Abstract
Since the beginning of psychology as a scientific endeavour, the question of whether the body plays a role in how a person experiences emotion has been the centre of emotion research. Patients with structural gastrointestinal disorders, such as Crohn's disease, provide an intriguing opportunity to study the influence of body signals on emotions and feelings. In the present study, emotionally salient films were presented to participants with Crohn's disease in either the active state (Crohn's-active, CA) or silent state (Crohn's-silent, CS), and to normal comparison (NC) participants. We hypothesized that CA participants would have increased feelings, compared with CS and NC participants, when viewing emotional films designed to elicit happiness, disgust, sadness and fear. Gastric myoelectrical activity (electrogastrogram, or EGG) was measured during the films, and after each film was presented, participants rated emotion intensity (arousal) and pleasantness (valence). All groups labelled the emotions similarly. In support of the hypothesis, CA participants showed an increase in subjective arousal for negative emotions compared with CS and NC participants. The CA participants also showed increased EGG during emotional film viewing, as well as a strong positive correlation of EGG with arousal ratings. Together, these findings can be taken as evidence that aberrant feedback from the gastrointestinal system up-regulates the intensity of feelings of negative emotions.
Keywords: feeling, emotion, arousal, Crohn's disease, electrogastrogram, film, psychophysiology, autonomic nervous system, stomach
INTRODUCTION
Since the beginning of psychology as a scientific endeavour, the question of whether the body plays a role in how a person experiences emotion has been the centre of emotion research. The observation that there are physiological changes during an emotional event is ubiquitous, and virtually every human culture has incorporated references to the body in their ‘emotional’ language. For example, the Chewong of central Malaysia associate emotions with the liver, the Gahuku-Gama of New Guinea associate emotions with the stomach and the Tahitians and Maori locate emotions in the intestines (Prinz, 2004).
Traditionally, there have been two opposing theoretical frameworks in emotion research. The first one, proposed by William James (1884) and Carl Lange (1887), posited that the conscious experience of emotion was dependent on the perception of body reactions. In this view, sensory feedback from the viscera—that is, feedback regarding the visceral changes themselves—accounts for the quality of emotional experiences. No genuine feeling can arise without these changes. Thus, for example, our feeling of fear owes much to our pounding heart and the ‘butterflies’ in our stomachs, and we grieve because we cry and not the other way around (Papanicolaou, 1988). In the James–Lange hypothesis, the body produces the feeling of the emotion.
Cannon (1929) counter-argued that it was not necessary for the feeling of emotions to be based on the body. The argument was based on experiments with cats that were induced into what was called sham rage (Bard, 1928; Cannon, 1927). In these experiments, decerebrated cats retained a behaviourally integrated rage. This response was not directed towards anything, and it appeared to lack any conscious experience of the genuine rage. It also was not a graded response: a full-blown rage response could be elicited by a mild stimulus. Cannon and Bard, a physiologist, analysed progressive cerebral transections in these cats, and the coordinated response subsided when the hypothalamus and thalamus were removed. They suggested that activity in the thalamus and hypothalamus was responsible for the quality of sensations of the emotions. The main tenet of this theory addresses the primary criticism of the James–Lange theory of emotions. According to Cannon and Bard, the autonomic nervous system has all-or-none responses, but we can have an infinite array of feelings. Also, according to Cannon, feelings can remain when the autonomic response has subsided. Another point was that visceral changes are too slow to be a source of emotional feelings that can be instantaneous, and artificial induction of changes typical of strong emotions do not produce them. Furthermore, Cannon argued that human beings with spinal cord or vagus nerve transections retained their emotional sensation, or that manipulation of the autonomic nervous system with epinephrine did not change the conscious experience of emotion (Marañon, 1924; Cannon, 1929; Saper, 2002). In his theory, bodily changes were the proper response manifestation of emotional awareness that was already achieved (Papanicolaou, 1988).
Recently, there has been a swell of support that has tipped the balance back towards the James–Lange view of emotions. Of particular note, Damasio (1994, 2001) has proposed that visceral states are mapped onto brain areas, producing images that give rise to feeling states and can also influence cognition to help steer the organism towards a beneficial response. This view has found support in neuroanatomical and neuroimaging studies, as summarized in the following text.
Afferent signals from the viscera enter the central nervous system at several morphological and functional levels to produce physiological responses and to influence overt behaviour and verbally reported subjective sensations. Visceral information arrives to the brain through the spinal cord and vagal afferents that terminate topographically in the nucleus of the solitary tract (NTS). NTS receives massive information from the body, and it is considered a major visceral relay area in ascending pathways (Adam, 1998; Craig, 2003). The most important projection from NTS is to the parabrachial nucleus (PBN). PBN projects to nuclei in the brainstem [such as periaqueductal grey (PAG) matter], hypothalamus, amygdala, basal forebrain and the posterior nuclei of the thalamus [ventroposterior medial nucleus (VPM) and posterior ventral medial nucleus (VMpo)], which in turn innervate the insular cortex (Craig and Andrew, 2002; Saper, 2002). Also, several functional neuroimaging studies have shown that the viscerosensory cortex, most notably the insular cortex, is activated during emotional processing, visceral stimulation or autonomic arousal (Phillips et al., 1997; Damasio et al., 2000; Small et al., 2003; Critchley et al., 2004). More specifically, activation of the anterior insula has been consistently associated with negative emotional states, including pain and distress (e.g. Iadarola et al., 1998; Evans et al., 2002) and hunger and thirst (Denton et al., 1999; Tataranni et al., 1999), and the insula has been implicated in the evaluation and representation of specific negative emotional states such as anger and disgust (Phillips et al., 1997; Calder et al., 2000; Damasio et al., 2000).
Nonetheless, it still has not been shown conclusively that the body is critical for the subjective feeling of emotion. For instance, Dana (1921) reported no reduction in the subjective intensity of emotions, and no lack of feelings, in an individual with a spinal cord lesion. In this study there was no standardized questionnaire, and the study relied on the report of one case. On the other hand, Hohmann (1966) showed that patients with spinal cord lesions reported decreased feelings of fear, anger and sexual excitement after their injuries. In order to corroborate Hohmann's study, Chwalisz et al. (1988) studied spinal cord lesion participants and normal comparison (NC) groups using several standardized questionnaires for reporting subjective emotions. The authors did not find any difference in subjective emotions between groups. More recently, Cobos et al. (2002) asked spinal cord injury patients and NC participants to rate emotionally charged pictures on arousal and valence. This study also failed to demonstrate between-group differences in such ratings. One limitation in all of these studies is that the subjects, even those with severe spinal cord transections, still have several alternative, patent pathways of communication between the body and the brain, such as the hypothalamic–pituitary–adrenal axis (HPA) and the vagus nerve, which subserve extensive interactions between brain and body. Thus, this evidence cannot fully adjudicate the issue of whether bodily feedback is necessary for normal emotions and feelings.
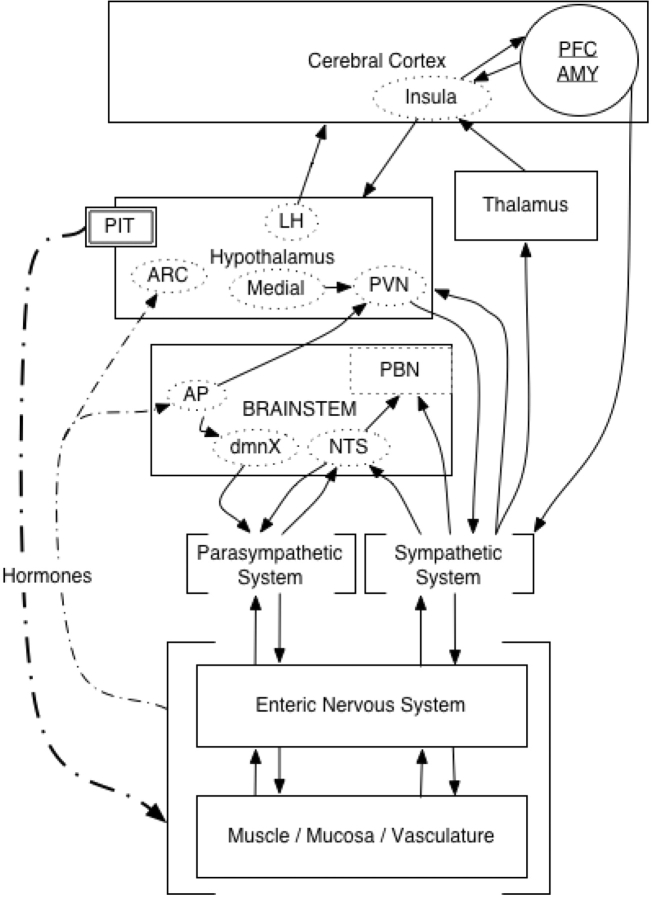
In emotion research, psychophysiological measures such as electrodermal activity, cardiac function, facial electromyogram and respiration have been used to assess body states during an emotional paradigm in experimental participants (e.g. Boiten, 1998; Bechara et al., 2000; Bradley, 2000; Tranel, 2000). One system that has almost never been considered in this literature is the gastrointestinal system. This could be due to the complex multiple bidirectional pathways between the central nervous system and the gastrointestinal system (Figure 1). The main biological function of the gastrointestinal system is to process ingested food and liquids. The coordination of digestion and the gastrointestinal system is complex and requires the coordinated activity of multiple neural (central nervous system, efferent autonomic nervous system and enteric nervous system) and hormonal signals (Woods, 2004; Quigley and Conklin, 2004).
