Abstract
Social neuroscience suggests medial pre-frontal cortex (mPFC) as necessary for social cognition. However, the mPFC activates less to members of extreme outgroups that elicit disgust, an emotion directed toward both people and objects. This study aimed to counteract that effect. Participants made either superficial categorical age estimations or individuating food-preference judgments about people, while fMRI recorded neural activity. Besides replicating the reduced mPFC activity to extreme outgroups that elicit disgust, this study demonstrates that the same type of judgment for these individuals is processed in a region anatomically distinct from social groups that elicit exclusively social emotions (pity, envy, pride). Finally, inferring individuating information (food preferences) increases mPFC activation above superficial categorical judgments. This evidence fits differentiated mPFC processing of extreme outgroups, which activate mPFC less than other groups, but suggests that individuation increases activation.
Keywords: MPFC, social emotions, Stereotype Content Model, Continuum Model
The medial prefrontal cortex (mPFC) reliably activates in social cognition tasks, among others (for reviews, see Amodio and Frith, 2006; Lieberman, 2007). However, in a dramatic reversal of this now-standard effect, our prior study documents the standard mPFC activation to photographs of people only from social groups that elicit exclusively social emotions (e.g. pity, envy), but dramatically reduced mPFC to social groups that elicit non-exclusively social emotions (e.g. disgust) (Harris and Fiske, 2006); these results occur when viewers think about the emotion these people evoke. Because of its role in social cognition, reduced mPFC activity may suggest that people belonging to these latter extreme outgroups may be perceived differently, maybe even as less human.1
Work on schema-triggered affect suggests that some type of affective response accompanies person perception (Fiske and Pavelchak, 1986). Emotion researchers often differentiate between complex emotions that occur later in cognitive development, and more basic emotions that may be considered hard-wired into our affective repertoire (Barrett, 2006; Niedenthal et al., 2006). Similar to this distinction, one can argue that some emotions, referred to as exclusively social emotions, occur only in the actual, imagined or implied presence of other people, while non-exclusively social emotions can occur either in the presence of people or objects. For instance, people do not report feeling envy toward a desired object such as a sports car; rather they report feeling envy toward the owner of the sports car. As for the car itself, they may actually report liking it. Thus, what separates exclusively social emotions from non-exclusively social emotions is the necessary requirement that people, human beings, be present for the former, not the latter, to occur.
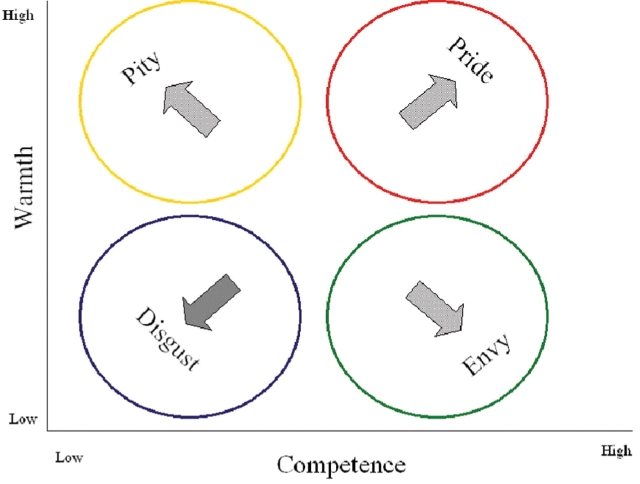
Two fundamental dimensions of social perception are perceived trait warmth and competence (Fiske et al., in press). The Stereotype Content Model (SCM; Fiske et al., 2002) predicts that perceived trait warmth and competence interact to differentiate affective reactions to members of distinct social groups (Figure 1). In a two-dimensional space of four quadrants, social groups perceived as high in both competence and warmth evoke from perceivers the ingroup social emotion of pride; groups perceived as high in competence but low in warmth receive the ambivalent social emotion of envy; groups perceived as high in warmth, but low in competence receive the paternalistic social emotion of pity. However, outgroups perceived as low in both warmth and competence elicit disgust, an emotion that is not exclusively social, being directed both at people and objects that seem repellant (Rozin and Fallon, 1987). Perceiving low–low outgroups as ‘disgusting’ suggests that people perceive these groups as so strikingly different that they do not evoke an exclusively social emotion.2 Infrahumanization theory (Leyens et al., 2001, 2003) states that people see some groups as less human than others; they judge outgroups themselves as not experiencing complex emotions to the same extent that the ingroup does. From the perceiver's own experience of emotion, SCM studies (Fiske et al., 2002; Cuddy et al., in press), including the initial neuro-imaging study (Harris and Fiske, 2006) suggest that extreme outgroups also do not elicit these complex, exclusively social emotions in the perceiver.
Fig. 1.
The emotions that result from the interaction of competence and warmth in the Stereotype Content Model. Pride, envy and pity are the three exclusively social emotions, while disgust is the non-exclusively social emotion.
