.005. Finally, an exploratory ERN × response condition × external motivation regression analysis produced only the ERN main effect. This pattern of results is consistent with our hypothesis that the conflict-monitoring process is associated with regulation according to internal cues and should be evident across conditions and participants.
Pe effects
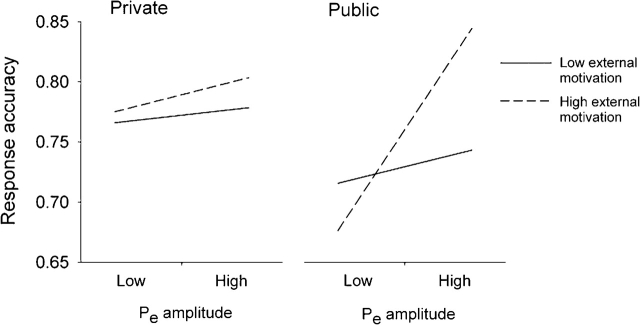
We predicted that the Pe amplitude would predict response accuracy on black–tool trials only among high-external-motivation participants who responded in public. We tested this specific prediction in a set of regression analyses in which Pe amplitude was the primary predictor and the interactions of response condition and external motivation were examined using dummy codes. The first regression tested whether (i) the Pe amplitude predicted black–tool accuracy for the public response/high-external-motivation group and (ii) this effect was different from the effect of Pe on accuracy for the combination of remaining groups (e.g. a 1 vs 3 comparison). This analysis revealed a strong effect for Pe amplitude among the public response/high-external-motivation group, β = 0.70, t(62) = 3.09, P < 0.005, whereas this effect was not significant for the combined group, β = 0.09, t(62) = 0.65, P = 0.52 (Figure 5). Moreover, the effect of Pe on accuracy was significantly larger for the public response/high-external motivation group than for the combination of remaining groups, β = −0.52, t(62) = −2.29, P < 0.03 and larger than each of the groups individually, Ps < 0.07. The Pe amplitude continued to predict accuracy on black–tool trials among the high-external-motivation or public response group when the ERN amplitude was covaried, β = 0.61, t(61) = 3.07, P = 0.007.
Fig. 5.
Predicted values for response accuracy on trials requiring stereotype inhibition (black-tool trials), illustrating the interaction of error-positivity (Pe) amplitude and sensitivity to normative pressure in Private vs. Public response conditions, computed at one standard deviation below and above each mean.
Additional analyses showed that Pe amplitude did not predict accuracy on black–gun trials for any of the four groups, Ps > 0.30, and the effect of the Pe amplitude on black–tool trial accuracy in the public response/high-external-motivation condition remained unchanged when accuracy on black–gun trials was covaried, β = 0.69, t(61) = 3.06, P < 0.005. Finally, an exploratory Pe × response condition × external motivation regression analysis predicting accuracy on black–tool trials produced only a significant main effect for Pe, β = 0.26, t(62) = −2.14, P < 0.04, which was qualified by the planned comparisons reported earlier. This pattern of results was consistent with our hypothesis that the error-perception process is associated with regulation according to external cues and should predict behaviour only in public among individuals sensitive to normative pressures.
DISCUSSION
The complementary roles of internal and external impetuses for self-regulation have been well-documented in the literature on intergroup behaviour (Crosby et al., 1980; Plant and Devine, 1998). Yet previous work has not addressed whether they reflect a single mechanism or alternative underlying mechanisms. On the basis of social psychological and neuroscientific theorizing, we proposed that externally driven regulation of race-biased behaviour should involve a distinct mechanism for processing external cues, beyond a mechanism for monitoring response conflicts associated with internalized cues. In support of this proposal, response regulation according to internal cues was associated with the ERN index of dACC activity, representing conflict monitoring, regardless of situational cues. In contrast, response regulation according to external cues was associated with the Pe, an ERP component previously associated with an rACC generator, and believed to represent error perception only among participants who were worried about social disapproval and whose responses were observed by an ostensibly non-prejudiced experimenter. Although our conclusions regarding the cortical sources of our ERP findings must be tempered by the fact that we did not collect EEG from a dense array and were therefore unable to conduct source localization analyses, these conclusions are consistent with previous research localizing the ERN and Pe waves to the dACC and rACC, respectively (e.g. Dehaene et al., 1994; van Veen and Carter, 2002), and with theories regarding the functions of dACC and rACC activations (e.g. Bush et al., 2000; Botvinick et al., 2001).