Fig. 1.
Schematic representation of anatomical connectivity between the viscera and central nervous system. Diagram based on Saper (2002) and Craig (2002). Note: AMY, amygdala; AP, area postrema; ARC, arcuate fasciculus; dmnX, dorsal, motor nucleus of vagal nerve; NTS, nucleus tractus solitarius; PBN, parabrachial, nucleus; PFC, prefrontal cortex; PIT, pituitary gland; PVN, paraventricular nucleus.
It has been shown that there are changes in the gastrointestinal system during stress. Acute stress tasks in rats (e.g. operant avoidance, radiation, handling, acoustic stimulation and tail shock) induce delayed gastric emptying. However, colonic motility is stimulated by the same acute stress tasks in rats (Taché, 1989; Enck and Holtmann, 1992). Similar types of changes have been observed in humans. Stern and coworkers reported changes in gastric myoelectrical activity in various stress-inducing tasks such as forehead cooling (Muth et al., 1999) and shock avoidance (Muth et al., 1998). Also, Blomhoff et al. (2000) presented emotionally charged words to patients with irritable bowel syndrome and to normal participants. The investigators measured rectal tone and brain event-related potentials. Overall changes in rectal tone occurred during exposure to emotionally charged words; however, neither word type (i.e. positive vs negative) nor group status predicted the direction of change.
Descriptions of changes in emotions and feelings due to changes in the gastrointestinal tract are rare. Adam (1983) showed that mild stimulation of the gastrointestinal tract in cats dampens negative emotional states, whereas strong stimulation of the gastrointestinal tract may be perceived as aversive. In human participants, Vianna and Tranel (in press) have shown that the amplitude of gastric myoelectrical activity correlates with subjective ratings of arousal, while normal participants watched emotion-eliciting film clips, suggesting that this psychophysiological index tracks arousal in emotion-inducing situations.
Inflammatory bowel diseases, such as Crohn's disease, present clear symptoms of altered gut motility and behaviour, and inflammation of the lining of digestive tract. Crohn's disease is also characterized by frequent attacks of diarrhoea, severe abdominal pain, nausea, fever and chills (Crawford, 1999), and it is diagnosed by the appearance of a set of clinical, endoscopic and histological features (Stenson, 1995). It is a chronic, relapsing inflammatory disease that may affect any portion of the gastrointestinal (GI) tract (Crawford, 1999). It is also characterized by recurrent flare-ups of symptoms and by post-operative recurrence after palliative surgery (Biancone et al., 2003).
Even though the exact cause of the disease is unknown, patients with Crohn's disease present clear pathological changes. For instance, aphthous ulcer, large ulcers, transmural inflammation, fissures and fistulas are all present in the disease (Tremaine, 1996, Stenson, 1995). It is thought that in all these processes the host mucosal immunity is stimulated and then fails to down-regulate. The aims of management of Crohn's disease are the elimination or amelioration of complaints and symptoms and the prevention or reduction of flare-ups (Schölmerich, 2004).
Structural gastrointestinal disorders such as Crohn's disease provide an intriguing opportunity for understanding the role of the gut in emotions and feelings. The patients do not have any comorbid psychiatric or psychological disorders at rates different from those in the general population (Fullwood and Drossman, 1995; Maunder, 1998). It has been shown already that patients with gastrointestinal disorders have decreased thresholds for gut sensation and pain in response to balloon distension in the gastrointestinal tract (Fullwood and Drossman, 1995). That is, patients with Crohn's disease have an inflamed gastrointestinal tract that induces abnormal motility and sensitivity. Hence, these patients have abnormal—specifically, increased—feedback of information from the gastrointestinal system to the central nervous system of a previously innocuous state. Therefore, it can be predicted that such patients might have a more intense feeling than normal comparison subjects, when presented with an emotional stimulus. And because these patients have a gastrointestinal system in a pathological state, it can be inferred that abnormal gastrointestinal feedback to the brain is responsible for such a difference. This is the issue we sought to address here.
In the present study, emotionally salient films were presented to participants diagnosed with Crohn's disease and to normal participants. While participants were viewing the films, gastric myoelectrical activity (electrogastrogram or EGG) was measured. Afterwards, participants were asked to rate the arousal and valence, and to label, the emotion felt while watching films. The decision to use films to elicit emotional states was motivated by the desire to use strong, robust stimuli to induce emotion. Films involve more than one perceptual modality (Oatley, 2004), and they typically have a storyline. The principal psychophysiological measure of interest was the EGG. The EGG is a reliable and non-invasive method of recording gastric myoelectrical activity (Nelsen and Kohatsu, 1968; Smout et al., 1980), and it has been shown to be highly correlated with subjective ratings of arousal (Vianna and Tranel, in press). The gastric myoelectrical activity paces the contraction of the stomach and originates in a pacemaker region lateral to the gastroesophageal junction and is characterized by regularly recurring potentials. The gastric slow wave is present all the time, and controls the frequency and propagation of the contractions of the stomach. The normal frequency of the electrogastric wave is three cycles per minute (c.p.m.), and is termed normogastria (Stern et al., 2000).
We included two types of Crohn's patients in our study, those with active disease (CA) and those whose disease was in a silent phase (CS). The hypothesis was that participants with active Crohn's disease would have increased feelings. Specifically, it was predicted that active Crohn's disease participants would subjectively report increased feelings when presented with emotionally charged films, compared with participants with silent Crohn's disease and normal comparison participants. In the active disease state, the gastrointestinal system is inflamed. Inflammation increases gastrointestinal sensitivity and motility (Coelho et al., 2000). Hence, the behaviour of the gastrointestinal system is aberrant, which could lead to increased feelings. In the remission state of the disease (silent Crohn's disease) the inflammation is quiescent. However, inflammation can cause structural changes to the gastrointestinal tract and plasticity in the nervous system (Lomax et al., 2005). Therefore, individuals with silent Crohn's disease were used so that we could investigate the influence of long-term changes in the gastrointestinal system and central nervous system. If subjects with silent Crohn's disease also have increased feelings in this experimental paradigm, it cannot be asserted that the gastrointestinal system is solely responsible for altered subjective feelings.
It was also predicted that subjects with active Crohn's disease would have higher amplitude stomach contractions, as measured by EGG, relative to those with silent Crohn's disease and NC subjects. Higher EGG amplitudes could be taken as evidence for an abnormal body status, at least insofar as the GI system is concerned, which allows the inference that any increases in the subjective ratings of feelings in patients with active Crohn's could be related to changes in the body (specifically, the gut).
METHODS
Participants
All procedures were approved by The University of Iowa Institutional Review Board for Human Subjects Research. Twenty NC participants (13 women, 7 men; mean age M = 38.8 years ± 3.4; mean education M = 15.9 years ± 0.3) were recruited for this study using an advertisement at the University of Iowa Hospitals and Clinics. No normal participant had a history of gastrointestinal, neurological or psychiatric problems at the time of enrolment, as determined by screening by a gastroenterologist (J.W.) or neuropsychologist (D.T.).
