Abstract
The discovery of the mirror neuron system (MNS) has led researchers to speculate that this system evolved from an embodied visual recognition apparatus in monkey to a system critical for social skills in humans. It is accepted that the MNS is specialized for processing animate stimuli, although the degree to which social interaction modulates the firing of mirror neurons has not been investigated. In the current study, EEG mu wave suppression was used as an index of MNS activity. Data were collected while subjects viewed four videos: (1) Visual White Noise: baseline, (2) Non-interacting: three individuals tossed a ball up in the air to themselves, (3) Social Action, Spectator: three individuals tossed a ball to each other and (4) Social Action, Interactive: similar to video 3 except occasionally the ball would be thrown off the screen toward the viewer. The mu wave was modulated by the degree of social interaction, with the Non-interacting condition showing the least suppression, followed by the Social Action, Spectator condition and the Social Action, Interactive condition showing the most suppression. These data suggest that the human MNS is specialized not only for processing animate stimuli, but specifically stimuli with social relevance.
Keywords: mirror neuron system, rhythm, social, EEG
INTRODUCTION
Social interaction is an essential part of being human. Some believe that we are born with an innate desire and ability for social interaction (Meltzoff and Moore, 1997). The intrinsic necessity for social interaction has been suggested as evolution's motivating factor for uniquely human skills including art, language, theory of mind and empathy (Ramachandran, 2000).
One system proposed to underlie many aspects of social cognition is the mirror neuron system (MNS) (Gallese et al., 2004). While recording single neurons in the macaque premotor cortex (Area F5), Rizzolatti and colleagues discovered a unique set of premotor neurons that appeared to respond both when a monkey performed an action and when it sat motionless observing another individual performing an action (Di Pellegrino et al., 1992; for a review, see Rizzolatti et al., 2001). These neurons were named ‘mirror neurons’ for their unique property of firing to both observed and performed actions.
Although single-unit recording is not typically performed in the human brain, indirect population-level measures support the existence of a functionally analogous system to the macaque MNS in the human inferior frontal gyrus (IFG) (Fadiga et al., 1995; Parsons et al., 1995; Iacoboni et al., 1999). Additionally, fMRI studies suggest that the frontal MNS in humans may be one part of a broader network of brain regions including the inferior parietal lobule (Parsons et al., 1995; Buccino et al., 2001;), the superior temporal sulcus (Iacoboni et al., 2001) and regions of the limbic system (Wicker et al., 2003; Singer et al., 2004; Morrison et al., 2004). This broader network, with its ability to match perceptions of the environment to internal sensorimotor representations, may play a key role in multiple aspects of social cognition from biological action perception to empathy.
Researchers speculate that the MNS in lower primates largely functions to facilitate action understanding (Rizzolatti et al., 2001), while in higher primates this same system is thought to have evolved to support imitation via on line activation of the motor properties of the mirror neurons (Rizzolatti and Arbib, 1998). In humans, it is speculated that mirror neurons further evolved to represent not only the physical aspects of an action but also the underlying intentions, thoughts and feelings that motivated that action, possibly through reciprocal connections with other brain regions such as the limbic system or medial prefrontal cortex. It has been suggested that this evolutionary bootstrapping provided the foundation for arguably unique human social skills such as theory of mind, empathy, and language (Gallese, 2001).
In support of the involvement of the MNS in understanding of intentions, a recent study found that activity in the IFG may be modulated by the underlying intention of an observed action (Iacoboni et al., 2005). Specifically, subjects were presented with the same action embedded in two different contexts (implying two different purposes for the action), and researchers found significant differences in the levels of activation between the two contexts. Further support for the role of the human MNS in social skills comes from studies on individuals with autism spectrum disorders (ASD). Following preliminary data from our laboratory suggesting a dysfunction of the MNS in individuals with ASD (Altschuler et al., 2000), five independent laboratories have published neurophysiological evidence supporting this claim (Nishitani et al., 2004; Dapretto et al., 2006; Oberman et al., 2005; Theoret et al., 2005; Villalobos et al., 2005).
Previous studies in our laboratory (Altschuler et al., 1997; Altschuler et al., 2000; Pineda et al., 2000; Oberman et al., 2005;) and other laboratories (Muthukumaraswamy and Johnson, 2004; Muthukumaraswamy et al., 2004) have investigated MNS in humans through analysis of electroencephalography (EEG) mu frequency band oscillations. At rest, sensorimotor neurons spontaneously fire in synchrony (Gastaut, 1951), leading to large-amplitude EEG oscillations in the 8–13 Hz (mu) frequency band recorded over scalp locations C3, Cz, and C4 (for a review see Pineda, 2005).