By examining the interaction of social situations and individual differences, we were able to isolate the effect of error perception on behaviour in order to obtain support for our hypothesis. This research is significant because it demonstrates that the function of the Pe, and its likely source in the rACC may be clarified by considering its role in social situations in which attitudes and goals are relevant. The present study is one of the few to examine neural activity associated with social pressures (see also, Berns et al., 2005) and is the first to examine explicit social pressure on neural responses in the context of race bias.
Role of rACC in self-regulation in social situations
Currently, the function of rACC activity is of much interest and some debate. One view of rACC activity (and, more broadly, medial frontal cortical activity) is that it reflects a unique neural module for the self and/or social cognition. An alternative view is that regions in frontal cortex provide a general function for integrating endogenous and exogenous information for orchestrating human behaviour. This generalist view argues that the extremely high associational connections within this region make it uniquely suited for computations of complex human social cognition and social behaviour (e.g. Amodio and Frith, 2006). Although our data are suggestive of an important role of rACC when regulating behaviour on the basis of social cues, they do not indicate that the rACC performs a uniquely social function or that other neural regions are not also involved. Hence, our findings are consistent with theorizing that the rACC is part of a general mechanism for complex forms of behavioural regulation. We speculate that this process is especially relevant to social interactions, owing to their complexity and the eminent significance of social relationships for human survival.
In speculating on the function of the rACC in social behaviour, it may be useful to consider its location relative to other regions of the MFC associated with self-processing and social processing. Extant source localization studies have identified Pe generators in somewhat different regions of the rACC. For example, van Veen and Carter (2002) localized Pe to an area just superior to the genu of the corpus collosum, whereas Hermann et al. (2004) localized Pe to a more posterior area of the rACC (but still anterior to the dACC source for the ERN). In both studies, localization was performed for error-related responses on the Eriksen flankers task (Eriksen and Eriksen, 1974). Discrepancies in localization may be due to several factors, such as variation between tasks, variation in electrode arrays, the use of different localization techniques and patterns of error variance in the data. Nevertheless, a consideration of the Pe source's proximity to areas implicated in mentalizing and theory of mind (e.g. para-ACC region bordering dorsal area of BA 32 and BA 9; see, for example, Frith and Frith, 1999) and self-knowledge and self-focused attention (most anterior para-ACC area bordering on BAs 32 and 10; see, for example, Gusnard et al., 2001) may inform our understanding of its function. For example, does the Pe wave reflect a form of conflict processing that elicits greater self-focused attention, mentalizing or other forms of social cognition? Further research is needed to address these questions.
Dissociable functions of dACC and rACC
Our analyses suggest that the functions of dACC and rACC may be conceptually dissociable, in that they perform different but complementary functions. In practice, however, the functions of the dACC and rACC may be thought of as hierarchically related. That is, theory and research indicate that the dACC is virtually always involved in the regulation of behavioural responses, particularly when the desired response competes with a pre-potent response. Indeed, dACC activity is commonly observed in tasks involving either an actual or implied response. On the other hand, rACC activity appears to be important for further guiding controlled response in accordance with more complex response contingencies, whereas it is not typically activated during simple behavioural responses that depend only on internalized task goals. Therefore, we speculate that the dACC may be required for the regulation of any type of behavioural response, and the rACC may be recruited to the extent that one's behaviour must be guided by more complex response contingencies (such as external social cues or task complexities). It is also possible that the rACC is important for stimulus processing in the absence of behaviour. However, it is difficult to design psychological tasks that are devoid of an implied behaviour, as much of the previous research has shown that simple exposure to a stimulus without a response requirement nevertheless activates a behavioural tendency (e.g. automotives; James, 1890; Bargh et al., 2001). Broadly speaking, our findings and interpretations are consistent with the view that the primary function of the frontal cortex is to orchestrate behaviour, and inferences regarding the function of observed neural activity may be best understood in this context.
