Abstract
Social relationships are particularly important during adolescence. In recent years, histological and MRI studies have shown that the brain is subject to considerable structural development during adolescence. Brain regions that are implicated in social cognition, including parts of prefrontal, parietal and superior temporal cortex, undergo the most pronounced and prolonged change. However, the development of social cognition during adolescence and its neural underpinnings remains poorly understood. Here, we begin by outlining how the brain changes between childhood and adulthood. We then describe findings that have emerged from behavioural and neuroimaging studies of the recognition of facial expression during adolescence. Finally, we present new data that demonstrate development of emotional perspective taking during adolescence. In this study, 112 participants, aged 8–36 years, performed a computerised task that involved taking an emotional perspective either from the participant's own point of view or from that of another person. The results showed that average difference in reaction time (RT) to answer questions in the first person perspective (1PP) and third person perspective (3PP) significantly decreased with age. The RT difference of adults tended to cluster close to the zero line (3PP = 1PP), while a greater proportion of pre-adolescents had higher difference values in both the positive (3PP > 1PP) and negative direction (1PP > 3PP) of the scale. The data suggest that the efficiency, and possibly strategy, of perspective taking develop in parallel with brain maturation and psychosocial development during adolescence.
Keywords: perspective taking, brain development, adolescence, social cognition, prefrontal cortex, parietal cortex
INTRODUCTION
Adolescence is the transitional period between late childhood and the beginning of adulthood, and marks the beginning of the reproductive lifespan in humans. Adolescence involves sexual maturity in terms of hormones and physical development of the body, and is also characterised by an increase in the complexity of group interactions and thus social behaviour (Lerner and Steinberg, 2004). Adolescence is a period of development and consolidation of the social self, of one's identity and understanding of the self in relation to the social world (Coleman and Hendry, 1990). Anecdotal evidence and self-report data suggest that children seem to become progressively self-conscious and concerned with other people's opinions as they go through puberty and the period of adolescence (Steinberg, 2005). The psychosocial context of adolescents is markedly different to that of children and adults. Relationships with peers, family and society go through distinct changes during this time. Adolescents begin to assert more autonomous control over their decisions, emotions and actions, and start to disengage from parental control. At the same time, the school context involves an intense socialisation process during which adolescents become increasingly aware of the perspectives of classmates, teachers and other societal influences (Berzonsky and Adams, 2003).
Recent evidence has shown that the brain goes through a remodelling process during adolescence. It is possible that neural plasticity facilitates the development of social cognitive skills required during the period of adolescence. In the following section, we describe evidence for neural development during adolescence.
DEVELOPMENT OF THE ADOLESCENT BRAIN
Recent structural MRI studies have demonstrated that the brain undergoes considerable development during adolescence. Both cross-sectional and longitudinal data demonstrate that changes in the frontal and parietal regions are especially pronounced and prolonged (Giedd et al., 1999; Sowell et al., 2003; Gogtay et al., 2004; Toga et al., 2006). Grey matter (GM) development in these areas is non-linear, in contrast to its linear development in the occipital lobes. The volume of GM in the frontal lobes increases during childhood with a peak occurring at around 12 years for males and 11 years for females, roughly coinciding with the age of puberty onset. This is followed by a decline in GM volume during adolescence (Giedd et al., 1999; Sowell et al., 2003; Gogtay et al., 2004; Toga et al., 2006). Similarly, parietal lobe GM volume increases during the pre-adolescent stage to a peak at around 12 years for males and 10 years for females, and is followed by a decline during adolescence (Giedd et al., 1999; Gogtay et al., 2004). While frontal and parietal cortex development is relatively rapid during adolescence, GM in the superior temporal cortex, including superior temporal sulcus (STS), reaches a peak at around 16 years and then follows a steady decline, not reaching maturity until relatively late (Toga et al., 2006). At the same time, there is an increase in prefrontal cortex (PFC) and parietal cortex white matter (WM) density from puberty onset, throughout adolescence and into adulthood (Giedd et al., 1996; 1999; Reiss et al., 1996; Sowell et al., 2001; Barnea-Goraly et al., 2005; for more detailed reviews of structural development in the brain, see Paus, 2005; Blakemore and Choudhury, 2006; Toga et al., 2006).
Earlier post-mortem investigations of human brain development revealed that two main cellular processes occur in the frontal cortex during adolescence: synaptogenesis followed by synaptic pruning (Huttenlocher, 1979; Huttenlocher et al., 1983); and axonal myelination (Yakovlev and Lecours, 1967). Myelinated axons appear white in MR images, whereas non-myelinated matter appears grey. Thus, the increase in WM seen in certain brain areas in MRI images during childhood and adolescence is thought to reflect the increase in myelination in those areas. The decrease in GM during adolescence might simply be a consequence of the increase in WM (since there is no increase in total brain volume). However, the non-linearity of GM development suggests it does not simply reflect the consequences of increased WM. Instead, it has been suggested that the pattern of GM development reflects, at least in part, the synaptic reorganisation that takes place during that period (Paus, 2005). The combined effect of these maturational processes might be to fine-tune neural circuitry in the PFC and other cortical regions, and thus increase efficiency of the cognitive systems they subserve (see Blakemore and Choudhury, 2006 for review).
CHANGES IN COGNITION: DEVELOPMENT OF EXECUTIVE FUNCTION
Structural development of these cortical regions may influence cognitive functioning during adolescence. A combination of behavioural and fMRI studies have demonstrated development of executive functions, that is, cognitive skills that enable the control and coordination of thoughts and behaviour, which are generally associated with the PFC (Luria, 1966; Shallice, 1982). Behavioural studies of performance on tasks including inhibitory control (Leon-Carrion et al. 2004; Luna et al. 2004a), processing speed (Luna et al. 2004a), prospective memory (MacKinlay et al., 2003), working memory (Anderson et al., 2001), decision-making (McGivern et al., 2002; Hooper et al. 2004; Luciana et al. 2005) and risk-taking (Gardner and Steinberg, 2005) continue to develop during adolescence. fMRI studies have shown that performance changes in executive function tasks are related to PFC development (Casey et al., 1997; Gaillard et al., 2000; Luna et al., 2001; Tamm et al., 2002; Bjork et al., 2004; Brown et al., 2005).