Crohn's disease patients were recruited through the Gastrointestinal Clinic at the University of Iowa Hospitals and Clinics. These participants were divided into active Crohn's disease group (CA, n = 8, four women, four men; mean age M = 40.3 years ± 3.8; mean education M = 15.0 years ± 0.8) and silent Crohn's disease group (CS, n = 12, seven women, five men; mean age M = 35.9 years ± 5.0; mean education M = 13.5 years ± 0.7). Crohn's disease participants were screened for any neurological or psychiatric disorder at the time of enrolment in the study (by D.T.), and none reported such a history. Participants also reported the drugs used for treating Crohn's disease. Six CA participants were taking mesalamine, one was taking prednisone and one was taking infliximab. Six CS participants reported taking infliximab, two were taking prednisone, two were taking mesalamine and two reported not taking any drugs.
Stimuli
Ten standardized films were used to elicit the discrete emotions of happiness, disgust, fear and sadness, as well as no emotion (neutral).1 These films were derived from published emotion research (Gross and Levenson, 1995; Boiten, 1998; Waldstein et al., 2000), and they have been used previously in our laboratory (Vianna and Tranel, in press). They were selected on the basis of being powerful inducers of target emotions, and fairly selective in terms of inducing the target emotion but no other emotions. The following films were used:
Neutral
‘Mosel River’ (Germany: The Rhine and Mosel; The Romantic Road, Steves, 1991). A scene showing the Mosel River. Length of film clip: 2.04 min.
‘Great Canadian Train Ride’ (‘The Great Canadian Train Ride’, Jones, 1993). A scene showing a train ride through Canada, explaining how the engineer controls the train. Length of film clip: 2.17 min.
Happiness
Tortilla Soup (Wang et al., 2001). A couple is cooking, laughing and hugging. Length of film clip: 2 min.
She's Having a Baby (Hughes, 1988). A woman tells her husband that she is pregnant, and they enjoy the several pregnancy stages. Length of film clip: 1.37 min.
Disgust
Sadness
The Champ (Marion and Newman, 1979). A boy sees his father dying and starts crying. Length of film clip: 2.52 min.
She's Having a Baby (Hughes, 1988). A woman is having surgical problems. A nurse comes out of surgery room and tells her husband. While the doctors are struggling with her life, the husband thinks about all the good moments they had together and wishes he could have a last chance to say he loves her. Length of film clip: 4.27 min.
Fear
The Shining (King et al., 1980). A boy is riding his tricycle in a corridor, when two girl apparitions materialize and frighten him with a bloody scene. Length of film clip: 1.25 min.
Pet Cemetery (King, 1989). A kid enters a house, fetches a scalpel and hides. An older man starts looking for the kid. Length of film clip: 4.07 min.
The film clips were digitized and stored in a G4 PowerMac computer. The clips were edited on iMovie (Apple Computer, 2003), and saved in Quicktime format (Apple Computer, 2004).
Subjective emotion ratings
Subjective reports of emotional experience were obtained for arousal and valence. We included both of these because they are the dimensions typically rated in emotion experiments (e.g. Bradley, 2000). A priori, we did not have predictions as to whether one or the other, or both, of these dimensions would turn out to differ in the CA participants; in principle, both could, although the preponderance of available evidence suggests that the arousal dimension might be more affected (e.g. in work linking the amygdala to emotional processing, the most salient relationship is with emotional arousal, rather than valence, as summarized in LaBar and Cabeza, 2006).
For arousal, participants rated their emotional experience during each film presentation on a scale of 1–7, with 1 corresponding to ‘I did not feel any emotion at all’ and 7 corresponding to ‘I felt an extremely intense emotion’. For valence, the rating scale also ranged from 1 to 7, with 1 corresponding to ‘extremely unpleasant’, 4 corresponding to ‘neutral’, and 7 corresponding to ‘extremely pleasant’. Participants also reported the primary emotion they felt during each clip, choosing one label for each clip from a list of emotion labels (happiness, fear, disgust, sadness and neutral).
Psychophysiology
The transducers, amplifiers, analog to digital converter (MP100WSW) and data acquisition (AcqKnowledge) were from Biopak, Inc. (Santa Barbara, CA, USA). All psychophysiological data were sampled at 200 Hz.
EGG was recorded using two disposable cutaneous Ag-AgCl electrodes. One electrode was placed above the umbilicus, and the second electrode was placed just below the lower left rib.
Skin conductance was recorded using disposable Ag-AgCl skin electrodes. Electrodes were placed on the thenar and hypothenar eminences (the eminences of the palm) of the right hand. Skin conductance output voltage was amplified by a factor of 5 μS/V and low-pass-filtered at 10 Hz. electrodermal activity (EDA) has been one of the most widely used psychophysiological measures, and is considered a reliable index of the sympathetic nervous system activity and subjective arousal (Edelberg, 1972; Venables and Christie, 1980; Dawson et al., 2000).
Procedure
Participants reported to the laboratory between 9 and 11 a.m., after a 2 h fast. Initially, the participants completed the Positive and Negative Affect Schedule questionnaire (PANAS; Watson et al., 1988). This questionnaire assesses background mood when the subjects started the experiment. Such background emotional states may influence how a person experiences the emotional stimuli (Schmuckle et al., 2002). This was important information, because a chronic disease (such as Crohn's) could influence general affective state (Katz et al., 2001). The PANAS, which is effective in assessing mood at the moment it is being completed, consists of 10 positive and 10 negative adjectives (Egloff, 1998; Watson et al., 1999).
After explanation of the experimental procedure, electrodes were placed on the participant. Participants were allowed to sit comfortably in a chair approximately 1 m away from a 20″ monitor screen. Before the experiment was initiated, the participants were asked to sit quietly, and a 2 min baseline measure of the psychophysiology was taken. Films were presented in a random order across participants. After each film clip was presented, the participant completed the subjective rating measures.
Data reduction
EGG
Data obtained during each film clip were subjected to fast fourier transform (FFT) with a Hamming window. The maximum spectral value within the normogastria range (2.5–3.5 c.p.m.) was calculated for each condition. As noted earlier, normogastria is associated with normal rhythm of the stomach of a healthy individual, and has been previously shown to correlate with subjective ratings of arousal (Vianna and Tranel, in press). Because of significant between-subject variability in the measure, the values for each participant were transformed into z-scores. This was done in order to observe changes of the psychophysiological signal during different emotional states in such a way that magnitude information could be preserved and compared across subjects and conditions.
EDA
Skin conductance activity was measured as the area under the curve over a given time interval (Damasio et al., 2000; Naqvi and Bechara, in press). Raw-skin conductance data were low-pass-filtered to remove high frequency noise. The slow downward drift in baseline skin conductance level was removed using a moving difference function with a difference interval of 0.05 s (10 points for a 200 Hz sampling rate). Because the film clips had somewhat different time lengths, the area under the curve was divided by the total film clip time. For statistical analysis the z-scores of the participants were calculated, reasons being the same as indicated for the EGG signal.
Statistical analysis
All data are presented as means ± SEM. Because specific questions were asked of the data, a contrast analysis of variance was applied to the subjective ratings of arousal and valence in order to determine whether there were between-group differences in the elicitation of emotion (Rosenthal et al., 2000). A contrast analysis of variance was also applied to investigate differences in the EGG. Mauchly sphericity test was performed, and when necessary, Huyn–Feldt correction was applied. Finally, correlations were conducted between EGG and the two emotion ratings, arousal and valence, following the general procedures outlined by Lang et al. (1993).
RESULTS
Baseline mood
PANAS scores were not different between the groups for both positive [NC: 33.0 ± 1.62; CA: 32.0 ± 4.21; CS: 29.6 ± 2.98; F(2, 37) = 1.57, P = 0.22] and negative [NC: 13.1 ± 0.92; CA: 12.0 ± 0.82; CS: 11.1 ± 0.55; F(2, 37) = 1.25, P = 0.30] scales. This finding supports the conclusion that the groups did not differ in their baseline emotional states at the beginning of the experiment.