If groups do not elicit an exclusively social emotion, can they also be individuated to the same extent as other social group actors? The Continuum Model of Impression Formation (CM; Fiske and Neuberg, 1990; Fiske et al., 1999) provides a theoretical backdrop within social psychology for person perception. According to this model, social perception occurs along a continuum from more categorical to more individuated impressions. When perceivers encounter social group members, they see them initially in terms of master statuses (age, race and gender in US samples; Fiske, 1998) that activate cognitive (stereotypic) and affective (prejudiced) responses. But motivated and informed perceivers can move along the continuum to form more nuanced, individuated impressions. The type of information that allows a more nuanced impression includes the person's idiosyncrasies: personality, accomplishments, skills and preferences (likes and dislikes). Particular judgments of preferences, for instance, may force one to go beyond the perceived social category to draw on information perceived to be unique to that social group member and experience an exclusively social emotion. Therefore, if perceivers initially consider individuating information when encountering an outgroup member who elicits a non-exclusively social emotion, they may activate mPFC where they did not before.
Additionally, inferring another's preferences causes perceivers to mentalize, that is, to consider what is inside another's mind. This type of mentalizing usually requires: observing behavior (buying broccoli), knowing the social consensus about the behavior (most people buy broccoli), knowing the actor's consistency (buying broccoli on every supermarket visit) and knowing the actor's distinctiveness in that behavior3 (always broccoli, never turnips; Kelley, 1972; McArthur, 1972). However, in social perception, this information is often not readily available. Thus, to judge preference, one must infer most of this information. Common sources of this inference include the behavior of the target's social group (stereotypes; Fiske et al., 1999) or even one's own behavior (simulation; Heal, 1996). Similarity to stereotypes or simulation could act as a proxy for the individual, which then allows the inference.
Recent behavioral work within social psychology suggests that similarity, inferring what is in another's mind and other dimensions significantly predict the degree to which outgroup members elicit the disgust emotion (Harris and Fiske, unpublished data). In two studies, participants first described a day in the life of a pictured social group member (only Study 1) before rating the person on a variety of dimensions derived from social neuroscience tasks that activated the mPFC (both studies). Results indicated that social group members who elicit disgust are perceived as less warm, competent, intelligent, articulate, similar and familiar. Further, participants reported more difficulty inferring what was in their heads, more difficulty inferring their dispositions, and the targets as experiencing more ups and downs in life—compared with social group members who elicited more social emotions (Harris and Fiske, unpublished data). This evidence, together with the social neuroscience evidence already mentioned, suggests a number of dimensions that may underlie the reduced mPFC activity observed in the initial neuro-imaging study.
Social neuroscience supports at least two of these dimensions in activating the mPFC. Work on similarity of the actor to the self provides an example (Mitchell et al., 2005). In one such paradigm, participants either decided how symmetrical a target's face appeared, or how pleased the target felt about having the picture taken. The correlation between the mPFC activity and similarity occurred only in this latter mentalizing task, suggesting that self-reflection indeed may be used as a proxy for the information about the target. This imaging work also implicitly posits a ventral–dorsal distinction for respectively similar–dissimilar social stimuli (Mitchell et al., 2005).
The role of inferred preferences in changing affective (prejudiced) neural signatures to outgroups also finds support in social neuroscience paradigms. For instance, faces of Black men can evoke a stronger amygdala and insula response in White participants than ingroup faces (Hart et al., 2000; Lieberman et al., 2005; Phelps et al., 2000; Wheeler and Fiske, 2005). This activation correlates with implicit measures of fear and prejudice, such as respectively the startle eye-blink response and the Implicit Association Test (IAT; Phelps et al., 2000; see also Amodio et al., (2003), for a similar correlation with event-related potentials). However, inferring the vegetable preference of the pictured Black man eliminates this differential amygdala and insula activation (Wheeler and Fiske, 2005). Thus, inferring preferences either affects the perceptual experience of the Black face or instantaneously recruits cognitive mechanisms that regulate the amygdala and insula activations, reducing these sub-cortical signatures of the prejudiced response. Similar processes may also occur for higher cortical areas.
In sum, the task of inferring preferences should re-activate the mPFC for social group members who otherwise elicit non-exclusively social emotions (e.g. disgust) for two reasons. First, the inference may depend on simulation scaled to similarity, indirectly suggesting social emotion elicitation. And second, even if self-generated, this information about another's preferences moves one further along the continuum to individuated impressions—reserved primarily for social targets who elicit exclusively social emotions and themselves reliably activate the mPFC. Therefore, we make two predictions: (i) significantly less mPFC activity to social group members who elicit non-exclusively social emotions, compared with those group members who elicit exclusively social emotions (a replication of the previous finding), and (ii) significantly more activity in the mPFC for (vegetable) preference judgments than for categorical (age) judgments.