NEURAL PLASTICITY AND THE DEVELOPMENT OF INTELLIGENCE
A recent longitudinal MRI study of participants aged between 3 and 29 years revealed that the trajectory of change in cortical thickness is associated with the development of IQ (Shaw et al., 2006). The relationship between cortical thickness and IQ, as indexed by Wechsler intelligence scales, was found to vary with age. Stratification of participants into three IQ bands (average, high and superior IQ) indicated that the maximum trajectory differences between groups were in superior frontal gyrus bilaterally extending into the medial PFC. The developmental shift in trajectory was most pronounced for the most intelligent children and adolescents: the children with the highest IQ had a thinner cortex in early childhood but cortical thickness then increased, peaking at around age 11, and then underwent the most dramatic cortical thinning thereafter. Shaw and colleagues proposed that intelligence levels relate to how the cortex changes during development.
While several studies have investigated the development of executive function in adolescence, as yet, few have looked at the development of social cognition during this period. In the next section, we describe studies that have focussed on the development of socio-emotional processing during adolescence.
DEVELOPMENT OF SOCIAL COGNITION
Emotion processing in adolescence
The environmental and biological changes at adolescence lead to new social encounters and heightened awareness and interest in other people. The importance of evaluating other people may be associated with increased attention to socially salient stimuli, particularly faces, and the processing of emotional information. Recognition of facial expressions of emotion is one area of social cognition that has been investigated during adolescence (Herba and Phillips, 2004). The amygdala, a brain region associated with emotion processing (Adolphs, 1999; Dolan, 2002; Phillips et al., 2003), was found to be significantly activated in response to the perception of fearful facial expressions in an fMRI study of adolescents aged between 12 and 17 years (Baird et al., 1999). The perception of happy faces compared with neutral was associated with significant bilateral amygdalar activation in a group of 12 adolescents aged 13–17 years (Yang et al., 2003). Sex-differences in amygdala-mediated cognitive development have also been reported to occur during adolescence (Killgore et al., 2001). While the left amygdala responded to fearful facial expressions in all children, left amygdala activity decreased over the adolescent period in females but not in males. Females also demonstrated greater activation of the dorsolateral PFC over this period, whereas males demonstrated less activation in this region with age. These findings were taken as evidence for an association between cerebral maturation and increased regulation of emotional behaviour; the latter mediated by prefrontal systems. A similar result was found in a recent study by Yurgelun-Todd and Killgore (2006) in a study of facial emotion processing in adolescents. In this study, bilateral prefrontal activity increased with age (from 8 to 15 years) for girls, whereas only the activity in right PFC was correlated with age in boys. It is possible that functional maturation associated with face emotion processing may be modulated by gender-specific hormonal profiles.
The effect of age on amygdala response to fearful facial expressions was addressed byThomas and colleagues (2001). Adults (mean age 24 years) relative to children (mean age 11 years) demonstrated greater amygdala activation to fearful facial expressions, whereas children relative to adults showed greater amygdala activation to neutral faces. It was argued that the children perceived the neutral faces as more ambiguous than the fearful facial expressions, with resulting increases in amygdala activation to the neutral faces. Age-related differences in neural strategies for emotion processing have been shown in an fMRI study of a group of adolescents (aged 7–17 years) and a group of adults (aged 25–36 years) who viewed faces showing emotional expressions. While viewing faces with fearful emotional expressions, compared with adults, adolescents exhibited greater activation of the amygdala, orbitofrontal cortex (OFC) and anterior cingulate cortex (ACC) (Monk et al. 2003). When subjects were asked to switch their attention between a salient emotional property of the face, like thinking about how afraid it makes them feel, and a non-emotional property, such as how wide the nose is, adults, but not adolescents, selectively engaged and disengaged OFC. In other words, the adult brain better modulated OFC activity based on attention demands, while the adolescent brain better modulated activity based on the demands of emotion. On the other hand, when there were no attentional demands, emotional content of the stimuli induced higher activity in ACC, OFC and amygdala in the adolescents compared with the adults. These fMRI results suggest that both the brain's emotion processing and cognitive appraisal systems develop during adolescence. This development has previously been interpreted in the context of the Social Information Processing Network (SIPN) model (Nelson et al., 2005).
The SIPN model posits that social information processing occurs by way of three interacting neural ‘nodes’, which afford the detection of social stimuli that are then integrated to a larger emotional and cognitive framework (Nelson et al., 2005). Nelson and colleagues propose that the ‘detection node’, comprising the intraparietal sulcus, STS, fusiform face area as well as temporal and occipital regions, deciphers social properties of the stimulus such as biological motion. The ‘affective node,’ including limbic areas of the brain including the amygdala, ventral striatum, hypothalamus and OFC, processes the emotional significance of the social stimulus. Finally, the ‘cognitive-regulatory node’, consisting of much of the PFC, is responsible for theory of mind, impulse inhibition and goal-directed behaviour. Development during adolescence of the nodes, the connections between them, the innervation by gonadal steroid receptors and the maturation of the neural substrates themselves, are proposed to explain development of social cognitive behaviours.
The imaginary audience
The emergence of the social self seems to be marked by a period of heightened self-consciousness, during which adolescents are thought to become increasingly preoccupied with other people's concerns about their actions, thoughts and appearance. This development has been described in terms of phases of egocentrism during childhood and adolescence (Elkind, 1967) and is based on Piaget's stages of cognitive growth (Inhelder and Piaget, 1958). It is proposed that after children develop internal representations of objects and referential thinking during early childhood, they reach the stage of the ‘emergence of concrete operations’. Between the ages of 7 and 11 years, children's abilities to deal with classes and hierarchies are proposed to be restricted to concrete, physical entities and do not extend to abstract thought. Children of this age group therefore manifest an inability to distinguish between a mental construction and perceptual phenomena. By age 11, the emergence of ‘formal operational thought’ enables children to differentiate between the perception of an object and their own mental construction of it, allowing them to objectify their own thoughts and reason about them. Piaget proposed that this new form of thinking allows children at early adolescence to conceptualise other people's thoughts and take their perspectives (Inhelder and Piaget, 1958).