Emotion induction
To evaluate the degree to which subjects experienced a specific feeling (ideally, the emotion targeted by the film), participants were asked to label the most intense emotion felt while watching each film. Also, a self-report of the intensity of each emotion during film presentation was used, that is, participants rated on a Likert scale (1–7) how much they felt each of the emotions (happiness, fear, sadness and disgust) during the film presentation.
Participants labelled the emotion felt while watching the movies very similarly to previous labelling rates for these films (Gross and Levenson, 1995). Also, the films elicited significantly (P < 0.01) the target emotion without eliciting other emotions (Table 1). The contrasts between intensity ratings for the different emotion types are within rows and across columns in Table 1. For the target emotion happiness, the NC group rated happiness higher than the other emotions [F(3, 95) = 86.43, P < 0.01, Bonferroni corrected]; the CA group rated happiness higher than the other emotions [F(3, 35) = 33.35, P < 0.01, post hoc Bonferroni corrected] and the CS group rated happiness higher than the other emotions [F(3, 55) = 51.82, P < 0.01, Bonferroni corrected]. For the target emotion disgust, the NC group rated disgust significantly higher than the other emotions [F(3, 95) = 39.13, P < 0.01, post hoc Bonferroni corrected]; the CA group rated disgust significantly higher than the other emotions [F(3, 35) = 69.4, P < 0.01, post hoc Bonferroni corrected] and the CS group rated disgust significantly higher than the other emotions [F(3, 55) = 22.82, P < 0.01, post hoc Bonferroni corrected]. For the target emotion fear, the NC group rated fear significantly higher than the other emotions [F(3, 95) = 76.92, P < 0.01, in a post hoc Bonferroni test, fear was significantly higher than all emotions, and disgust was significantly higher than happiness]; the CA group rated fear significantly higher than the other emotions [F(3, 35) = 19, P < 0.01, post hoc Bonferroni corrected] and the CS group rated fear significantly higher than the other emotions [F(3, 55) = 20.17, P < 0.01, post hoc Bonferroni corrected]. For the target emotion sadness, the NC group rated sadness significantly higher than the other emotions [F(3, 95) = 88.85, P < 0.01, post hoc Bonferroni corrected]; the CA group rated sadness significantly higher than the other emotions [F(3, 35) = 7.84, P < 0.01, post hoc Bonferroni corrected] and the CS group rated sadness significantly higher than the other emotions [F(3, 55) = 36.96, P < 0.01, post hoc Bonferroni corrected]. For the target emotion neutral (no emotion), the NC group rated happiness higher than the other emotions [F(3, 95) = 22.09, P < 0.01, Bonferroni corrected]; the CA group rated happiness higher than the other emotions [F(3, 35) = 33.85, P < 0.01, post hoc Bonferroni corrected] and the CS group rated happiness higher than the other emotions [F(3, 55) = 18.72, P < 0.01, Bonferroni corrected]. Neutral film clips were expected to have the least intense ratings in all emotions as the question does not have a specific rating of neutrality. However, the happiness ratings of the neutral clips were significantly different from the other emotion ratings, indicating that participants reported small degrees of happiness associated with the neutral clips. The appropriate (target) ratings for all films were at least one unit higher than the other ratings, so the films can be considered to have elicited successfully the target emotions (cf. Gross and Levenson, 1995).
Table 1.
Frequency of participants’ labelling the emotion felt while watching the film as the target emotion (i.e. the one intended by the experimenters) and discrete intensity ratings of each emotion while watching each film. For nearly all cells, the target emotion intensity was rated significantly higher (P < 0.01) than the other emotions (indicated bya). Disgust intensity was rated higher than happiness in fearful stimuli for the normal comparison group (indicated byb)
| Group | Target emotion | Labellling of target emotion (in %) | Discrete intensity ratings for each emotion |
|||
|---|---|---|---|---|---|---|
| Happy | Fear | Sad | Disgust | |||
| NC | Neutral | 71.4 | 2.14 (0.24)a | 1.00 (0.00) | 1.09 (0.09) | 1.02 (0.06) |
| Happy | 86.7 | 3.91 (0.25)a | 1.08 (0.07) | 1.37 (0.11) | 1.13 (0.06) | |
| Disgust | 80.0 | 1.81 (0.23) | 1.42 (0.10) | 1.82 (0.21) | 4.52 (0.31)a | |
| Fear | 100 | 1.20 (0.11) | 4.55 (0.21)a | 1.70 (0.16) | 2.17 (0.17)b | |
| Sad | 97.6 | 1.54 (0.16) | 2.11 (0.17) | 5.11 (0.22)a | 1.47 (0.15) | |
| CA | Neutral | 69.2 | 2.78 (0.72)a | 1.00 (0.0) | 1.00 (0.0) | 1.00 (0.0) |
| Happy | 85.7 | 4.50 (0.58)a | 1.20 (0.14) | 1.20 (0.22) | 1.00 (0.0) | |
| Disgust | 64.3 | 1.00 (0.0) | 1.00 (0.0) | 1.60 (0.45) | 5.60 (0.38)a | |
| Fear | 91.7 | 1.00 (0.0) | 4.12 (0.47)a | 1.25 (0.16) | 1.87 (0.39) | |
| Sad | 85.7 | 1.30 (0.57) | 2.20 (0.57) | 5.60 (0.41)a | 1.10 (0.11) | |
| CS | Neutral | 60.0 | 2.85 (0.54)a | 1.00 (0.0) | 1.00 (0.0) | 1.00 (0.0) |
| Happy | 75.0 | 3.90 (0.52)a | 1.00 (0.0) | 1.05 (0.06) | 1.00 (0.0) | |
| Disgust | 80.0 | 1.75 (0.44) | 1.20 (0.11) | 1.80 (0.35) | 4.65 (0.61)a | |
| Fear | 80.0 | 1.20 (0.15) | 3.90 (0.56)a | 1.35 (0.19) | 1.90 (0.42) | |
| Sad | 100 | 1.70 (0.34) | 1.55 (0.27) | 4.55 (0.47)a | 1.15 (0.14) | |
Subjective ratings of arousal and Valence
Recall that our principal hypothesis was that participants with CA would have increased feelings compared with participants with CS and NC participants. The findings for the arousal ratings supported the hypothesis. Specifically, there were significant between-group differences in arousal ratings for all three negative emotions, and in all cases, the CA group demonstrated higher ratings (Table 2): fear [F(1, 41) = 4.69, P = 0.03], sad [F(1, 41) = 5.84, P = 0.02]; and disgust [F(1, 41) = 5.86, P = 0.02]. The differences for neutral [F(1, 41) = 0.03, P = 0.87] and happy [F(1, 41) = 1.69, P = 0.2] were not significant.
Table 2.
Ratings of arousal and valence for NC, CA and CS groups for the different emotional stimuli. *indicates a significant difference (P < 0.05) between groups in a contrast analysis
| Emotion | Arousal |
Valence |
||||
|---|---|---|---|---|---|---|
| NC | CA | CS | NC | CA | CS | |
| Neutral | 2.02 (0.25) | 1.93 (0.37) | 1.86 (0.18) | 4.63 (0.13) | 4.21 (0.14) | 4.45 (0.19) |
| Happiness | 3.80 (0.33) | 4.28 (0.22) | 3.54 (0.41) | 5.27 (0.23) | 5.36 (0.19) | 4.90 (0.20) |
| Disgust | 4.50 (0.34) | 5.36 (0.22)* | 4.09 (0.34) | 2.88 (0.29) | 2.71 (0.44) | 3.18 (0.29) |
| Fear | 4.52 (0.33) | 5.29 (0.29)* | 3.74 (0.46) | 2.79 (0.17) | 2.89 (0.33) | 3.41 (0.20) |
| Sad | 4.76 (0.37) | 5.78 (0.23)* | 4.59 (0.34) | 2.66 (0.14) | 2.64 (0.19) | 3.54 (0.36) |
For the valence ratings, the between-group differences were not significant: neutral [F(1, 41) = 2.13, P = 0.15], happy [F(1, 41) = 0.65, P = 0.42], fear [F(1, 41) = 0.45, P = 0.50], disgust [F(1, 41) = 0.44, P = 0.51] and sad [F(1, 41) = 1.84, P = 0.18].