It is notable that our use of individual differences in the present study was not designed to dissociate dACC and rACC functions. That is, we included participants who were, on average, internally motivated to respond without prejudice. We expected that the dACC would be activated across conditions, given that all participants had the goal of responding accurately (i.e. in an unbiased manner) on the task. Our hypothesis pertained to the emergence of the rACC as a predictor of behavioural control in the condition in which responses were regulated according to external social cues. Thus, we did not attempt to examine individuals for whom only the rACC would predict response regulation because, as outlined above, it is unlikely that the rACC would be involved in the absence of dACC activition during behaviours involving response conflict.
Theoretical implications for models of self-regulation
Although extant theorizing has emphasized the significance of both internal and external factors in self-regulation (see, for example, Baumeister and Vohs, 2004), few theories have examined their interplay (cf. Deci and Ryan, 2000). A limitation in this line of inquiry is the lack of a theoretical model outlining the mechanisms underlying internal vs external forms of self-regulation. Having associated internally and externally motivated regulation with previously identified ERP indices of dACC and rACC activity, respectively, we can bring findings from the cognitive neuroscience literature to bear on the interplay between internal and external impetuses for self-regulation. On the one hand, our theoretical analysis suggests a mechanistic explanation for some previous findings. For example, the findings that individual differences in internal and external motivations to respond without prejudice are generally uncorrelated (Plant and Devine, 1998; Amodio et al., 2003), and that externally motivated regulation tends to be less stable and less consistent than internally motivated regulation (e.g. Ryan and Connell, 1989), can be explained by our evidence for alternative neurocognitive substrates associated with conflict monitoring and error perception.
On the other hand, our findings suggest new hypotheses regarding self-regulation and intergroup behaviour. For example, given the stronger anatomical connections of the dACC to motor areas in the brain, one might expect that internally driven regulation would be more evident in behavioural responses than would externally driven regulation. In contrast, externally driven regulation may have more significant effects in responses involving more cognitively complex forms of judgement and decision-making. As another example, previous evidence that conflict monitoring operates independently of conscious awareness, whereas error-perception processes are associated with conscious awareness, suggests that attentional load would be more detrimental to regulation according to external cues than to internal cues. By the same token, individuals may be more attentive to regulatory failures when responding on the basis of external compared with internal cues, and failures to regulate according to external cues may lead to more elaborate and potentially more substantial reparatory processing in service of future prejudice reduction.
Finally, it is notable that the social neuroscience approach was critical to the present research. Our conclusion that error perception predicts self-regulatory processes specifically in response to external cues required the integration of social psychology and neuroscience at all levels of research (theory, method, measurement, analysis and interpretation). This research, and other work evidencing neural processes especially relevant to social behaviour (e.g. Eisenberger et al., 2003; Richeson et al., 2003), suggests that a consideration of social psychological factors will enhance the design of neuroscientific research and the interpretation of neural activation patterns.
Acknowledgments
We thank Brandice Baranowski, Marissa Langhoff and Amanda Nelson for their assistance in data collection. This article is based on research conducted in fulfilment of J. K.'s honours thesis at the University of Wisconsin, Madison.