The development of adolescent egocentrism is therefore thought to be a dialectic process: it is the ability to represent other people's thoughts as distinct from their own and therefore decentre themselves that also drives the new form of egocentrism. In other words, as soon as they are able to understand that other people have distinct thoughts and perspectives, they become preoccupied with the notion that other people's thoughts are focused on their own behaviour or appearance (Elkind, 1967). Elkind's original theoretical model of adolescent egocentrism delineates two ideation patterns thought to arise as a consequence, and to characterise common adolescent social behaviours: the ‘imaginary audience’ and the ‘personal fable’. The notion of the imaginary audience refers to adolescents’ beliefs that they are the object of other people's scrutiny. According to Elkind's theory, this belief results in increased self-consciousness, a tendency to anticipate the reactions of other people in relation to the self, and a feeling of being the focus of attention, regardless of whether a real audience exists or not in the situation. The personal fable, a related construct, denotes adolescents’ convictions of their own personal uniqueness, giving rise to the sense of being ‘special’ (Elkind, 1967).
Since this original account of adolescent egocentrism, social psychological studies have investigated the imaginary audience with questionnaires and qualitative approaches. The exact age, validity and explanation (e.g. Lapsely and Murphey, 1985; Frankenberger, 2000; Vartinian and Powlishta, 2001; Bell and Bromnick, 2003) of Elkind's account of adolescent egocentrism have been challenged, and the theory has since evolved.
Perspective taking
The ability to take another's perspective is crucial for successful social communication. Reasoning about others, and understanding what they think, feel or believe, involves stepping into their ‘mental shoes’ and taking their perspective (Gallese and Goldman, 1998). The distinction between the phenomenal and representational levels of self-other relationships is worth noting. As detailed by Frith and de Vignemont (2005) and Vogeley and colleagues (2004), one can take different perspectives in terms of spatial representations, such that the locations of other entities in space are represented by the beholder in different reference frames. In an egocentric frame of reference, the location of an object is represented in relation to the subject, i.e. in relation to the personal agent (e.g. is the line on your right or left?), whereas in the allocentric frame of reference, the location of one object in relation to another object is represented by the agent (e.g. is the line on the right or left of the square?). Thus, while the egocentric perspective relates that which is seen to the agent who sees it, the allocentric perspective is independent of the agent's position. At the phenomenal level, however, the first-person perspective (1PP) (e.g. is the line on your right or left?) and the third-person perspective (3PP) (e.g. is the line on his right or left?) are both centred on an agent. Perspective taking at this phenomenal level requires ‘the translocation of the egocentric viewpoint’ from the 1PP to the 3PP (Vogeley and Fink, 2003).
Perspective taking includes awareness of one's own subjective space or mental states (‘first-person perspective’ or 1PP) and the ability to ascribe locations, mental states or emotions to another person (‘third-person perspective’ or 3PP). Perspective taking is related to first-order theory of mind in that it involves surmising what another person is thinking or feeling (Harris, 1989). It requires the ability to distinguish the self from someone else and appreciate another's intentions or beliefs. The ability to adopt another's viewpoint may underpin the ability to read other minds and understand another's feelings (Humphrey, 1976). Thus, emotional perspective taking, considering how ‘she’ would feel rather than how ‘I’ would feel necessitates a shift in the egocentric perspective, from one's own to another person's egocentric perspective.
Mechanisms of perspective taking
There is currently much debate surrounding the mechanism of perspective taking. How do we automatically switch roles from the self to the other in everyday social interactions? One prevalent view is that we understand others by mentally simulating their actions (Goldman, 1989; Harris, 1995). In support of this ‘simulation theory’, a growing body of evidence from neurophysiological studies has demonstrated that common brain areas are activated both when we execute an action and when we observe another person perform the same action (Grafton et al., 1996; Rizzolatti et al., 1996a, b; Decety et al., 1997; Buccino et al., 2001). Simulation theorists draw on the existence of a mirror neuron system to suggest that simulating other people's actions is an ontogenic precursor to understanding their thoughts and emotions (Gallese and Goldman, 1998). On the other side of the debate is ‘theory theory’, or the idea that we use a common sense psychological theory, or folk psychology, to understand other minds, rather than internally simulating them (e.g. Gopnik and Meltzoff, 1997).
Perspective taking and the brain
The brain regions that undergo the most significant development during adolescence overlap with those that have been linked to the ability to take other people's perspectives and infer mental states. Functional neuroimaging studies have revealed that medial PFC, IPL and STS are associated with making the distinction between 3PP and 1PP at the motor (Ruby and Decety, 2001), visuo-spatial (Vogeley et al., 2004), conceptual (Vogeley et al., 2001; Ruby and Decety, 2003) and emotional (Ruby and Decety, 2004) level. While these have shown common activations in 1PP and 3PP conditions in prefrontal and parietal areas (Ruby and Decety, 2001; Vogeley et al., 2004), and common deactivations in areas such as lateral superior temporal cortex (Vogeley et al., 2004), differential activity between the two perspective conditions has also been reported. Taking someone else's perspective, whether it involves thinking about how another person would think or feel, or imagining them making an action, relative to one's own perspective, was associated with increased activity in medial superior frontal gyrus, left STS, left temporal pole and right IPL (Ruby and Decety, 2001, 2003, 2004). IPL has also been implicated in the distinction between self and other at the sensorimotor level (Farrer and Frith, 2002) as well as at a higher social cognitive level (Uddin et al., 2006). In line with simulation theory, therefore, common neural networks are recruited for 1PP and 3PP. However, Vogeley and colleagues (2001) suggest that the differential brain activity implies that simulation cannot be the only mechanism at play. Perhaps the distinct brain regions activated in 3PP and 1PP indicate who the agent is in the interaction.