Gastric myoelectrical activity (EGG)
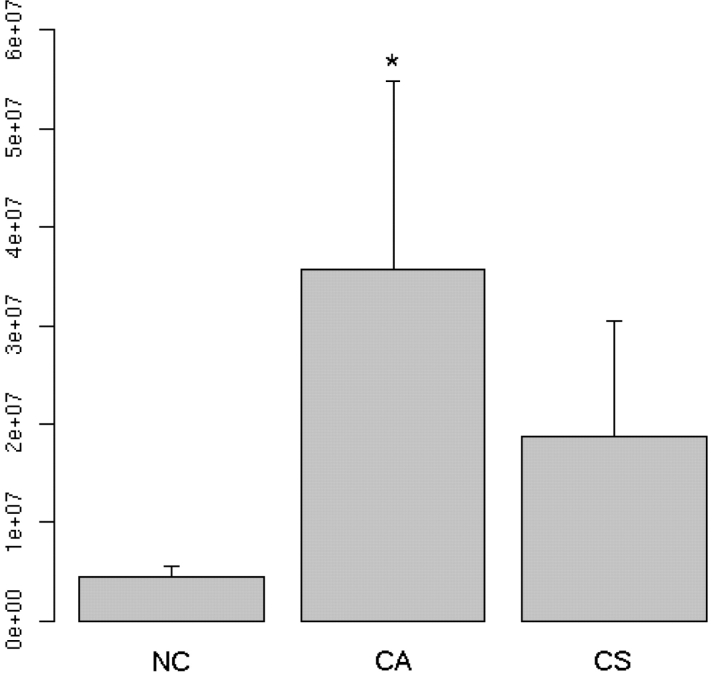
Consistent with our prediction, the CA group demonstrated higher baseline EGG peak amplitude, compared with the CS and NC groups [F(1, 38) = 9.23, P = 0.0018; Figure 2]. Also, peak EGG during the experiment correlated strongly with arousal ratings in the CA group (r = 61, P = 0.04, Figure 3a), but not with valence ratings (r = 0.34, P = 0.16). In the CS group, peak EGG did not correlate significantly with arousal (r = −0.28, P = 0.78, Figure 3b) or with valence (r = 0.14, P = 0.39).
Fig. 2.
Baseline EGG amplitude of NC, CA and CS participants. EGG amplitude was significantly higher in the CA group, compared with the other two groups.
Fig. 3.
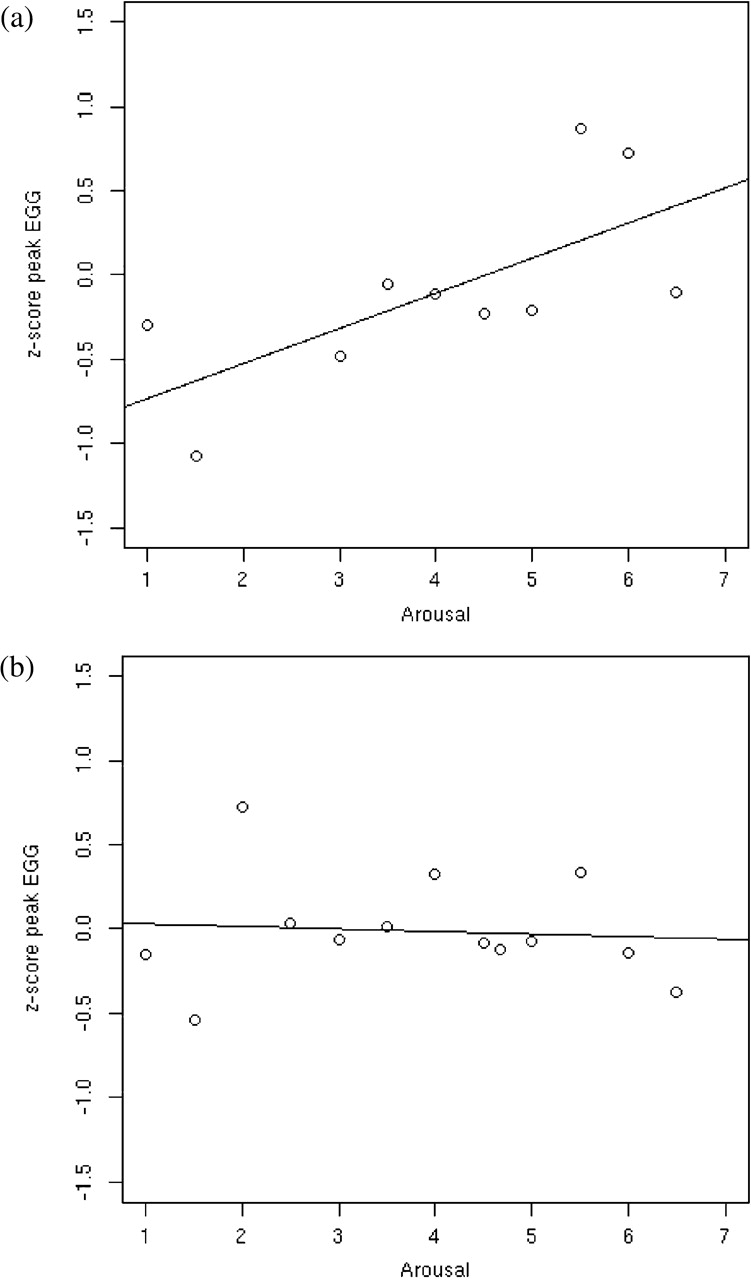
Correlation of EGG peak amplitude and subjective ratings of arousal in the two Crohn's disease groups: (a) CA disease: r = 0.61, (b) CS disease: r = −0.28.
Due to the nature of the EGG signal (high variability between subjects and a low signal-to-noise ratio), it was not possible to perform a meaningful correlation analysis between the peak EGG amplitude signal value and the arousal ratings. In order to have a suitable correlation analysis, a larger number of participants in the CA group would be needed. Therefore, a direct analysis showing that higher EGG peak amplitude correlates with higher arousal ratings was not possible. We performed correlations between arousal ratings and the z-score of the EGG peak amplitude of each participant. However, with the z-score transformation, the magnitude of the signal is lost. The information retained is the difference of EGG peak amplitude for each emotion. Because each group has on average a different magnitude of the EGG peak amplitude, the correlation analysis was performed for each group. As noted earlier, there were high correlations between the z-score of EGG amplitude and arousal ratings for the CA (reported immediately above) and NC (reported in Vianna and Tranel, in press) groups.
To summarize, the inference that increased EGG amplitude is leading to increased arousal in the CA group is based on the following observations:
On average, the ratings of arousal in the CA group are higher than NC;
On average, EGG peak amplitude in the CA group is higher than NC and
For both the CA and NC groups, the difference between emotions, or z-scores, of the EGG peak amplitude correlated with arousal ratings.
EDA
A consistent correlation of subjective ratings of arousal and EDA has been reported in emotion research (Burch and Greiner, 1960; Edelberg, 1972; Venables and Christie, 1980; Lang et al., 1993; Bradley, 2000; Dawson, 2000; Tranel, 2000). In our study, the normal participant group evidenced a strong positive correlation between EDA and ratings of arousal (r = 0.82, P = 0.001). This strong correlation was also observed in the CS group (r = 0.67, P = 0.03). In contrast, the CA group did not evidence a significant correlation between subjective ratings of arousal and EDA (r = 0.34, P = 0.16).
DISCUSSION
The findings from this study were supportive of the hypothesis: individuals with CA reported more intense feelings (increased arousal) while viewing emotionally charged film clips, compared with participants with CS and normal participants. This pattern was seen for all negative emotions included in the study (fear, disgust and sad), but not for the neutral and happy conditions. This is not surprising, because negative emotions reliably elicit higher arousal ratings than neutral and happiness (e.g. Bradley, 2000; Carretie et al., 2001). It was also shown that the baseline EGG peak amplitude just before the experiment started was higher in CA participants than in any other group, that is, CA participants had aberrant gastrointestinal activity before any interaction with the stimuli had begun. Thus, an inference can be made that aberrant (increased) gastrointestinal activity leads to increased feelings in the CA participants.