METHOD
Participants
A total of 18 undergraduates from Princeton University participated for course credit. Participants were right-handed and reported no abnormal neurological condition, head trauma or brain lesions. All participants had normal or corrected vision and provided informed consent. The mean age was 20, with 10 women and seven ethnic minorities (four Asian, two Black, and one Hispanic).
Stimuli
Sixty-four color photographs of both dehumanized and humanized social group members appeared in the study. Half of these images had previously been pretested to fall into the low–low cell of the SCM (homeless people and drug addicts), reliably eliciting the non-exclusively social emotion disgust, while the remaining pictures fell into one of the other three SCM cells (college students, Americans,4 business people, rich people, disabled people and elderly people), reliably eliciting exclusively social emotions.
Scanning parameters
All fMRI scanning was conducted at Princeton's Center for the Study of Brain, Mind, and Behavior, which uses a 3.0 Tesla Siemens Allegra head-dedicated MR scanner. A Dell computer projected to a screen mounted at the rear of the scanner bore presented the stimuli, which participants viewed while supine through a series of mirrors. Responses were recorded using bimanual fiber-optic response pads (Current Designs Inc. url: http://www.curdes.com/response). Prior to the functional echo planar image (EPI) acquisitions, subjects received a short series of structural MRI scans to allow for subsequent functional localization. These scans took ∼12 min and included: (i) a brief scout for landmarking, (ii) a high-resolution whole-brain MPRAGE sequence for later localization and intersubject registration. Functional imaging then proceeded using an EPI sequence that allowed for whole-brain coverage in a relatively short period of time (thirty-two 3 mm axial slices; 1mm gap, TR: 2 s; TE: 30 ms). In-plane resolutions were 3 × 3 mm (196 mm FOV, 64 × 64 matrix).
Procedure
The study was a 2 × 2 repeated measures design. Participants made one of two types of judgments5 (individuating preference or categorical), while viewing in the scanner either of two types of individual social group members—those eliciting exclusively social emotion (SE) or non-exclusively social emotion (NE). The judgments asked participants either to infer a vegetable preference (vegetable condition) or to make a categorical judgment of middle aged or not (age condition), while viewing each photograph. In the vegetable condition, participants reported whether the social group member who followed the picture of the vegetable would like that particular vegetable, while in the age condition they had to decide if the person was over 35 years old. They made ‘Yes/No’ responses on a button box. Before entering the scanner, participants familiarized themselves with the tasks by practicing on a computer with a set of neutral faces not presented in the actual study.
Inside the scanner, participants saw the photographs of the social group members in a series of 8 runs of 16 photographs each in a slow event-related design. Both tasks were performed for half of each run. Participants were prompted with the cue ‘Over 35?’ or ‘Likes Veggie?’ for the age and vegetable tasks, respectively, before seeing the line drawing of a vegetable for 1 s6 followed by a picture of either a NS social group member or SE social group member for 2 s. An 11-s fixation cross (colored red if they were doing the age task or green if they were doing the vegetable task) separated each pair of vegetable and social group member. After eight such pairs, a task switch was signaled by a cue, and the participant performed the second task for another 8 pairs. Each of the 64 photographs appeared once for each type of judgment, and never in the same run. All pictures were randomly sequenced for each run, and run order was randomized for each participant. After the scanning session, participants were probed for suspicion; none were suspicious. They were then thoroughly debriefed, given course credit and thanked.
Preprocessing
Both image preprocessing and statistical analysis used Brain Voyager QX (www.brainvoyager.de). Before statistical analysis, image preprocessing consisted of: (i) slice acquisition order correction, (ii) 3D rigid-body motion correction, (iii) voxelwise linear detrending across time, (iv) temporal bandpass filtering to remove low and high frequency (scanner and physiology related) noise. Distortions of EPI images were corrected with a simple affine transformation. Functional images were registered to the structural images and interpolated to cubic voxels. After coregistering participants’ structural images to a standard image using a 12-parameter spatial transformation, their functional data were similarly transformed, along with a standard moderate degree of spatial smoothing (Gaussian 8 mm FWHM).
Data analysis
Data were analyzed using the general linear model available on the Brain Voyager software package. A series of regressions examined BOLD brain activity to each of the four kinds of stimuli resulting from the task × actor interaction. We computed contrast maps for the two main effects averaged across all participants, and examined the simple effects within the relevant regions significantly activated in these main effects just for the time (2 s) when the pictures of the social group members were displayed. All data are presented with their coordinates based on a standard system (Talairarch and Tournoux, 1988). In addition, we report cluster averages and provide the coordinates at the center of the cluster, not maximum values. Random effects analyses were performed on all imaging data.
RESULTS
All reported imaging results are significant at P = 0.005 with at least 10 contiguous voxels unless otherwise noted.