The medial PFC also plays a role in differentiating between the self and an unknown other such as George W. Bush (Kelley et al., 2002), and between the self and a personally known other such as a close friend (Heatherton et al., 2006). Medial PFC has been consistently associated with mentalising (cf. Amodio and Frith, 2006). Mentalising refers to the inferences we naturally make about other people's intentions, beliefs and desires, which we then use to predict and make sense of their behaviour (Fletcher et al., 1995). A number of neuroimaging studies, using a wide range of tasks, have reported activation in what seems to be a highly circumscribed mentalising network, comprising the medial PFC, STS especially around the temporo-parietal junction (TPJ), and the temporal poles adjacent to the amygdala (Fletcher et al., 1995; Brunet et al., 2000; Castelli et al., 2000; Gallagher et al., 2000; Vogeley et al., 2001; see Frith and Frith, 2003, 2006, for detailed reviews of the differential roles of these brain areas social cognition).
Recently, using fMRI, Aichhorn and colleagues (2006) investigated whether, like social perspective taking and theory of mind, visual perspective taking is associated with medial PFC activity. They developed a paradigm that strictly necessitated a shift of perspective in a computerised visuo-spatial task. The results demonstrated that medial PFC was not involved in visual perspective taking, but that posterior regions of the STS and TPJ were involved in judging what another can see. Drawing on results of differential neural networks shown to be involved in visual compared with social perspective taking in various studies, Aichhorn and colleagues (2006) suggested that different aspects of theory of mind are required in different types of perspective taking. They suggest that the dorsal posterior STS/TPJ region is involved in realising that minds represent the world differently and storing ‘cold’ facts about the mind. Medial PFC, on they other hand, is proposed to be involved representing ‘hot’ information such as predicting the emotional consequences of behaviour (Aichhorn et al., 2006).
Development of perspective taking during adolescence
A large body of research has focussed on the development of theory of mind during childhood (e.g. Wimmer and Perner, 1983; Perner et al., 1987; Gopnik and Astington, 1988) and its impairment in autism (e.g. Baron-Cohen et al., 1985). Since Piaget's studies using the three mountain problem (Piaget and Inhelder, 1948, 1956), only a handful of social psychology studies have investigated perspective taking in early to middle childhood (e.g. Bosacki and Astington, 1999) and, to our knowledge, none has considered its development during adolescence. Studies have shown that theory of mind develops in infancy by the age of 5 years (Barresi and Moore, 1996; Leslie, 1994). So, what are the cognitive consequences of the continued development of its underlying neural circuitry? Clearly, the development of associated abilities will be subtle. Given that the social environment dramatically changes during adolescence, and that the brain undergoes a restructuring process, it might be expected that social cognitive abilities such as perspective taking develop during adolescence. We therefore investigated development of perspective taking during adolescence.
Subjects
We recruited 107 right-handed participants, comprising 30 pre-adolescent children (12 males, mean age 8.6 years, s.d. = 0.46), 40 adolescents (19 males, mean age 12.8 years, s.d. = 1.20) and 37 adults (19 males, mean age 24.0 years, s.d. = 4.05) (Choudhury et al., 2005). The study was approved by the local ethics committee. Written consent was obtained from each participant, and from his or her parent or guardian for subjects under 16.
Perspective taking task
The task involved answering questions that required the participant to imagine either how s/he would feel (for 1PP scenarios), or how a protagonist (for 3PP scenarios) would feel in various scenarios (Figure 1). The participant was asked to choose as quickly as possible one of two emotional faces in answer to each question (from a total of five possible emotional faces). Each participant's non-directional reaction time difference between 3PP and 1PP (ΔRT) was calculated and analysed using a one-way ANOVA to test the effects of age and gender on ΔRT.
Fig. 1.
Development of perspective taking during adolescence. Total of 120 stimuli, each of which consisted of a one-line sentence describing an everyday scenario, were presented on a laptop computer screen together with a question concerning how the participant himself or herself (for 1PP scenarios), or how a protagonist (for 3PP scenarios), would feel in such circumstances. The participant pressed the space bar after reading the question at his or her own pace. This elicited the presentation of two possible response choices in the form of simple cartoon faces, each representing one of five possible emotions: very happy, happy, neutral, sad, afraid and angry. Faces were used so that verbal ability did not affect response time. The participant was asked to choose one of the two possible faces in answer to each question, as quickly and accurately as possible. The questions were delivered in four blocks of 30 question and answer stimuli, in a pseudorandom order that was counterbalanced between participants. Each block of questions lasted approximately 2 min. Reaction times (RT), taken as the time in milliseconds (ms) between the presentation of the answer screen (two faces) and the key press for the chosen answer, were recorded. A practice condition was included and instructions emphasised that the participant should pay careful attention to the person whose perspective they were required to take.
Results: development of efficiency for perspective taking
While there was no significant effect of gender, there was a significant main effect of age group showing that ΔRT decreased significantly with age (F(2,104) = 10.82; P < 0.0001; Table 1). Post hoc Bonferroni tests indicated that the mean ΔRT was significantly larger in pre-adolescents compared with adults (P < 0.0001) and in adolescents compared with adults (P < 0.005).
Table 1.