Footnotes
1 As with most behavioural tasks used to elicit ERNs, participants are typically aware of their errors on the WIT despite the absence of feedback. However, the awareness of an error does not mean that the ERN is produced by error perception. Indeed, research designed to address this issue found no association between awareness of errors and ERN (Neiuwenhuis et al., 2001). Rather, conscious awareness of an error likely occurs after the ERN and during the time frame of the Pe wave. The present task was not designed to dissociate awareness from the ERN. Although we are not in a position to make strong claims regarding the role of awareness, it is unlikely that awareness of errors would drive ERN amplitudes.
2 The electrical dipoles produced by the rACC and dACC have different orientations (dependent on the orientation of their respective dendritic columns, which in turn varies as a function of cortical topography), and the negative poles of each have been shown to orient toward the same scalp region (e.g. van Veen and Carter, 2002). It is for this reason that the ERN and Pe are typically maximal at the same frontocentral scalp location.
3 It is notable that the public vs private response manipulation affected participants’ self-reported stereotype endorsement but not their accuracy on the weapons-identification task. On the surface, our results appear inconsistent with the findings of Lambert et al. (2003). However, the participants in the present study completed the task while being observed by a non-prejudiced experimenter, whereas participants in the Lambert et al. (2003) completed the task prior to an upcoming inter-racial interaction. The difference between an actual vs anticipated public response condition may be critical, as past research suggests that stress and anxiety associated with a particular task or event is more likely to impair behavioural regulation before or after an acute stressor rather than during its onset (Selye, 1956; McEwen and Seeman, 1999; Aston-Jones and Cohen, 2005). Thus, it is unclear whether our results directly contradict the findings of Lambert et al. (2003).
4 We were careful in this research to focus our analyses on theoretically relevant waveforms that were interpretable in the context of the behavioural task. Our hypotheses and analyses pertained to responses to black–gun vs black–tool trials because these trials provided theoretically clear manipulations of high vs low conflict. White faces are included in the task to provide a response alternative, and because white faces are not known to be associated with either tools or guns, they provide an important ‘filler’ condition. As in the past research (Amodio et al., 2004), successful stereotype inhibition behaviour (i.e. accuracy on black–tool trials) was not predicted by ERNs from white–gun trials, but was associated with ERN amplitudes from white–tool trials (ß = 0.30, t = 2.49, P = 0.02). This effect is believed to be driven by participants’ vigilance for tool targets, because an error in tool trials could indicate race bias (if preceded by a black face). Because faces were presented very quickly (200 ms), a heightened vigilance to tools is an advantageous strategy. In support of this interpretation, black–tool ERNs continued to predict stereotype inhibition accuracy when the effect of white–tool ERNs was covaried in a partial correlation, r(63) = −0.23, P = 0.06, whereas the effect of white–tool ERNs on accuracy was not significant when black–tool ERNs were covaried, r(63) = −0.18, P = 0.14. Similarly, the Pe associated with the white–tool trials predicted accuracy among high external motivation participants responding in public, r(18) = 0.48, P = 0.03, yet black–tool Pe amplitudes continued to predict accuracy when white–tool Pe scores were covaried in a partial correlation, r(17) = 0.58, P < 0.01. It is likely that the ERN and Pe waves from black–tool and white–tool trials both reflect a degree of vigilance for tool targets, but that the black–tool ERPs represent additional processing of the conflict between a stereotyping tendency and the desire to respond without bias.