Task main effect
In order to replicate previous work concerning mental inference tasks, we examined the first main effect: vegetable preference vs age judgments, collapsed over photographs. The main effect contrast of task revealed a significant area of mPFC more active for the vegetable preference judgments than the age judgments, [t (17) = 3.85, partial η2 = 0.71, at x = −3, y = 53, z = 20; 1043 voxels]. Because of this large area of activation, we reduced our threshold for significance to P < 0.002, resulting in two distinct areas of mPFC activation for the preference task over the age task. The first is a more ventral area [t (17) = 3.75, partial η2 = 0.68, at x = 2, y = 56, z = 12; 69 voxels], while the second is a more dorsal area [t (17) = 3.66, partial η2 = 0.65, at x = −8, y = 51, z = 23; 22 voxels; see Figure 2]. Also significantly different in this contrast at our a priori threshold were areas of superior frontal gyrus, middle temporal gyrus and parahippocampal gyrus (Table 1). Areas that were conversely more active during the age judgments than the preference judgments included bilateral precentral gyrus, middle frontal gyrus, inferior parietal lobule, cingulate and insula (Table 1).
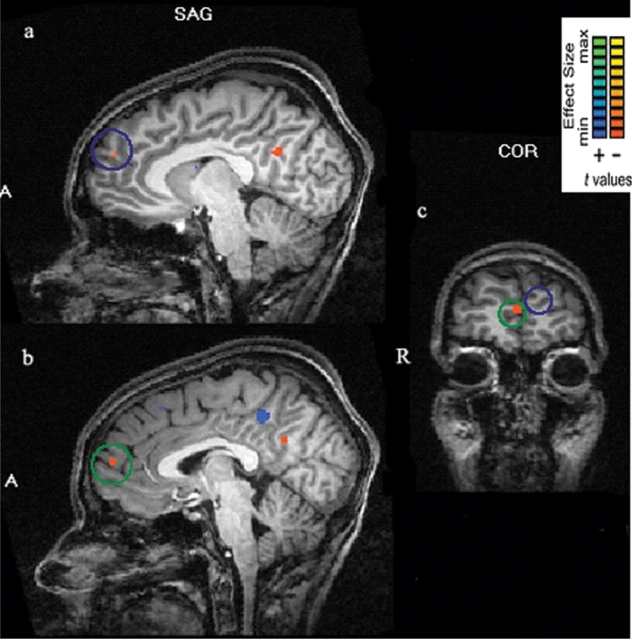
Fig. 2.
Ventral and dorsal distinction in the mPFC for the task main effect. Greater activity when subjects performed the individuation task than the categorization task. (a) Sagittal slice showing more dorsal mPFC activation at x = −8, y = 51, z = 23. (b) Sagittal slice showing more ventral mPFC activation at x = 2, y = 56, z = 12. (c) Coronal slice showing both the more dorsal (blue) and more ventral (green) mPFC activations.
Table 1.
Regions of brain activity associated with task main effect contrast
| Anatomical label | x | y | z | cluster size | t-value |
|---|---|---|---|---|---|
| Vegetable > Age | |||||
| Medial prefrontal cortex (ventral) | 2 | 56 | 12 | 69 | 3.75 |
| Medial prefrontal cortex (dorsal) | −8 | 51 | 23 | 22 | 3.66 |
| Left superior frontal gyrus (BA 9) | −3 | −53 | 27 | 800 | 3.85 |
| Right middle temporal gyrus (BA 21) | 53 | −9 | −16 | 237 | 3.45 |
| Left parahippocampal gyrus (BA 34) | −19 | −9 | −17 | 28 | 3.42 |
| Age > Vegetable | |||||
| Left middle frontal gyrus (BA 6) | −21 | 0 | 62 | 22 | 3.35 |
| Left middle frontal gyrus (BA 10) | −37 | 44 | 22 | 30 | 3.35 |
| Precentral gyrus (BA 6; left) | −41 | −6 | 37 | 168 | 3.36 |
| Precentral gyrus (BA 6; right) | 42 | 0 | 29 | 1700 | 3.75 |
| Left precentral gyrus (BA 4) | −39 | −13 | 54 | 189 | 3.37 |
| Right inferior parietal lobule (BA 40) | 38 | −47 | 42 | 3229 | 3.81 |
| Right cingulate (BA 31) | 3 | −38 | 41 | 657 | 3.52 |
| Left insula | −33 | 14 | 8 | 1110 | 3.64 |
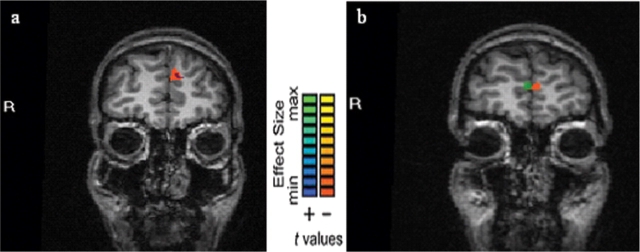
Follow-up simple effects analyses support differential processing by task within types of social group member. A more ventral area of mPFC was active when the two types of judgments were contrasted just for the SE social group members [t (17) = 3.37, partial η2 = 0.56, at x = −4, y = 57, z = 12; 56 voxels; see Figure 3A]; a more dorsal area of mPFC was active when this contrast was performed just for the NS actors [t (17) = 3.35, with an effect size as indicated by partial η2 = 0.55, at x = −6, y = 51, z = 25; 468 voxels; see Figure 3B].