The use of mu suppression as an index of mirror neuron activity is validated by anatomical and physiological evidence of strong cortico–cortico connections between human and non-human primate ventral premotor cortex (including the region thought to contain mirror neurons) and primary sensorimotor cortex where the mu rhythm is generated and recorded (Muakkassa and Strick, 1979; Godschalk et al., 1984; Matelli et al., 1986; Ghosh et al., 1987; Nishitani and Hari, 2000; Tokuno and Nambu, 2000; Dum and Strick, 2002; Shimazu et al., 2004).
Functional correlations also support the use of mu suppression as an index of mirror neuron activity. First, studies dating back to 1954 find that, similar to mirror neurons, mu oscillations respond specifically to self-performed, observed and imagined actions (Gastaut and Bert, 1954; Cochin et al., 1998; Babiloni et al., 2002; Pineda et al., 2000). Additionally, both mirror neurons (Rizzolatti and Fadiga, 1998) and mu oscillations only respond to animate stimuli (Altschuler et al., 1997; Oberman et al., 2005), and respond more to target-directed actions as compared to non-goal-directed actions (Muthukumaraswamy et al., 2004). Finally, both mirror neurons (Buccino et al., 2001) and mu oscillations seem to respond in a somatotopic manner (Pfurtscheller et al., 1997). Taken together, these findings suggest that changes in mu suppression can provide an inexpensive, non-invasive method to study human mirror neuron functioning (Oberman et al., 2005; Muthukumaraswamy et al., 2004).
In the original studies of mu suppression by Gastaut and Bert (1954), it was reported that the amount of suppression was related to the degree to which the observer related to the image on the screen. Although it is widely accepted that the MNS is selective for animate stimuli and proposed to be involved in many aspects of social cognition, the degree to which the MNS is sensitive to differences in degree of social interaction is unclear.
One study, conducted by Iacoboni et al. (2004), which largely highlights the role of the dorsomedial prefrontal and medial parietal cortex (the ‘default state areas’) in the processing of social interactions also finds differences in the degree of activity in the IFG when an observer watches a scene depicting two individuals interacting as compared to a scene depicting one individual engaging in everyday activities. However, as these scenes differed in complexity, content, as well as degree of social interaction, it is hard to discern what property of the stimulus the IFG was sensitive to. The goal of the current study was to highlight the role of the MNS in the processing of social stimuli. Specifically, we investigated both the degree to which the mu rhythm would be modulated based on the degree of social content of a given human action as well as the degree to which the observer is involved in the observed scene.
MATERIALS AND METHODS
Subjects
The sample was composed of 20 college students (10 male, 10 female) ranging in age from 18–34 years (mean = 21.05 years, s.d. = 3.38) recruited from the University of California, San Diego Psychology Department subject pool. Participants received class credit for their participation, and all gave written consents & to participate in the study. This study was reviewed and approved by the University of California, San Diego Human Research Protections Program.
Procedure
EEG data were collected while subjects watched four different videos: (1) Visual White Noise: full-screen television static (mean luminance 3.7 cd/m2) was presented as a baseline condition; (2) Non-interacting: three individuals tossed a ball up in the air to themselves; (3) Social Action, Spectator: three individuals tossed a ball to each other; and (4) Social action, Interactive: similar to video 3 except occasionally the ball would be thrown off the screen, seemingly toward the viewer, as if the viewer were part of the game. All videos were 80 s in length and were matched for low-level visual properties such as amount of movement and complexity of the scene. All conditions were presented twice in order to obtain enough clean EEG data for analyses and the order of the conditions was counterbalanced across subjects.
To ensure that the participants attended to the video stimuli, during all of the conditions except the baseline, they were asked to engage in a continuous performance task. Between four and six times during the 80 s video, the actors in the video stopped throwing the ball and would simply hold it in their hands for a period of 2 s. Subjects were asked to count the number of times the ball stopped being thrown and report the number of stops to the experimenter at the end of the block.
EEG data acquisition and analysis
Disk electrodes were applied to the face above and below the left eye to monitor the electrooculogram (EOG) and behind each ear (mastoids) for use as reference electrodes. Data were collected from 13 electrodes embedded in a cap, at the following scalp positions: F3, Fz, F4, C3, Cz, C4, P3, Pz, P4, T5, T6, O1 and O2, using the international 10–20 method of electrode placement. Following placement of the cap, electrolytic gel was applied at each electrode site and the skin surface was lightly abraded to reduce the impedance of the electrode–skin contact. The impedances on all electrodes were measured and confirmed to be less than 5 kΩ both before and after testing. Once the electrodes were in place, subjects were seated inside an acoustically and electromagnetically shielded testing chamber. EEG was recorded and analyzed using a Neuroscan Synamps system (band pass 0.1–30 Hz). Data were collected for approximately 160 s per condition at a sampling rate of 500 Hz.