Reaction times (ms) in the two conditions (3PP and 1PP) and the difference in RT between them
| Mean ± SE RT in 3PP | Mean ± SE RT in 1PP | Mean ± SE ΔRT | |
|---|---|---|---|
| Pre-adolescent (N = 30) (mean age 8.6 years) | 1697.8 ± 63.3 | 1678.9 ± 62.3 | 139.3 ± 19.7 |
| Adolescent (N=40) (mean age 12.8 years) | 1236.4 ± 45.4 | 1181.1 ± 40.8 | 110.2 ± 14.1 |
| Adult (N=37) (mean age 24.0 years) | 889.9 ± 33.3 | 880.3 ± 33.5 | 49.7 ± 6.3 |
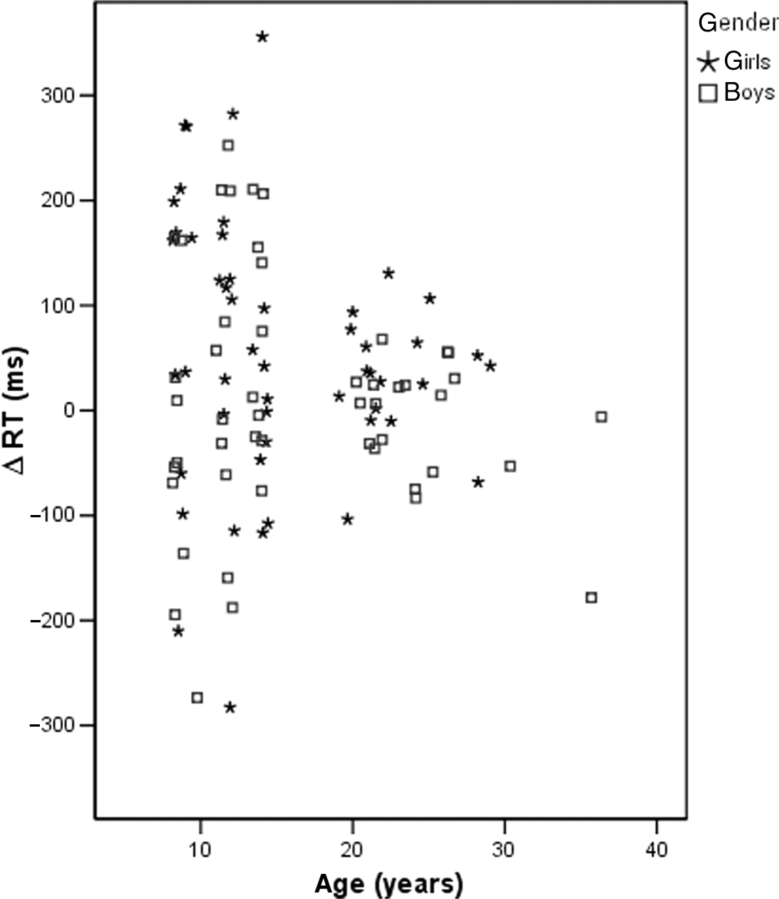
ΔRT was plotted to investigate directionality (Figure 2). As shown in the graph, ΔRT among both groups of younger participants was larger and spread almost equally in both directions (i.e. 3PP > 1PP and 1PP > 3PP), whereas among adults there was little difference with ΔRT values clustering around the zero mark of the difference scale (i.e. 3PP = 1PP). This finding suggests that the efficiency, or strategy, of perspective taking develops during adolescence, perhaps in parallel with the underlying neural circuitry.
Fig. 2.
Difference between 3PP and 1PP (ΔRT) against age, showing direction of ΔRT. Differences are larger and more scattered in both directions for the younger participants. With increasing age, differences get closer to zero. The data indicate that children and young adolescents may have a less systematic processing strategy.
Discussion
The synaptic reorganisation in the frontal and parietal cortices during adolescence is likely to have implications for social cognitive processes that depend on these brain regions, such as mentalising, perspective taking and related processes. This conjecture is supported by the findings from our perspective taking study. If we assume that, among the age groups tested, adults are most experienced in social interaction and have mature frontal and parietal neural circuitry, then a low difference in ΔRT (3PP = 1PP) is likely to indicate the highest proficiency in perspective taking. In contrast, the most pronounced difference in RT between 1PP and 3PP, seen in the pre-adolescent group, would therefore indicate relatively inefficient processing. It might be speculated therefore, that prior to adolescence, the unsystematic direction of ΔRT reflects an immature cognitive mechanism for perspective taking.
Whether this response pattern among pre-adolescents is a result of a relative difficulty in differentiating between the first- and third-person, or that children of this age group are less inclined, or find it more difficult, to step into another person's ‘mental shoes’, requires further investigation. The differences between age groups may also be influenced by differences in social experience. Compared with children and adolescents, adults are generally more skilled at instinctively inferring the perspectives of other people. Perhaps adults show no difference between RTs for 1PP and 3PP as a result of their mature neural circuitry supporting social cognition, as well as their greater social experience.
We have described how neuroimaging and behavioural data demonstrate that executive functions, recognition of facial emotions and emotional perspective taking develop during adolescence, in terms of cognitive and neural strategies. This may be interpreted in terms of the SIPN model (Nelson et al., 2005). Executive function abilities such as risk assessment, decision making and impulse inhibition, and social cognitive abilities such as facial emotion recognition and perspective taking, are associated with components of the three nodes of the SIPN, including PFC, amygdala and STS. These social cognitive processes show development, while the neural substrates themselves show plasticity in terms of actual structure or changes in activity with age in the social cognitive tasks we have reviewed.
However, the SIPN is perhaps also limiting as it neglects the role of the parietal cortex. Parietal cortex (particularly IPL) undergoes a developmental trajectory similar to that of PFC and is associated with processes related to social cognition, such as perspective taking in the motor, conceptual and emotional domains (Ruby and Decety, 2001, 2003, 2004) and imitation of other people's actions (Decety et al., 2002; Jackson et al., 2006). Parietal development has already been linked to the improvement in abstract reasoning skills during adolescence (Luna et al., 2004b; Qin et al., 2004). Further investigations are required to determine how parietal development influences social cognitive development, and which regions are particularly involved.
New studies might consider what mechanisms are directing social cognitive development. The SIPN proposes a ‘multi-step’ route, in which neurally based nodes process social stimuli in a sequential manner. Somewhere in this route, however, between detecting that a stimulus is animate and imbuing it with emotional significance, the brain must assign it to the correct agent. In other words, in the framework of the SIPN, an additional ‘agency node’ linked to IPL might be involved in distinguishing whether the action is related to the self or to another before the limbic node would process approach or avoidance decisions and before the cognitive-regulatory node would perform higher level social processing. Indeed, as mentioned above, IPL seems to be involved in distinguishing between self and other, in terms of imagining how someone would think or feel (Ruby and Decety, 2003, 2004), making an action (Farrer and Frith, 2002) or imagining making an action (Ruby and Decety, 2001).