Fig. 3.
Task simple effects mPFC activations. (a) mPFC activation greater for vegetable preference judgments than categorical age judgments for NS social group members. The area in orange shows the mPFC activation for this simple effect, while the area in blue shows the dorsal mPFC activation for the task main effect. (b) mPFC activation greater for vegetable preference judgments than categorical age judgments for SE social group members. The area in orange shows the mPFC activation for this simple effect, while the area in green shows the ventral mPFC activation for the task main effect.
Social-group-member effects
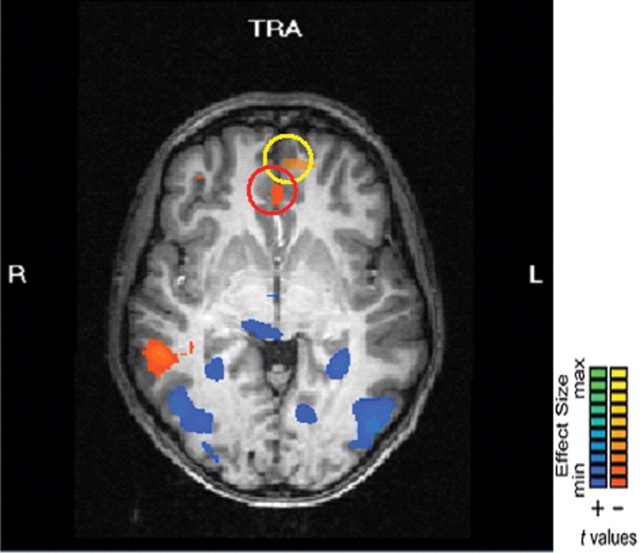
Our second analysis sought to replicate the initial finding of reduced mPFC activity to social group members who elicit disgust (Harris and Fiske, 2006). To test this, we examined the main effect contrast of social group member (SE vs NS) collapsed across tasks. While no results emerged at our standard threshold, when the P-value was raised to P < 0.02, a significant area of the mPFC was more active to SE actors than NS actors [t (17) = 2.51, P = 0.02, partial η2 = 0.32, at x = 0, y = 38, z = −1; 505 voxels]. This area is adjacent but posterior to and more medial than the area found in the initial dehumanization study (Figure 4; Harris and Fiske, 2006) sharing its z (−1) Talairach plane. Also significantly more active to the humanized actors at the a priori threshold was an area of right superior temporal sulcus (rSTS) and medial occipital cortex; while left parahippocampal gyrus and bilateral occipital cortex were more active to dehumanized actors (Table 2).
Fig. 4.
Greater activity to groups that elicit exclusively social emotions (SE) than to groups that elicit non-exclusively social emotions (NS). Yellow circle: Harris & Fiske, 2006, Psych. Sci., mPFC activation to exclusive social emotion groups compared to fixation at x = −9, y = 50, z = −2. Red circle: Present study mPFC activation to exclusive social emotion groups compared to non-exclusive social emotion groups at x = 0, y = 38, z = −1.
Table 2.
Regions of brain activity associated with social group member main effect contrast
| Anatomical label | x | y | z | cluster size | t-value |
|---|---|---|---|---|---|
| SE > NS | |||||
| Medial prefrontal cortex | 0 | 38 | −1 | 505 | 2.51 |
| Right superior temporal sulcus | 54 | −36 | −3 | 397 | 3.52 |
| Occipital cortex | 1 | −79 | 19 | 4531 | 4.40 |
| NS > SE | |||||
| Left parahippocampal gyrus | −30 | −40 | −6 | 702 | 3.55 |
| Occipital cortex (right) | 34 | −73 | 15 | 3407 | 4.00 |
| Occipital cortex (left) | −40 | −72 | 13 | 7126 | 3.93 |
The simple effects contrast within the age task condition revealed no significant difference at our a priori threshold. When this threshold was raised to P < 0.02, however, again an area of mPFC was more active to SE actors than NS actors [t (17) = 2.56, P = 0.02, with an effect size as indicated by partial η2 = 0.34, at x = −13, y = 53, z = 15; 69 voxels]. This finding, taken with the main effect result, replicates the initial neuro-imaging study of greater mPFC activation to SE vs NS social group members, although the location is adjacent to our previous result. Even though the P-value is high for neuro-imaging studies, the effect size is medium, and the area size is nontrivial.