Another interesting result that came out of our data is that EDA, arguably the gold standard psychophysiological index in emotion research, was not a good index of subjective ratings of arousal for the participants with CA. In normal participants and participants with CS, EDA did provide a good index of subjective ratings of arousal. Again, we can take these outcomes to suggest that the aberrant increase of the gastrointestinal activity (as shown by baseline EGG peak amplitude) in the CA participants is driving the subjective response. Indeed, when participants with CA disease are asked about their state, they reported a diffuse uncomfortable sensation from the belly. However, after each film, no participant reported an increase or decrease of the gastrointestinal sensations. Also, it must be noted that neither the PANAS ratings nor the subjective ratings of arousal in the neutral and happy conditions were different from the normal population, and therefore, the heightened gastrointestinal sensations did not generally bias the ratings of the CA participants.
Because the gastrointestinal system has the enteric nervous system that accompanies its tract, the central nervous system does not need to control every motor neuron or instance of gut behaviour. Command signals are transmitted by the efferent autonomic nervous system that activates integrated circuits positioned in the gut wall, eliciting a patterned response. Hence, a small number of efferent fibres control large portions of the gastrointestinal system (Wood et al., 1999). Moreover, the system set in this manner can serve as an amplifying system, that is, it receives a small signal and produces a large response that can be ‘sensed’ by the central nervous system. Therefore, as the gastrointestinal system is inflamed, any behaviour from this system can elicit an aberrant or even painful response. It is well-established that hypersensitivity can occur after an inflammatory event (Al-Chaer and Traub, 2002). This ‘higher sensitivity’ can be interpreted by the central nervous system as the appropriate amplified response producing a more intense feeling.
An interesting observation that comes out of our data is that Crohn's disease individuals who are in remission, i.e. CS, did not show a correlation between EGG and subjective ratings of emotional arousal. However, the subjective ratings of arousal and valence of the CS disease participants did not differ from the ratings of NC participants.
Crohn's disease subjects in remission have all been through an active state at one point in their life. As described earlier, Crohn's disease is a chronic inflammatory disease. There is an interaction between immune and non-immune cells, and this is amplified during an inflammatory process (Mawe et al., 2004). For instance, elevated interleukin-1 can enhance the release of prostaglandins that contribute to the amplification of tissue injury, which can lead to progressive alterations in smooth muscle contractility (Hosseini et al., 1999). Inflammation also can lead to hyperalgesia, by sensitization of gastric afferents, and changes in the efferent pathways (Kang et al., 2005). These changes are persistent and can occur at sites in the bowel distant from affected site (Barbara et al., 1997; Mawe et al., 2004; Lomax et al., 2005). The inflamed system undergoes plasticity throughout all levels of the nervous system (Porreca et al., 2002), and this reorganization might be changing the gut–brain relationship.
It also must be noted that half of the participants in remission were taking infliximab to control the disease.2 Infliximab is a recombinant antibody that binds to tumour necrosis factor-α (TNF-α) antagonizing its effects at the cellular level (Panaccione et al., 2005). Indeed, high levels of TNF-α have been correlated with nausea (by means of tachygastria; Hermann et al., 2003), and administration of recombinant anti-TNF-α antibody can block this increased gastric myoelectrical activity effect. This blunting effect was seen only with the EGG activity of CS subjects. The role of TNF-α on emotion is not well-established. For instance, Connor et al. (1998) demonstrated that TNF-α can decrease the time spent on the open arm of the elevated plus maze (‘an anxiogenic-like effect’) in an animal model. On the other hand, Yamada et al. (2000) showed that knockout mice for TNF-α receptor also showed a decrease in the time spent on the open arm of the elevated plus maze. However, a role for cytokines, in particular TNF-α, has been suggested in stress, depression and sickness behaviour (Konsman et al., 2002; Grippo et al., 2005). Sickness behaviour is seen in the initial phase of infection of during acute flares of an inflammatory chronic disease. Its manifestations are fever, lethargy, increased sleep, reduced social activity, reduced mobility, anhedonia, decreased learning and anorexia (Dunn and Swiergiel, 1998). Grippo et al. (2005), in a chronic mild stress model in rats, showed that increased levels of cytokines (such as TNF-α and IL-1β) observed were related to the degree of anhedonia in rats. Also, therapeutic doses of systemic cytokines in humans have produced anorexia, depressed mood, disordered sleep, poor motivation and impaired thought processing (Schiller et al., 1991; Wilson et al., 2002).
As noted, the subjective ratings of arousal did not correlate with EGG in the CS participants, unlike the case for CA participants and NC participants (Vianna and Tranel, in press). Changes in the gastric myoelectrical activity due to infliximab administration plus a reorganization of the nervous system due to the prior inflamed state might be driving this ‘uncoupling’ of the gut activity from feelings in the group of CS participants. The possibility of ‘uncoupling’ gut activity (as seen with CS participants) or electrodermal activity (as seen with CA participants) with subjective ratings of arousal can be very important in understanding the role of the body in emotion and feelings, and might have a consequence in several psychopathological diseases such as functional or somatic diseases.
It would not be controversial to assert that the gastrointestinal system has some role in emotion and feeling, and this would surely not come as news to the average person on the street. However, there has been a remarkable scarcity of empirical data regarding the interplay between the gastrointestinal system and various emotions and feelings, and this is even more the case at the level of systems neuroscience. The main goal of this study was to address the question of how the gut plays a role in emotions and feelings. For this, we hypothesized that an abnormal gastrointestinal system would lead to increased reported feelings.
Our hypothesis was supported only for negative emotions. As discussed earlier, this result could be due to the nature of the stimuli presented, that is, negative stimuli induced higher arousal ratings than positive or neutral stimuli, an effect that is generally reported in emotion studies (Bradley, 2000; Damasio et al., 2000). However, the gastrointestinal system might have a more substantial role in negatively valenced emotional situations. This idea would agree with the flight-or-fight response developed by Cannon. Interestingly, Cannon's early work is on the gastrointestinal system. In the fight-or-flight response, the body must adapt rapidly for the new threatening situation. Changes in the gut might subserve a further role of amplifying the signal to guide the organism to a better and faster response.
Acknowledgments
Supported by Program Project Grant NINDS NS19632.
Footnotes
1 The rationale for choosing these target emotions was to include the “primary” emotions with the exception of anger. Anger was excluded because in pilot work, subjects tended to rate anger as disgust, as in “I am disgusted at the man beating his wife.” In this sense the disgust has a social connotation, and this was outside the scope of the current project.
2 One of the CA participants was also taking infliximab. However, the participant was in the early phase of the treatment with the drug, and their EGG pattern was different from the ones seen in the CS group; thus, we believe that the drug had not yet exerted a ‘dampening’ effect on the EGG.