A recent behavioural study demonstrated that, like perspective taking, the ability to imagine making an action develops during adolescence (Choudhury et al., in press). Two motor imagery tasks thought to tap action representations were administered to 40 young adolescents (24 males; mean age 13.1 years) and 33 adults (15 males; mean age 27.5 years). The tasks relied on the chronometry of executed (E) and imagined (I) hand actions. Typically, the timing of E and I are highly correlated owing to representations of motor constraints (Decety and Jeannerod, 1995; Sirigu et al., 1995, 1996). However, the correlation between E and I is significantly lower for parietal lesion patients (Sirigu et al., 1996; Wolpert et al., 1998), children with developmental co-ordination disorder (Wilson et al., 2001) and adults with schizophrenia (Maruff et al., 2003). The results of our study showed that there was a significant increase in the execution imagery time correlation between adolescence and adulthood, perhaps reflecting refinement of the action representation in the parietal cortex system.
While parietal cortex is involved in storing representations of one's own actions and differentiating between self and other, it has been proposed that STS is associated with the prediction of actions based on past actions, while medial PFC is involved in anticipating future consequences of actions based on internal mental states (Frith, in press). We know from executive function studies that the ability to suppress impulses develops during adolescence, perhaps in line with PFC maturation (Casey et al., 1997; Rubia et al., 2001; Adleman et al., 2002; Tamm et al., 2002). As the ability to inhibit one's own impulses develops and the ability to understand other people's mental states is refined, a change in the strategy for perspective taking might take place. Perhaps children and young adolescents exert relatively poor inhibition over their own egocentric bias, and therefore rely on predicting other people's emotional perspectives based on the consequences of their own past actions. Maturation of PFC circuitry might facilitate a strategic shift such that older adolescents and adults can predict the feelings of other people based on how they anticipate the other person would feel if s/he were to make the action in the given scenario. Development of IPL might enable older adolescents and adults to keep the self/other distinction intact, while in pre-adolescents, immature circuitry in the area might lead to a blurring between 1PP and 3PP and the imposition of the self-perspective onto the other.
CONCLUSION
Changes in social behaviour are driven by both social and biological factors. During adolescence, it is likely that peer interactions and societal influences as well as genetically determined hormonal milieu influence social behaviour. However, since the recent discovery that the brain matures considerably during adolescence, evidence has emerged pointing to the role of neural maturation in the development of social cognition during adolescence.
Perspective taking is a cognitive mechanism that underlies everyday social interaction. The significant decrease in the difference between RTs for 1PP and 3PP during adolescence found in the current study may reflect cognitive and behavioural features both experimentally (Steinberg, 2005) and anecdotally associated with adolescent development (Time Magazine, 7 June 2004). Our data suggest that, prior to adolescence, children are less efficient and have a less systematic style of processing the emotional perspectives of other people. Future neuroimaging studies are necessary to test our prediction that this reflects a developmental shift in the neural strategy required for perspective taking.
To what extent the developing brain interacts with socio-cultural influences in the environment of adolescents is a question for future research. Further studies are also needed to investigate the interaction between sexual maturity and social cognition. It is unknown, for example, how sex hormones influence the organisation of the brain's connectivity, and how this interacts with social cognition. Finally, as the recent study on IQ and cortical thickness (Shaw et al., 2006) highlights, the role of individual differences in cognitive skills must be taken into account.
Acknowledgments
This research was funded by the Medical Research Council, Child Health Research Appeal Trust and The Royal Society.
Footnotes
Conflict of Interest
None declared.
REFERENCES
- Adleman NE, Menon V, Blasey CM, et al. A developmental fMRI study of the Stroop color-word task. NeuroImage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Social cognition and the human brain. Trends in Cognitive Sciences. 1999;3:469–79. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- Aichhorn M, Perner J, Kronbichler M, Staffen W, Ladurner G. Do visual perspective tasks need theory of mind? Neuroimage. 2006;30:1059–68. doi: 10.1016/j.neuroimage.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anderson V, Anderson P, Northam E, Jacobs R, Catroppa C. Development of executive functions through late childhood and adolescence in an Australian sample. Developmental Neuropsychology. 2001;20:385–406. doi: 10.1207/S15326942DN2001_5. [DOI] [PubMed] [Google Scholar]
- Baird AA, Gruber SA, Fein DA, et al. Functional magnetic resonance imaging of facial affect recognition in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:195–9. doi: 10.1097/00004583-199902000-00019. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cerebral Cortex. 2005;15:1848–54. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Barresi J, Moore C. Intentional relations and social understanding. Behavioral and Brain Sciences. 1996;19:107–54. [Google Scholar]
- Bell JH, Bromnick RD. The social reality of the imaginary audience: a grounded theory approach. Adolescence. 2003;38:205–19. [PubMed] [Google Scholar]
- Berzonsky MD, Adams GR. The Blackwell Handbook of Adolescence. Oxford: Blackwell; 2003. [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. Journal of Neuroscience. 2004;24:1793–802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. Journal of Child Psychology and Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Bosacki S, Astington JW. Theory of mind in preadolescence: Relations between social understanding and social competence. Social Development. 1999;8:237–55. [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cerebral Cortex. 2005;15:275–90. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Brunet E, Sarfati Y, Hardy-Bayle MC, Decety J. A PET investigation of the attribution of intentions with a nonverbal task. Neuroimage. 2000;11:157–66. doi: 10.1006/nimg.1999.0525. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, et al. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. European Journal of Neuroscience. 2001;13:400–4. [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, et al. A pediatric functional MRI study of prefrontal activation during performance of a Go-No-Go task. Journal of Cognitive Neuroscience. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Castelli F, Happe F, Frith U, Frith C. Movement and mind: A functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12:314–25. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Choudhury S, Charman T, Bird V, Blakemore SJ. Development of action representation during adolescence. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2006.07.010. in press. [DOI] [PubMed] [Google Scholar]