REFERENCES
- Allport GW. The Nature of Prejudice. MA: Addison-Wesley; 1954. Reading. [Google Scholar]
- Amodio DM., Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Harmon-Jones E, Devine PG. Individual differences in the activation and control of affective race bias as assessed by startle eyeblink responses and self-report. Journal of Personality and Social Psychology. 2003;84:738–53. doi: 10.1037/0022-3514.84.4.738. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Harmon-Jones E, Devine PG, Curtin JJ, Hartley SL, Covert AE. Neural signals for the detection of unintentional race bias. Psychological Science. 2004;15:88–93. doi: 10.1111/j.0963-7214.2004.01502003.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual Review of Neuroscience. 2005;28:403–50. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A. Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cerebral Cortex. 2001;11:837–48. doi: 10.1093/cercor/11.9.837. [DOI] [PubMed] [Google Scholar]
- Bargh JA, Gollwitzer PM, Lee-Chai A, Barndollar K, Trötschel R. The automated will: Nonconscious activation and pursuit of behavioral goals. Journal of Personality and Social Psychology. 2001;81:1014–27. [PMC free article] [PubMed] [Google Scholar]
- Bates JF, Goldman-Rakic PS. Prefrontal connections of medial motor areas in the rhesus monkey. Journal of Comparative Neurology. 1993;336:211–28. doi: 10.1002/cne.903360205. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Vohs KD. Handbook of Self-regulation: Research, Theory, and Applications. New York: Guilford Press; 2004. [Google Scholar]
- Berns GS, Cohen JD, Mintun MA. Brain regions responsive to novelty in the absence of awareness. Science. 1997;276:1272–5. doi: 10.1126/science.276.5316.1272. [DOI] [PubMed] [Google Scholar]
- Berns GS, Chappelow J, Zink CF, Pagnoni G, Martin-Skurski ME, Richards J. Neurobiological correlates of social conformity and independence during mental rotation. Biological Psychiatry. 2005;58:245–53. doi: 10.1016/j.biopsych.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7:184–8. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Blanchard EA, Lilly T, Vaughn LA. Reducing the expression of racial prejudice. Psychological Science. 1991;2:101–5. [Google Scholar]
- Botvinick MM, Nystrom LE, Fissel K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–81. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–49. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Crosby F, Bromley S, Saxe L. Recent unobtrusive studies of Black and White discrimination and prejudice: a literature review. Psychological Bulletin. 1980;87:546–63. [Google Scholar]
- Deci EL, Ryan RM. The “what” and “why” of goal pursuits: human needs and the self-determination of behavior. Psychological Inquiry. 2000;11:227–68. [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–5. [Google Scholar]
- Devine PG. Prejudice and stereotypes: their automatic and controlled components. Journal of Personality and Social Psychology. 1989;56:5–18. [Google Scholar]
- Devine PG, Elliot AJ. Are racial stereotypes really fading? The Princeton Trilogy revisited. Personality and Social Psychology Bulletin. 1995;21:1139–50. [Google Scholar]
- Devine PG, Plant EA, Amodio DM, Harmon-Jones E, Vance SL. The regulation of explicit and implicit race bias: the role of motivations to respond without prejudice. Journal of Personality and Social Psychology. 2002;82:835–48. [PubMed] [Google Scholar]
- Dovidio JF, Brigham JC, Johnson BT, Gaertner SL. Stereotyping, prejudice and discrimination: another look. In: McCrae CN, Stangor C, Hewstone M, editors. Stereotypes and Stereotyping. New York: Guilford; 1996. pp. 276–319. [Google Scholar]
- Dunton BC, Fazio RH. An individual difference measure of motivation to control prejudiced reactions. Personality and Social Psychology Bulletin. 1997;23:316–26. [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends in Cognitive Sciences. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of target letters in a non-search task. Perception and Psychophysics. 1974;16:143–9. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinincal Neurophysiology. 1991;78:447–55. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Forster KI, Forster JC. DMDX: A Windows display program with millisecond accuracy. Behavioral Research Methods, Instruments, and Computers. 2003;35:116–24. doi: 10.3758/bf03195503. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds—a biological basis. Science. 1999;286:1692–5. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, Stein EA. A midline dissociation between error-processing and response-conflict monitoring. NeuroImage. 