No areas of mPFC were active at our standard threshold for this contrast within the vegetable judgments, but when our P-value was again raised to P < 0.02, a significant area of the mPFC was more active to SE actors than NS actors [t (17) = 2.56, partial η2 = 0.34, P = 0.02, at x = −11, y = 38, z = −3; 127 voxels]. However, this time this activation is more ventral and posterior than the activation for the similar contrast in the age judgments. This suggests that the different task recruits a different area of the mPFC to perform this judgment. In addition, this activation is in the neighborhood of similar activations when participants perform outcome monitoring tasks (Walton et al., 2004; Coricelli et al., 2005; Knutson et al., 2005), suggesting that having participants infer vegetable preferences engaged differential processing of these social group members. Moreover, these ventral and dorsal areas both overlap with the areas that were active for the initial task main effect contrast.
In short, the medium but reliable social-group-member effect that replicates our previous work appears in the age-judgment condition. However, a different social-group-member effect also appears in the vegetable-preference condition in a different area of the mPFC. All these effects are medium-sized, but useful because of the replication, the nontrivial voxel-areas represented, and the dissociation within the mPFC.
DISCUSSION
Our results essentially replicated the previous finding of reduced mPFC to actors that elicit disgust, an emotion that is not exclusively social, using different photographs and more participants. Social group members perceived as low in warmth and competence are processed differently from all other social group members. This latter finding is implied by the differential mPFC activation when participants are performing exactly the same tasks for SE vs NS targets.
Additionally, a task that caused participants to individuate by inferring the vegetable preference of the social targets activated the mPFC more for these targets than a task in which the participants relied on categorical information, consistent with the Continuum Model of Impression Formation (CM, Fiske and Neuberg, 1990; Fiske et al., 1999). The interesting finding here is the differentiation within mPFC for this greater activation between the two types of social group members. Our results also show this increase for the SE targets in an anatomically more ventral area of the mPFC, an area implicated in the processing of targets more similar to the self, while the increase for the NS targets was in a more dorsal area. This area is not in the same anatomical location as Mitchell et al. (2005) dorsal different-others area, but is an anatomically distinct region from the SE activation.
The more interesting result, though, is that the manipulated social goal of the perceiver can spark mPFC activity to the social group members who elicit disgust. This suggests that other processes may also activate the mPFC for these actors, consistent with the CM, such as direct instructions to individuate, instructions to form an accurate impression, accountability of impression to another person or outcome dependency (Fiske et al., 1999). These hypotheses have yet to be tested.
These initial age-preference findings are encouraging preliminaries to a broader search for mechanisms. However, a note of caution: Because nothing is known about the actual preferences of the targets, this information must be inferred by our participants. The CM makes no claim about the accuracy of the information, so inferred information works just as well as actual, accurate information. In fact, the CM and other models of impression formation and person perception within social psychology emphasize that people commonly infer this information from behavior, stereotypes, etc. (see Fiske, 2004 for a review). Work in attribution theory details the processes people use to reach these inferences (Heider, 1958; Jones and Davis, 1965; Kelley, 1972), and subsequent neuro-imaging work shows these processes do involve the mPFC (Harris et al., 2005).
These findings highlight a variety of concerns to be addressed in future research. The first and most pressing surrounds a more precise understanding of the functional role of the different anatomical areas of mPFC. This large strip of frontal cortex appears necessary for social cognition (Amodio and Frith, 2006), but serves many other cognitive and regulatory functions. A clearer understanding of the function of mPFC would allow us to draw further conclusions about the mechanism that differentiates social group members that elicit emotions such as disgust, or other non-exclusively social emotions. As mentioned, a similar–different ventral–dorsal distinction respectively in mPFC has been suggested (Mitchell et al., 2005). These findings can be considered consistent with this literature, but a more comprehensive understanding of the mPFC would further illuminate various possible dimensions along which more ventral vs more dorsal areas are recruited.
A second major concern to address in future research is identifying the exact dimensions along which social group members low on both warmth and competence are perceived as different. The anatomical differences found in this study imply that the same task is performed differently in the brain for these targets. A more thorough understanding of how they are perceived differentially would illuminate the mechanism that allows this differential processing to occur. Indeed, the initial neuro-imaging study (Harris and Fiske, 2006) claimed that this difference suggests possible less-human perception or dehumanization (Allport, 1954; Bar-Tal, 1989; Haslam et al., 2005; Opotow, 1990; Staub, 1990; Struch and Schwartz, 1989); further research should follow-up on this claim, tying behavioral measures to mPFC activity. The present study is consistent with this claim, though the dimension of similarity is not parsed out from a dehumanization account. Future research should parse these two overlapping but nonetheless independent dimensions. Regardless, these results do provide additional support for the critical role of social goals in changing perceptions of social groups for which people feel extreme prejudice.