EEG frequency oscillations in the 8–13 Hz range recorded over occipital cortex are influenced by states of expectancy and awareness (Klimesch et al., 1998). Since the μ frequency band overlaps with the posterior alpha band, it is possible that recordings from C3, Cz and C4 might be contaminated by this posterior activity. In order to reduce this influence, the first and last 10 s of each block of data were removed from all subjects to eliminate the possibility of attentional transients due to initiation and termination of the stimulus. A 1 min segment of data following the initial 10 s was obtained and combined with the other trial of the same condition, resulting in one 2 min segment of data per condition. Eye blink and head movements were manually identified in the EOG recording and EEG artifacts during these intervals were removed prior to analysis according to standard criteria (Goldensohn et al., 1999). Data were only analyzed if there was sufficient clean data with no movement or eye blink artifacts.
Although data were obtained from electrodes across the scalp, mu rhythm is defined as oscillations measured over sensorimotor cortex; thus, only data from C3, Cz and C4 are presented. A repeated-measures within-subject ANOVA was conducted to compare the suppression values across the three experimental conditions.
Data were segmented into epochs of 2 s beginning at the start of the segment. Fast Fourier transforms in the 8–13 Hz range were performed on the cleaned and epoched data (1024 points). A cosine window was used to control for artifacts resulting from data splicing. Mu suppression was calculated as the ratio of the power during the experimental conditions relative to the power during the baseline condition. A ratio was used to control for variability in absolute mu power as a result of individual differences such as scalp thickness and electrode placement and impedance, as opposed to mirror neuron activity. Since ratio data are inherently non-normal as a result of lower bounding, a log transform was used for analysis. A log ratio of less than zero indicates suppression, whereas a value of zero indicates no suppression and values greater than zero indicate enhancement.
RESULTS
Behavioral performance
All subjects performed with 100% accuracy on the continuous performance task during all conditions. We infer that any differences found in mu suppression are, therefore, not due to differences in attending to the stimuli.
Mu suppression
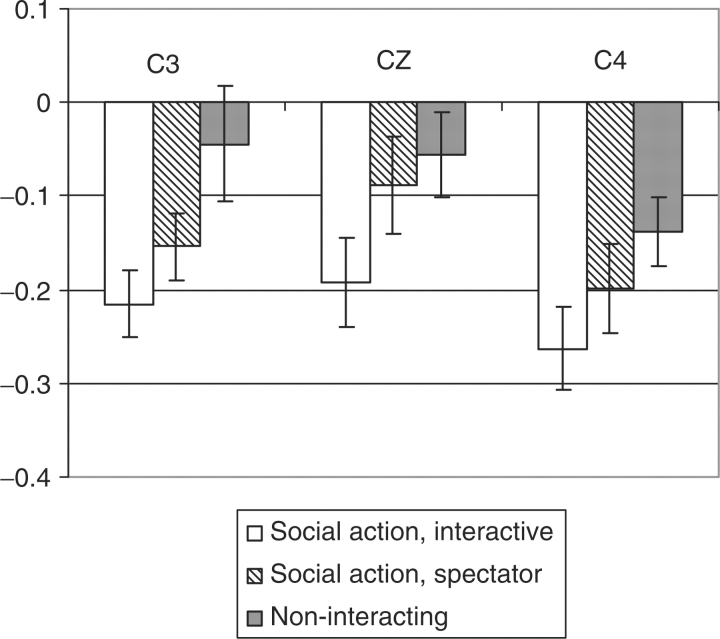
A highly significant main effect of condition was obtained (Figure 1) (F (2,58) = 17.75, P < 0.0001). Follow-up pair wise comparisons revealed a linear trend with the Social Action, Interactive condition, showing the greatest amount of suppression (M = −0.22) followed by the Social Action, Spectator condition (M = −0.15) with the Non-interacting condition (M = −0.08) showing the least amount of suppression.
Fig. 1.
Mu suppression during the observation of human action. Bars represent the mean log ratio of power in the mu frequency (8–13 Hz) during the Social Action, Interactive (white), Social Action, Spectator (gray stripe), and Non-interacting (dark gray) over the power in the baseline condition for scalp locations C3, Cz and C4. Error bars represent the standard error of the mean. For all values, a mean log ratio less than zero indicates mu suppression.