- Choudhury S, Blakemore SJ, Charman T. Development of perspective taking during adolescence. Cognitive Neuroscience Society. 2005;B53:56. [Google Scholar]
- Coleman JC, Hendry L. The Nature of Adolescence. 2nd edn. Florence, KY: Taylor & Frances/Routledge; 1990. [Google Scholar]
- Decety J, Chaminade T, Grezes J, Meltzoff AN. A PET exploration of the neural mechanisms involved in reciprocal imitation. Neuroimage. 2002;15:265–72. doi: 10.1006/nimg.2001.0938. [DOI] [PubMed] [Google Scholar]
- Decety J, Jeannerod M. Mentally simulated movements in virtual reality: does Fitts's law hold in motor imagery? Behavioural Brain Research. 1995;72:127–34. doi: 10.1016/0166-4328(96)00141-6. [DOI] [PubMed] [Google Scholar]
- Decety J, Grezes J, Costes N, et al. Brain activity during observation of actions. Influence of action content and subject's strategy. Brain. 1997;120:1763–77. doi: 10.1093/brain/120.10.1763. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298:1191–4. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- Elkind D. Egocentrism in adolescence. Child development. 1967;38:1025–34. [PubMed] [Google Scholar]
- Farrer C, Frith CD. Experiencing oneself vs. another person as being the cause of an action: the neural correlates of the experience of agency. NeuroImage. 2002;15:596–603. doi: 10.1006/nimg.2001.1009. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happe F, Frith U, et al. Other minds in the brain: A functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–28. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Frankenberger KD. Adolescent egocentrism: a comparison among adolescents and adults. Journal of Adolescence. 2000;23:343–54. doi: 10.1006/jado.2000.0319. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society B Biological Science. 2003;358:459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD. In Proceedings of the Royal Society B Biological Science. (in press) [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50:531–4. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Frith U, de Vignemont F. Egocentrism, allocentrism, and Asperger syndrome. Consciousness and Cognition. 2005;14:719–38. doi: 10.1016/j.concog.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54:180–5. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happé F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of theory of mind in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gallese V, Goldman A. Mirror neurons and the simulation theory of mindreading. Trends in Cognitive Sciences. 1998;2:493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Developmental Psychology. 2005;41:625–35. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, et al. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cerebral Cortex. 1996;6:551–60. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence:a longitudinal MRI study. Nature Neuroscience. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Science United States of America. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman A. Mind and Language. Vol. 4. 1989. Interpretation psychologized; pp. 161–85. [Google Scholar]
- Gopnik A, Astington JW. Children's nderstanding of representational change and its relation to the understanding of false belief and the appearance-reality distinction. Child Development. 1988;59:26–37. doi: 10.1111/j.1467-8624.1988.tb03192.x. [DOI] [PubMed] [Google Scholar]
- Gopnik A, Meltzoff AN. Words, Thoughts, and Theories. Cambridge, MA: MIT Press; 1997. [Google Scholar]
- Grafton ST, Arbib MA, Fadiga L, Rizzolatti G. Localization of grasp representations in humans by positron emission tomography. Observation compared with imagination. Experimental Brain Research. 1996;112:103–111. doi: 10.1007/BF00227183. [DOI] [PubMed] [Google Scholar]
- Harris P. From simulation to folk psychology: the case for development. In: Davies M, Stone T, editors. Folk Psychology. Oxford: Blackwell; 1995. pp. 207–31. [Google Scholar]
- Harris P. Children and Emotion. Oxford: Blackwell Publishers; 1989. [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. Social Cognitive and Affective Neuroscience. 2006;1:18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herba C, Phillips M. Annotation: development of facial expression recognition from childhood to adolescence: behavioural and neurological perspectives. Journal of Child Psychology and Psychiatry. 2004;45:1185–98. doi: 10.1111/j.1469-7610.2004.00316.x. [DOI] [PubMed] [Google Scholar]
- Hooper CJ, Luciana M, Conklin HM, Yarger RS. Adolescents’ performance on the development of decision making and ventromedial prefrontal cortex. Developmental Psychology. 2004;40:1148–58. doi: 10.1037/0012-1649.40.6.1148. [DOI] [PubMed] [Google Scholar]
- Humphrey NK. The social function of intellect. In: Bateson PPG, Hinde RA, editors. Growing Points in Ethology. Cambridge: Cambridge University Press; 1976. [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex – developmental changes and effects of aging. Brain Research. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, De Courten C, Garey LJ, Van Der Loos H. Synaptic development in human cerebral cortex. International Journal of Neurology. 1983;16–17:144–54. [PubMed] [Google Scholar]
- Inhelder B, Piaget J. The Growth of Logical Thinking from Childhood to Adolescence. New York: Basic Books; 1958. [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. Neural circuits involved in imitation and perspective-taking. Neuroimage. 2006;31:429–39. doi: 10.1016/j.neuroimage.2005.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Oki M, Yurgelun-Todd DA. Sex-specific developmental changes in amygdale responses to affective faces. Neuroreport. 2001;12:427–33. doi: 10.1097/00001756-200102120-00047. [DOI] [PubMed] [Google Scholar]
- Lapsley DK, Murphy MN. Another look at the theoretical assumptions of adolescent egocentrism. Developmental Review. 1985;5:201–17. [Google Scholar]
- Leon-Carrion J, Garcia-Orza J, Perez-Santamaria FJ. The development of the inhibitory component of the executive functions in children and adolescents. International Journal of Neuroscience. 2004;114:1291–311. doi: 10.1080/00207450490476066. [DOI] [PubMed] [Google Scholar]
- Lerner R, Steinberg L, editors. Handbook of Adolescent Psychology. 2nd edn. New York: Wiley; 2004. [Google Scholar]