Finally, our main message is that social cognition always depends on context (Todorov et al., 2006). Even reactions as immediate as disgust to a dirty, unkempt homeless person or an IV-drug-injector can be altered if one plays the role of a soup-kitchen volunteer attempting to feed the hungry, or a social worker leading someone on the path away from drug-addiction.
Acknowledgments
We thank the Princeton University Center for Brain, Mind, and Behavior for their generous support of this research project. We would also like to thank the Princeton Imaging Group, the Fiske lab, as well as Jonathan Cohen, James Haxby, Sabina Kastner and Matthew Lieberman for their feedback.
Footnotes
Conflict of Interest
None declared.
1 In addition, the same self-reported disgust emotion when evoked by objects did not activate this or any area of the mPFC—again suggesting that there was nothing specific about the emotions per se that caused the activation pattern.
2 Participants in other studies that use the SCM framework also attribute disgust to low–low social groups, not simply in our neuro-imaging studies (e.g. Fiske et al., 2002; Cuddy et al., in press).
3 Consistency and distinctiveness are the key elements, often but not always along with consensus information, that allow one to make a dispositional attribution.
4 Social group members that composed the category Americans were traditional American heroes such as firefighters, astronauts and athletes.
5 These vegetable and age judgments were the same tasks employed by Wheeler & Fiske (2005) in their examination of amygdala activation to Black vs White faces.
6 Participants were instructed to ignore the vegetable presented before the picture of the social group member when they were making the age judgments.
REFERENCES
- Allport GW. The Nature of Prejudice. Reading, MA: Addison-Wesley; 1954. [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews, Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Harmon-Jones E, Devine PG. Individual differences in the activation and control of affective race bias as assessed by the startle eyeblink response and self-report. Journal of Personality and Social Psychology. 2003;84:738–53. doi: 10.1037/0022-3514.84.4.738. [DOI] [PubMed] [Google Scholar]
- Barrett LF. Solving the emotion paradox: categorization and the experience of emotion. Personality and Social Psychology Review. 2006;10:20–46. doi: 10.1207/s15327957pspr1001_2. [DOI] [PubMed] [Google Scholar]
- Bar-Tal D. Delegitimization: The extreme case of stereotyping and prejudice. In: Bar-Tal D, Graumann C, Kruglanski A, Stroebe D, editors. Stereotyping and Prejudice: Changing Conceptions. NY: Spinger-Verlag; 1989. [Google Scholar]
- Coricelli G, et al. Regret and its avoidance: a neuroimaging study of choice behavior. Nature Neuroscience. 2005;8:1255–62. doi: 10.1038/nn1514. [DOI] [PubMed] [Google Scholar]
- Cuddy AJC, Fiske ST, Glick P. The BIAS map: behaviors from intergroup affect and stereotypes. Journal of Personality and Social Psychology. doi: 10.1037/0022-3514.92.4.631. (in press). [DOI] [PubMed] [Google Scholar]
- Fiske ST. Stereotyping, prejudice, and discrimination. In: Gilbert DT, Fiske ST, Lindzey G, editors. Handbook of Social Psychology. 4th edn. Vol. 2. New York: McGraw-Hill; 1998. pp. 357–411. [Google Scholar]
- Fiske ST. Social Beings: A Core Motives Approach to Social Psychology. New York: Wiley; 2004. [Google Scholar]
- Fiske ST, Pavelchak MA. Category-based versus piecemeal-based affective responses: developments in schema-triggered affect. In: Sorrentino RM, Higgins ET, editors. Handbook of Motivation and Cognition: Foundations of Social Behavior. NY: Guilford Press; 1986. pp. 167–203. [Google Scholar]
- Fiske ST, Neuberg S. A continuum of impression formation, from category-based to individuating processes: influences of information and motivation on attention and interpretation. In: Zanna MP, editor. Advances in Experimental Psychology. Vol. 23. New York: Academic Press; 1990. pp. 1–74. [Google Scholar]
- Fiske ST, Lin M, Neuberg S. The continuum model: Ten years later. In: Trope Y, Chaiken S, editors. Dual-process Theories in Social Psychology. New York, NY: Guilford Press; 1999. pp. 231–54. [Google Scholar]
- Fiske ST, Cuddy AJC, Glick P. Universal dimensions of social perception: warmth, then competence. Trends in Cognitive Science. doi: 10.1016/j.tics.2006.11.005. (in press). [DOI] [PubMed] [Google Scholar]
- Fiske ST, Cuddy AJC, Glick P, Xu J. A model of (often mixed) stereotype content: competence and warmth respectively follow from perceived status and competition. Journal of Personality and Social Psychology. 2002;82:878–902. [PubMed] [Google Scholar]