REFERENCES
- Adam G. Intestinal afferent influence on behavior. In: Holzl R, Whitehead WE, editors. Psychophysiology of the Gastrointestinal Tract: Experimental and Clinical Applications. New York: Plenum; 1983. pp. 351–60. [Google Scholar]
- Adam G. Visceral Perception: Understanding Internal Cognition. New York: Plenum Press; 1998. [Google Scholar]
- Al-Chaer ED, Traub RJ. Biological basis of visceral pain: recent developments. Pain. 2002;96:221–5. doi: 10.1016/S0304-3959(02)00046-5. [DOI] [PubMed] [Google Scholar]
- Apple Computer, Inc. iMovie (Version 3.3). Software. 2003. [Google Scholar]
- Apple Computer, Inc. Quicktime Player (Version 6.5). Software. 2004. [Google Scholar]
- Bard P. A diencephalic mechanism for the expression of rage with special reference to the sympathetic nervous system. American Journal of Physiology. 1928;84:490–515. [Google Scholar]
- Barbara G, Vallanca BA, Collins SM. Persistent intestinal neuromuscular dysfunction after acute nematode infection in mice. Gastroenterology. 1997;113:1224–32. doi: 10.1053/gast.1997.v113.pm9322517. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Biancone L, Tosti C, Fina D, et al. Review article: maintenance treatment of Crohn's disease. Alimentary Pharmacology & Therapeutics. 2003;17(Suppl. 2):31–37. doi: 10.1046/j.1365-2036.17.s2.20.x. [DOI] [PubMed] [Google Scholar]
- Blomhoff S, Spetalen S, Jacobsen MB, Vatn M, Malt UF. Intestinal reactivity to words with emotional content and brain information processing in irritable bowel syndrome. Digestive Diseases & Sciences. 2000;45:1160–5. doi: 10.1023/a:1005502119461. [DOI] [PubMed] [Google Scholar]
- Boiten FA. The effects of emotional behaviour on components of the respiratory cycle. Biological Psychiatry. 1998;49:29–51. doi: 10.1016/s0301-0511(98)00025-8. [DOI] [PubMed] [Google Scholar]
- Bradley MM. Measuring emotion: Behavior, feeling, and physiology. In: Lane R, Nadel L, editors. Cognitive Neuroscience of Emotion, Series in Affective Science. New York: Oxford University Press; 2000. pp. 242–76. [Google Scholar]
- Burch NR, Greiner TH. A bioelectric scale of human alertness: concurrent recordings of the EEG and GSR. Psychiatric Research Report. 1960;12:183–93. [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manes F, Antoun N, Young AW. Impaired recognition and experience of disgust following brain injury. Nature Neuroscience. 2000;3:1077–8. doi: 10.1038/80586. [DOI] [PubMed] [Google Scholar]
- Cannon WB. The James-Lange theory of emotions: a critical examination and an alternative theory. American Journal of Psychology. 1927;39:106–24. [PubMed] [Google Scholar]
- Cannon WB. Bodily changes in pain, hunger, fear and rage. Appleton, New York. Cannon, W.B., Washburn, A.L. (1912). An explanation of hunger. American Journal of Physiology. 1929;29:441–54. [Google Scholar]
- Carretie L, Mercado F, Tapia M, Hinojosa JA. Emotion, attention, and the ‘negative bias’, studied through event-related potentials. International Journal of Psychophysiology. 2001;41:75–85. doi: 10.1016/s0167-8760(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Diener E, Gallagher D. Autonomic arousal feedback and emotional experience: evidence from the spinal cord injured. Journal of Personality Social Psychology. 1988;54:820–28. doi: 10.1037//0022-3514.54.5.820. [DOI] [PubMed] [Google Scholar]
- Cobos P, S’anchez M, Garcia C, Vera MN, Vila J. Revisiting the James versus Cannon debate on emotion: startle and autonomic modulation in patients with spinal cord injuries. Biological Psychology. 2002;61:251–69. doi: 10.1016/s0301-0511(02)00061-3. [DOI] [PubMed] [Google Scholar]
- Coelho A-M, Fioramonti J, Bueno L. Systemic lipopolisaccharide influences rectal sensitivity in rats: role of mast cells, cytokines, and vagus nerve. American Journal of Physiology and Gastrointestinal and Liver Physiology. 2000;279:G781–90. doi: 10.1152/ajpgi.2000.279.4.G781. [DOI] [PubMed] [Google Scholar]
- Connor TJ, Song C, Leonard BE, Merali Z, Anisman H. An assessment of the effects of central interleukin-1-, -2, -6, and tumor necrosis factor-alpha administration on some behavioural, neurochemical, endocrine, and immune parameters in rat. Neuroscience. 1998;84:923–33. doi: 10.1016/s0306-4522(97)00533-2. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3(8):655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. A new view of pain as a homeostatic emotion. Trends in Neurosciences. 2003;26:303–7. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- Craig AD, Andrew D. Responses of spinothalamic lamina I neurons to repeated brief contact heat stimulation in the cat. Journal of Neurophysiology. 2002;87:1902–14. doi: 10.1152/jn.00578.2001. [DOI] [PubMed] [Google Scholar]
- Crawford JM. The gastrointestinal tract. In: Cotran RS, Kumar V, Collins T, editors. Pathologic Basis of Disease. 6th edn. Philadelphia: WB Saunders; 1999. pp. 775–43. [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes’ Error. New York: Putnam; 1994. [Google Scholar]
- Damasio AR. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. New York: Harcourt Brace; 1999. [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3:1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Dana CL. The anatomic seat of the emotions: a discussion of the James–Lange theory. Archives of Neurology and Psychiatry. 1921;6:634–39. [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, Bernston GG, editors. The Handbook of Psychophysiology. 2nd edn. New York: Cambridge University Press; 2000. [Google Scholar]
- Denton D, Shade R, Zamarippa F, et al. Neuroimaging of genesis and satiation of thirst and interoceptor-driven theory of origins of primary consciousness. Proceedings of the National Academy of Sciences USA. 1999;96:5304–9. doi: 10.1073/pnas.96.9.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. The role of cytokines in infection-related behavior. Annals New York Academy of Sciences. 1998;840:577–85. doi: 10.1111/j.1749-6632.1998.tb09596.x. [DOI] [PubMed] [Google Scholar]
- Edelberg R. Electrical activity of the skin: its measurements and uses in psychophysiology. In: Greenfield NS, Sternbach RA, editors. Handbook of Psychophysiology. New York: Holt, Rinehart and Winston; 1972. pp. 367–418. [Google Scholar]
- Egloff B. The independence of positive and negative affect depends on the affect measure. Personality and Individual Differences. 1998;25:1101–9. [Google Scholar]
- Enck P, Holtmann G. Stress and gastrointestinal motility in animals: a review of the literature. Journal of Gastrointestinal Motility. 1992;1:83–90. [Google Scholar]
- Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RS, Corfeld DR. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. Journal of Neurophysiology. 2002;88:1500–11. doi: 10.1152/jn.2002.88.3.1500. [DOI] [PubMed] [Google Scholar]
- Fullwood A, Drossman DA. The relationship of psychiatric illness with gastrointestinal disease. Annual Review of Medicine. 1995;46:483–96. doi: 10.1146/annurev.med.46.1.483. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiology & Behavior. 2005;84:697–706. doi: 10.1016/j.physbeh.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotion elicitation using films. Cognition and Emotion. 1995;9:87–108. [Google Scholar]
- Harkin D. Animal Hospital. TV series. BBC. London; 1995. [Google Scholar]
- Hermann GE, Tovar C, Rogers CF. LPS-induced suppression of gastric motility relieved by TNFR:Fc construct in dorsal vagal complex. American Journal of Physiology. 2003;284:G634–39. doi: 10.1152/ajpgi.00412.2001. [DOI] [PubMed] [Google Scholar]
- Hohmann G. Some effects of spinal cord lesions on experienced emotional feelings. Psychophysiology. 1966;3:526–34. doi: 10.1111/j.1469-8986.1966.tb02690.x. [DOI] [PubMed] [Google Scholar]
- Hosseini JM, Goldhill JM, Bossone C, Pineiro-Carrero VM, Shea-Donohue T. Progressive alterations in circular smooth muscle contractility in tnbs-induced colitis in rats. Neurogastroenterology & Motility. 1999;11:347–56. doi: 10.1046/j.1365-2982.1999.00165.x. [DOI] [PubMed] [Google Scholar]
- Hughes J. She's having a baby. Paramount Pictures. Los Angeles; 1988. [Google Scholar]
- Iadarola MJ, Berman KF, Zeffiro TA, et al. Neural activation during acute capsaicin-evoked pain and allodynia assessed with PET. Brain. 1998;121:931–47. doi: 10.1093/brain/121.5.931. [DOI] [PubMed] [Google Scholar]
- James W. What is an emotion? Mind. 1884;9:188–205. [Google Scholar]
- Jones DP. The great Canadian train ride. Los Angeles; 1993. [Google Scholar]
- Kang YM, Lamb K, Gebhart GF, Bielefeldt K. Experimentally induced ulcers and gastric sensory-motor function in rats. American Journal Physiology. 2005;288:G284–91. doi: 10.1152/ajpgi.00250.2004. [DOI] [PubMed] [Google Scholar]