- Leslie AM. Pretending and believing: Issues in the theory of ToMM. Cognition. 1994;50:211–38. doi: 10.1016/0010-0277(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Luciana M, Conklin HM, Cooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Development. 2005;76:697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004a;75:1357–72. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B. Algebra and the adolescent brain. Trends in Cognitive Sciences. 2004b;8:437–9. doi: 10.1016/j.tics.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn K, Munoz D, et al. Maturation of widely distributed brain function subserves cognitive development. NeuroImage. 2001;12:786–93. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Luria AR. Higher Cortical Functions in Man. Oxford, UK: Basic Books Inc; 1966. [Google Scholar]
- Mackinlay R, Charman T, Karmiloff-Smith A. Remembering to remember: a developmental study of prospective memory in a multitasking paradigm. Poster presented at the Society for Research in Child Development, Biennial Meeting; 24–27 April 2003; Tampa, Florida. [Google Scholar]
- Maruff P, Wilson P, Currie J. Abnormalities of motor imagery associated with somatic passivity phenomena in schizophrenia. Schizophrenia Research. 2003;60:229–238. doi: 10.1016/s0920-9964(02)00214-1. [DOI] [PubMed] [Google Scholar]
- McGivern RF, Andersen J, Byrd D, Mutter KL, Reilly J. Cognitive efficiency on a match to sample task decreases at the onset of puberty in children. Brain & Cognition. 2002;50:73–89. doi: 10.1016/s0278-2626(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. NeuroImage. 2003;20:420–8. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Nelson E, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–74. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences. 2005;9:60–8. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Perner J, Leekam S, Wimmer H. Three-year-olds' difficulty with false belief: The case for a conceptual deficit. British Journal of Developmental Psychology. 1987;5:125–37. [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry. 2003;54:504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Piaget J, Inhelder B. The Child's Conception of Space. London: Routledge and Paul Kegan; 1948, 1956. [Google Scholar]
- Qin Y, Carter CS, Silk EM, et al. The change of the brain activation patterns as children learn algebra equation solving. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5686–91. doi: 10.1073/pnas.0401227101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119:1763–74. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Cognitive Brain Research. 1996a;3:131–41. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Matelli M, et al. Localization of grasp representations in humans by PET: 1. Observation versus execution. Experimental Brain Research. 1996b;111:246–252. doi: 10.1007/BF00227301. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, et al. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13:250–61. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nature Neuroscience. 2001;4:546–50. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. What you believe versus what you think they believe: a neuroimaging study of conceptual perspective-taking. European Journal of Neuroscience. 2003;17:2475–80. doi: 10.1046/j.1460-9568.2003.02673.x. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. Journal of Cognitive Neuroscience. 2004;16:988–99. doi: 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. (Series B: Biological sciences).Philosophical transactions of the Royal Society of London. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–9. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Cohen L, Duhamel J, et al. Congruent unilateral impairments for real and imagined hand movements. NeuroReport. 1995;6:997–1001. doi: 10.1097/00001756-199505090-00012. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Duhamel J, Cohen L, Pillon B, Dubois B, Agid Y. The mental representation of hand movements after parietal cortex damage. Science. 1996;273:1564–7. doi: 10.1126/science.273.5281.1564. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. Journal of Neuroscience. 2001;21:8819–29. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the life span. Nature Neuroscience. 2003;6:309–15. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. Journal of American Academy of Child and Adolescent Psychiatry. 2002;41:1231–8. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, et al. Amygdala response to facial expressions in children and adults. Biological Psychiatry. 2001;49:309–16. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends in Neurosciences. 2006;29:148–59. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Molnar-Szakacs I, Zaidel E, Iacoboni M. rTMS to the right inferior parietal lobule disrupts self-other discrimination. Social Cognitive and Affective Neuroscience. 2006;1:65–71. doi: 10.1093/scan/nsl003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian LR, Powlishta KK. Demand characteristics and self-report measures of imaginary audience sensitivity: implications for interpreting age differences in adolescent egocentrism. The Journal of Genetic Psychology. 2001;162:187–200. doi: 10.1080/00221320109597960. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Fink GR. Neural correlates of the first-person-perspective. Trends in Cognitive Sciences. 2003;7:38–42. doi: 10.1016/s1364-6613(02)00003-7. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Bussfeld P, Newen A, et al. Mind reading: Neural mechanisms of theory of mind and self-perspective. Neuroimage. 2001;14:170–81. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- Vogeley K, May M, Ritzl A, Falkai P, Zilles K, Fink GR. Neural correlates of first-person perspective as one constituent of human self-consciousness. Journal of Cognitive Neuroscience. 2004;16:817–27. doi: 10.1162/089892904970799. [DOI] [PubMed] [Google Scholar]
- Wilson PH, Maruff P, Ives S, Currie J. Abnormalities of motor and praxis imagery in children with DCD. Human Movement Science. 2001;20:135–59. doi: 10.1016/s0167-9457(01)00032-x. [DOI] [PubMed] [Google Scholar]
- Wimmer H, Perner J. Beliefs about beliefs: representations and constraining functions of wrong beliefs in young children's understanding of deception. Cognition. 1983;13:103–128. doi: 10.1016/0010-0277(83)90004-5. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Goodbody S-J, Husain M. Maintaining internal representations: the role of the human superior parietal lobe. Nature Neuroscience. 1998;1:529–33. doi: 10.1038/2245. [DOI] [PubMed] [Google Scholar]
- Yakovlev PA, Lecours IR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. Oxford: Blackwell; 1967. pp. 3–70. [Google Scholar]
- Yang TT, Menon V, Reid AJ, Gotlib IH, Reiss AL. Amygdalar activation associated with happy facial expressions in adolescents: a 3-T functional MRI study. Journal of the American Academy of child and Adolescent Psychiatry. 2003;48:979–85. doi: 10.1097/01.CHI.0000046886.27264.BA. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Killgore WD. Fear-related activity in the prefrontal cortex increases with age during adolescence: a preliminary fMRI study. Neuroscience Letters. 2006;406:194–9. doi: 10.1016/j.neulet.2006.07.046. [DOI] [PubMed] [Google Scholar]