2003;20:1132–11. doi: 10.1016/S1053-8119(03)00334-3. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Fencsik DE. Functions of the medial frontal cortex in the processing of conflict and errors. Journal of Neuroscience. 2001;21:9430–7. doi: 10.1523/JNEUROSCI.21-23-09430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhing WJ, Goss B, Coles MG.H, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–90. [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann MJ, Rommler J, Ehlis AC, Heidrich A, Fallgatter AJ. Source localization (LORETA) of the error-related-negativity (ERN/Ne) and positivity (Pe) Cognitive Brain Research. 2004;20:294–9. doi: 10.1016/j.cogbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- James W. The Principles of Psychology. Dover: New York; 1890/1950. [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the International Federation. Encephalography and Clinical Neurophysiology. 1958;10:371–5. [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–6. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2001;37:216–23. [PubMed] [Google Scholar]
- Lambert AJ, Payne BK, Jacoby LL, Shaffer LM, Chasteen AL, Khan SR. Stereotypes as dominant responses: On the “social facilitation” of prejudice in anticipated public contexts. Journal of Personality and Social Psychology. 2003;84:277–295. doi: 10.1037//0022-3514.84.2.277. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. Journal of Comparative Neurology. 1993;338:114–40. doi: 10.1002/cne.903380109. [DOI] [PubMed] [Google Scholar]
- Luu P, Flaisch T, Tucker DM. Medial frontal cortex in action monitoring. Journal of Neuroscience. 2000;20:464–9. doi: 10.1523/JNEUROSCI.20-01-00464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychological Science. 2003;14:47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Masterman DL, Cummings JL. Frontal-subcortical circuits: the anatomic basis of executive, social, and motivated behaviors. Journal of Psychopharmacology. 1997;11:107–14. doi: 10.1177/026988119701100203. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- Monteith MJ. Self-regulation of stereotypical responses: implications for progress in prejudice reduction. Journal of Personality and Social Psychology. 1993;65:469–85. [Google Scholar]
- Monteith MJ, Deneen NE, Tooman GD. The effect of social norm activation on the expression of opinions concerning gay men and Blacks. Basic and Applied Social Psychology. 1996;18:267–87. [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GPH, Kok A. Error-related brain potentials are differently related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. 2001;38:752–60. [PubMed] [Google Scholar]
- Pailing PE, Segalowitz SJ, Dywan J, Davies PL. Error negativity and response control. Psychophysiology. 2002;39:198–206. doi: 10.1017/S0048577202010247. [DOI] [PubMed] [Google Scholar]
- Payne BK. Prejudice and perception: The role of automatic and controlled processes in misperceiving a weapon. Journal of Personality and Social Psychology. 2001;81:181–92. doi: 10.1037//0022-3514.81.2.181. [DOI] [PubMed] [Google Scholar]
- Plant EA, Devine PG. Internal and external motivation to respond without prejudice. Journal of Personality and Social Psychology. 1998;75:811–32. doi: 10.1177/0146167205275304. [DOI] [PubMed] [Google Scholar]
- Plant EA, Devine PG. Responses to other-imposed pro-Black pressure: acceptance or backlash? Journal of Experimental Social Psychology. 2001;37:486–501. [Google Scholar]
- Plant EA, Devine PG, Brazy PC. The bogus pipeline and motivations to reduce prejudice: revisiting the fading and faking of racial prejudice. Group Processes and Intergroup Relations. 2003;6:187–200. [Google Scholar]
- Richeson JA, Baird AA, Gordon HL, et al. An fMRI examination of the impact of interracial contact on executive function. Nature Neuroscience. 2004;6:1323–8. doi: 10.1038/nn1156. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex. Philosophical Transactions of the Royal Society. 1996;351:1433–44. doi: 10.1098/rstb.1996.0128. [DOI] [PubMed] [Google Scholar]
- Ryan RM, Connell JP. Perceived locus of causality and internalization: Examining reasons for acting in two domains. Journal of Personality and Social Psychology. 1989;57:749–61. doi: 10.1037//0022-3514.57.5.749. [DOI] [PubMed] [Google Scholar]
- Selye H. The Stress of Life. New York: McGraw-Hill; 1956. [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. NeuroImage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–59. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]