Experimental manipulation confirmation
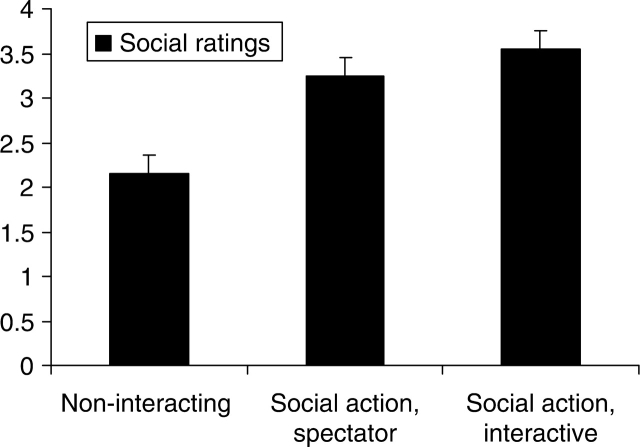
In order to assess whether subjects perceived the stimuli as containing different levels of social content, a separate group of 20 individuals rated the videos on social content on a 5-point Likert scale. A highly significant main effect of condition was obtained (Figure 2): (F (2,18) = 57.86, P < 0.0001). Follow-up pair wise comparisons revealed a linear trend with the Social Action, Interactive condition, rated as having the greatest social content (M = 3.55) followed by the Social Action, Spectator condition (M = 3.25), with the Non-interacting condition (M = 2.15) being rated as the least social.
Fig. 2.
Ratings of perceived social content. Bars represent the mean rating on a 5-point Likert scale of social content of the three experimental videos. Error bars represent the standard error of the mean. Zero on the Likert scale was labeled ‘No Social Content,’ while 5 was labeled ‘Extremely Social.’
DISCUSSION
Results of this study, showing modulations in mu wave suppression to observed actions based on the degree of social interaction, suggest that MNS is sensitive to the presence of social cues in a stimulus. The finding that the system is most active (resulting in the greatest amount of mu wave suppression) when the stimulus is not only social but interactive is consistent with the anecdotal report by Gastaut and Bert (1954) that the blocking of the mu wave occurs when an individual ‘identifies himself with an active person represented on the screen’ as well as with the previous fMRI data showing increased activity in IFG in response to social interaction (Iacoboni et al., 2004). These findings suggest that the greater the degree of identification of the viewer with the stimuli, the greater the degree of social interaction perceived.
This study provides the necessary link between the known action observation/execution properties of the monkey MNS and the theorized social functions of the human MNS. The selectivity of the human MNS for animate stimuli had been previously suggested based on studies finding no significant mu suppression to the observation of inanimate stimuli matched for low-level visual properties (Altschuler et al., 1997; Oberman et al., 2005). Additionally, previous fMRI studies had indicated that the human MNS was sensitive to intentions and goals, with the IFG modulating its degree of activity based on the intention of the observed action (Iacoboni et al., 2005). The current study suggests that this system is also sensitive to the degree of sociality, as evidenced by modulations in the degree of mu suppression between the three experimental conditions. This characteristic may provide a necessary link between simple action observation and more complex social skills such as theory of mind, empathy and language.
Since the mu frequency band overlaps the posterior alpha band, it is possible that, despite efforts to control for this, recordings from C3, Cz and C4 might be contaminated by this posterior activity. As all conditions involved visual stimuli and the eyes were open throughout the study, we would not expect a systematic difference between conditions in posterior alpha activity. Additionally, by eliminating the first and last 10 s of each block and including a continuous performance task during all three experimental conditions, we reduced the possibility of confounds such as changes in attention affecting our mu power results. Consistent with this, no electrodes other than C3, Cz and C4 showed a consistent pattern of suppression in the frequency band of interest. These results suggest that the modulations of mu rhythms observed in C3, Cz and C4 were not likely to be mediated by posterior alpha activity or differences in attentional demands between the three experimental conditions.
Although mu wave suppression is considered a valid index of mirror neuron activity (Muthukumaraswamy et al., 2004; Oberman et al., 2005), owing to the low spatial resolution of EEG it is difficult to differentiate between activity selective to the premotor MNS and activity in other regions that are part of a larger action observation/execution network that may modulate the activity in the premotor MNS (Muthukumaraswamy and Johnson, 2004; Muthukumaraswamy et al., 2004). Further investigations with higher-spatial-resolution techniques, such as fMRI and high-resolution EEG, may be able to dissociate between these two sources of activation and confirm at what stage of processing the information regarding social content is processed.
Acknowledgments
The authors would like to thank Bill Skinner for his assistance with data collection and revisions of previous versions of this manuscript. Data presented in this manuscript will also be presented in San Francisco at the 13th annual meeting of the Cognitive Neuroscience Society in April 2006.