- Katz RC, Flasher L, Cacciapaglia H, Nelson S. The psychosocial impact of cancer and lupus: a cross validation study that extends the generality of “benefit-finding” in patients with chronic disease. Journal of Behavioral Medicine. 2001;24:561–71. doi: 10.1023/a:1012939310459. [DOI] [PubMed] [Google Scholar]
- King SW, Kubrick S, Johnson D, Richards MP. The Shining. Warner Bros. Hollywood; 1980. [Google Scholar]
- King SW, Lambert MD, Zinnemann TP. Pet Cemetery. Paramount Pictures. Hollywood; 1989. [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Trends in Neurosciences. 2002;25:154–59. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1983;30:261–73. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–73. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lange CG. In: Über Gemuthsbewegungen. Thomas T, editor. Leipzig: 1887. [Google Scholar]
- Lomax A, Fernandez E, Sharkey K. Plasticity of the enteric nervous system during intestinal inflammation. Neurogastroenterology and Motility. 2005;17:4–15. doi: 10.1111/j.1365-2982.2004.00607.x. [DOI] [PubMed] [Google Scholar]
- Marañon G. Contribution a l’etude de l’action emotive de l’adrenaline. Revue Française D’Endocrinologie. 1924;2:301–25. [Google Scholar]
- Marion F, Newman WW, Zeffirelli FD. The Champ. Metro-Goldwyn Mayer. Hollywood; 1979. [Google Scholar]
- Maunder RG. Panic disorder associated with gastrointestinal disease: review and hypotheses. Journal of Psychosomatic Research. 1998;44:91–105. doi: 10.1016/s0022-3999(97)00133-5. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Collins SM, Shea-Donohue T. Changes in enteric neural circuitry and smooth muscle in the inflamed gut. Neurogastroenterology and Motility. 2004;16(Suppl. 1):133–36. doi: 10.1111/j.1743-3150.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- Muth ER, Koch KL, Stern RM, Thayer JF. Effect of autonomic nervous system manipulations on gastric myoelectrical activity and emotional responses in healthy human subjects. Psychosomatic Medicine. 1999;61:297–303. doi: 10.1097/00006842-199905000-00008. [DOI] [PubMed] [Google Scholar]
- Muth ER, Thayer JF, Stern RM, Friedman B, Drake C. The effect of autonomic nervous system activity on gastric myoelectrical activity: does the spectral reserve hypothesis hold for the stomach? Biological Psychology. 1998;47:265–78. doi: 10.1016/s0301-0511(97)00030-6. [DOI] [PubMed] [Google Scholar]
- Naqvi N, Bechara A. Psychophysiological approaches to the study of decision-making. In: Senior C, Russel T, Gazzaniga M, editors. Methods in mind: The study of human cognition. Cambridge: MIT Press; (in press) [Google Scholar]
- Nelsen T, Kohatsu S. Clinical electrogastrography and its relationship to gastric surgery. American Journal of Surgery. 1968;116:215–22. doi: 10.1016/0002-9610(68)90496-0. [DOI] [PubMed] [Google Scholar]
- Oatley K. Emotional payoffs. Trends in Cognitive Sciences. 2004;8:55–7. [Google Scholar]
- Panaccione R, Ferraz JG, Beck P. Advances in medical therapy of inflammatory bowel disease. Current Opinion in Pharmacology. 2005;5:566–72. doi: 10.1016/j.coph.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC. Emotion: a reconsideration of the somatic theory. New York: Gordon and Breach; 1988. [Google Scholar]
- Phillips ML, Young AW, Senior C, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–98. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends in Neuroscience. 2002;25:319–25. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Prinz J. Gut Reactions: A Perceptual Theory of Emotion. New York: Oxford University Press; 2004. [Google Scholar]
- Quigley EMM, Conklin JL. The “big” brain and the “little” brain: interactions between the enteric and central nervous systems in the regulation of gut function. In: Quigley EMM, Pfeiffer RF, editors. Neuro-gastroenterology. Philadelphia: Butterworth Heinemann; 2004. pp. 3–14. [Google Scholar]
- Rosenthal R, Rosnow RL, Rubin DB. Contrasts and Effect Sizes in Behavioral Research. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annual Review of Neuroscience. 2002;35:433–69. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Schiller JH, Storer BE, Witt PL, et al. Biological and clinical effects of intravenous tumor necrosis factor-alpha administered three times weekly. Cancer Research. 1991;51:1651–58. [PubMed] [Google Scholar]
- Schmuckle SC, Egloff B, Burns LR. The relationship between positive and negative affect in the positive and negative affect schedule. Journal of Research in Personality. 2002;36:463–75. [Google Scholar]
- Schölmerich J. Review article: systemic and topical steroids in inflammatory bowel disease. Alimentary Pharmacology & Therapeutics. 2004;20(Suppl. 4):66–74. doi: 10.1111/j.1365-2036.2004.02059.x. [DOI] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39:701–11. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Smout AS, Van der Schee E, Grashuis J. What is measured in electrogastrography? Digestive Diseases & Sciences. 1980;25:179–87. doi: 10.1007/BF01308136. [DOI] [PubMed] [Google Scholar]
- Stenson WF. Inflammatory bowel disease. In: Yamada T, Alpers DH, Owyang C, Powell DC, Silverstein FE, editors. Textbook of Gastroenterology. 2nd edn. Vol. 2. Philadelphia: JB Lippincott Co; 1995. pp. 1748–1806. [Google Scholar]
- Stern RM, Koch KL, Muth ER. The gastrointestinal system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophsyiology. 2nd edn. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- Steves R. Germany: The Rhine and Mosel; the romantic road. Questar Inc. Los Angeles; 1991. [Google Scholar]
- Taché Y. Stress induced alterations of gastric emptying. In: Bueno L, Collins S, Junien JL, editors. Stress and Digestive Motility. John Libbey Eurotext; 1989. pp. 123–32. [Google Scholar]
- Tataranni PA, Gautier JF, Chen K, Ueker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proceedings of the National Academy of Sciences USA. 1999;96:4569–74. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D. Electrodermal activity in cognitive neuroscience: Neuroanatomical and neurophysiological correlates. In: Lane R, Nadel L, editors. Cognitive Neuroscience of Emotion, Series in Affective Science. New York: Oxford University Press; 2000. pp. 192–224. [Google Scholar]
- Tremaine WJ. Pathology and pathophysiology of symptoms. In: Prantera C, Korelitz BI, editors. Crohn's Disease. New York: Marcel Dekker, Inc.; 1996. pp. 93–112. [Google Scholar]
- Venables PH, Christie MJ. Electrodermal activity. In: Martin I, Venables PH, editors. Techniques of Psychophysiology. Chichester, UK: Wiley; 1980. pp. 3–67. [Google Scholar]
- Vianna EPM, Tranel D. Gastric myoelectrical activity as an index of emotional arousal. International Journal of Psychophysiology. doi: 10.1016/j.ijpsycho.2005.10.019. (in Press) [DOI] [PubMed] [Google Scholar]
- Wang H-L, Lee A, Schamus J. Tortilla soup. Samuel Goldwyn Company. Los Angeles; 2001. [Google Scholar]
- Waters J. Pink Flamingos. New Line Studios. Hollywood; 1973. [Google Scholar]
- Waldstein SR, Kop WJ, Schmidt LA, Hauffer AJ, Krantz DS, Fox NA. Frontal electrocortical and cardiovascular reactivity during happiness and anger. Biological Psychiatry. 2000;55:3–23. doi: 10.1016/s0301-0511(00)00065-x. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark L, Tellegen A. Development and validation of brief measures of positive and negative affect: the panas scales. Journal of Personality Social Psychology. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Watson D, Wiese D, Vaidya J, Tellegen A. The two general activation systems of affect: structural findings, evolutionary considerations, and psychobiological evidence. Journal of Personality and Social Psychology. 1999;76:820–38. [Google Scholar]
- Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition—the case for a head-to-toe inflammatory paradigm. Journal of the American Geriatrics Society. 2002;50:2041–56. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- Wood JD. Enteric nervous control of motility in the upper gastrointestinal tract in defensive states. Digestive Diseases & Sciences. 1999;44(8 Suppl):44S–52S. [PubMed] [Google Scholar]
- Woods SC. Metabolic signals in the control of food intake. In: Stricker EM, Woods SC, editors. Handbook of Behavioral Neurology. 2nd edn. New York: Kluwer Academic/Plenum; 2004. pp. 243–74. [Google Scholar]
- Yamada K, Iida R, Miyamoto Y, Saito K, Sekikawa K, Seishima M, Nabeshima T. Neurobehavioral alterations in mice with a targeted deletion of the tumor necrosis factor- gene: implications for emotional behavior. Journal of Neuroimmunology. 2000;111:131–38. doi: 10.1016/s0165-5728(00)00375-1. [DOI] [PubMed] [Google Scholar]