- Harris LT, Fiske LT. Dehumanizing the lowest of the low: neuroimaging responses to extreme outgroups. Psychological Science. 2006;17:847–53. doi: 10.1111/j.1467-9280.2006.01793.x. [DOI] [PubMed] [Google Scholar]
- Harris LT, Todorov A, Fiske ST. Attributions on the brain: Neuro-imaging dispositional inferences beyond theory of mind. Neuroimage. 2005;28:763–9. doi: 10.1016/j.neuroimage.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Whalen PJ, Shin LM, McInerney SC, Fischer H, Rauch SL. Differential response in the human amygdala to racial outgroup vs ingroup face stimuli. Brain Imaging. 2000;11:2351–5. doi: 10.1097/00001756-200008030-00004. [DOI] [PubMed] [Google Scholar]
- Haslam N. Dehumanization: An Integrative Review. 2005. Manuscript under review. [DOI] [PubMed] [Google Scholar]
- Haslam N, Bain P, Douge L, Lee M, Bastian B. More human than you: attributing humanness to self and others. Journal of Personality and Social Psychology. 2005;89:937–50. doi: 10.1037/0022-3514.89.6.937. [DOI] [PubMed] [Google Scholar]
- Heal J. Simulation, theory, and content. In: Carruthers P, Smith P, editors. Theories of Theories of Mind. Cambridge: Cambridge University Press; 1996. pp. 75–89. [Google Scholar]
- Heider F. The Psychology of Interpersonal Relations. New York: Wiley; 1958. [Google Scholar]
- Jones EE, Davis KE. From acts to dispositions: the attribution process in person perception. In: Berkowitz L, editor. Advances in Experimental Social Psychology. Vol. 2. New York: Academic Press; 1965. pp. 220–6. [Google Scholar]
- Kelley HH. Attribution in social interaction. In: Jones EE, Kanouse DE, Kelley HH, Nisbett RE, Valins S, Weiner B, editors. Attribution: Perceiving the Cause of Behavior. Hillsdale, NJ: Lawrence Elbaum & Associates; 1972. pp. 1–26. [Google Scholar]
- Knutson B, Taylor J, Kaufmann N, Peterson R, Glover G. Distributed neural representation of expected value. Journal of Neuroscience. 2005;25:4806–12. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyens JP, Rodriguez-Perez A, Rodriguez-Torres R, et al. Psychological essentialism and the differential attribution of uniquely human emotions to ingroups and outgroups. European Journal of Social Psychology. 2001;31:395–411. [Google Scholar]
- Leyens J, Ph., Cortes BP, Demoulin S, et al. Emotional prejudice, essentialism, and nationalism. European Journal of Social Psychology. 2003;33:703–18. [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annual Review of Psychology. 2007;58 doi: 10.1146/annurev.psych.58.110405.085654. (in press) [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Hariri A, Jarcho JM, Eisenberger NI, Bookheimer SY. An fMRI investigation of race-related amygdala activity in African American and Caucasian American individuals. Nature Neuroscience. 2005;8:720–2. doi: 10.1038/nn1465. [DOI] [PubMed] [Google Scholar]
- McArthur LA. The how and what of why: some determinants and consequences of causal attribution. Journal of Personality and Social Psychology. 1972;72:171–93. [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17:1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Niedenthal P, Krauth-Gruber S, Ric F. Psychology of Emotion: Interpersonal, Experiential and Cognitive Approaches. NY: Psychological Press; 2006. [Google Scholar]
- Opotow S. Moral exclusion and injustice: an introduction. Journal of Social Issues. 1990;46:1–20. [Google Scholar]
- Phelps EA, O'Connor KJ, Cunningham WA, et al. Performance on indirect measures of race evaluation predicts amygdala activation. Journal of Cognitive Neuroscience. 2000;12:729–38. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- Rozin P, Fallon AE. A perspective on disgust. Psychological Review. 1987;94:23–41. [PubMed] [Google Scholar]
- Staub E. The Roots of Evil: The Origins of Genocide and Other Group Violence. NY: Cambridge University Press; 1989. [Google Scholar]
- Struch N, Schwartz SH. Intergroup aggression: its predictors and distinctness from in-group bias. Journal of Personality and Social Psychology. 1989;56:364–73. doi: 10.1037//0022-3514.56.3.364. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- Todorov A, Harris LT, Fiske ST. Toward behaviorally inspired social neuroscience. Brain Research. 2006;1079:76–85. doi: 10.1016/j.brainres.2005.12.114. [DOI] [PubMed] [Google Scholar]
- Walton ME, Devlin JT, Rushworth MF. Interaction between decision making and performance monitoring within prefrontal cortex. Nature Neuroscience. 2004;7:1259–65. doi: 10.1038/nn1339. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Fiske ST. Controlling racial prejudice: social-cognitive goals affect amygdala and stereotype activation. Psychological Science. 2005;16:56–63. doi: 10.1111/j.0956-7976.2005.00780.x. [DOI] [PubMed] [Google Scholar]