Footnotes
Conflict of Interest
None declared.
REFERENCES
- Altschuler EL, Vankov A, Hubbard EM, Roberts E, Ramachandran VS, Pineda JA. Mu wave blocking by observer of movement and its possible use as a tool to study theory of other minds. Poster Session Presented at the 30th Annual Meeting of the Society for Neuroscience; New Orleans: LA. 2000. Nov, [Google Scholar]
- Altschuler EL, Vankov A, Wang V, Ramachandran VS, Pineda JA. Person see, person do: Human cortical electrophysiological correlates of monkey see monkey do cells. Poster Session Presented at the 27th Annual Meeting of the Society for Neuroscience; New Orleans: LA. 1997. [Google Scholar]
- Babiloni C, Babiloni F, Carducci F, et al. Human cortical EEG rhythms during the observation of simple aimless movements: a high-resolution EEG study. NeuroImage. 2002;17:559–72. [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, et al. Action observation activates premotor and parietal areas in a somatotopic manner: a fMRI study. European Journal of Neuroscience. 2001;13:400–4. [PubMed] [Google Scholar]
- Cochin S, Barthelemy C, Lejeune B, Roux S, Martineau J. Perception of motion and qEEG activity in human adults. Electroencephalography and Clinical Neurophysiology. 1998;107:287–95. doi: 10.1016/s0013-4694(98)00071-6. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Experimental Brain Research. 1992;91:176–80. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Motor areas in the frontal lobe of the primate. Physiology and Behaviour. 2002;77:677–82. doi: 10.1016/s0031-9384(02)00929-0. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolati G. Motor facilitation during action observation: a magnetic stimulation study. Journal of Neurophysiology. 1995;73:2608–11. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- Gallese V. The ‘Shared Manifold’ hypothesis. Journal of Consciousness Studies. 2001;8:33–50. [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends in Cognitive Sciences. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Gastaut HJ. The electrical activity of the brain. Annual Review of Physiology. 1951;13:297–326. doi: 10.1146/annurev.ph.13.030151.001501. [DOI] [PubMed] [Google Scholar]
- Gastaut HJ, Bert J. EEG changes during cinematographic presentation. Electroencephalography and Clinical Neurophysiology. 1954;6:433–44. doi: 10.1016/0013-4694(54)90058-9. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Brinkman C, Porter RA. A quantitative study of the distribution of neurons projecting to the precentral motor cortex in the monkey (M. fascicularis) The Journal of Comparative Neurology. 1987;259:424–44. doi: 10.1002/cne.902590309. [DOI] [PubMed] [Google Scholar]
- Godschalk M, Lemon RN, Kupyers HG, Ronday HK. Cortical afferents and efferents of monkey postarcuate area: an anatomical and electrophysiological study. Experimental Brain Research. 1984;56:410–24. doi: 10.1007/BF00237982. [DOI] [PubMed] [Google Scholar]
- Goldensohn ES, Legatt AD, Koszer S, Wolf SM. Goldensohn's EEG interpretation: problems of overreading and underreading. Armonk: Futura Publishing; 1999. [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one's own mirror neuron system. PLOS Biology. 2005;3:1–7. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–8. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Koski L, Brass M, Bekkering H, Woods RP, Dubeau M-C, et al. Re-afferent copies of imitated actions in the right superior temporal cortex. Proceedings of the National Academy of Science; 2001. pp. 13995–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Lieberman MD, Knowlton BJ, et al. Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. Neuroimage. 2004;21:1167–73. doi: 10.1016/j.neuroimage.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Russegger H, Pachinger T, Schwaiger J. Induced alpha band power changes in the human EEG and attention. Neuroscience Letters. 1998;244:73–6. doi: 10.1016/s0304-3940(98)00122-0. [DOI] [PubMed] [Google Scholar]
- Matelli M, Carmarda M, Glickstein M, Rizzolatti G. Afferent and efferent projections of the inferior area 6 in the macaque monkey. The Journal of Comparative Neurology. 1986;251:281–298. doi: 10.1002/cne.902510302. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Explaining facial imitation: a theoretical model. Early Dev. Parenting. 1997;6:179–92. doi: 10.1002/(SICI)1099-0917(199709/12)6:3/4<179::AID-EDP157>3.0.CO;2-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison I, Lloyd D, Di Pellegrino G, Roberts N. Vicarious responses to pain in anterior cingulate cortex: Is empathy a multisensory issue? Cognitive, Affective, and Behavioral Neuroscience. 2004;4:270–8. doi: 10.3758/cabn.4.2.270. [DOI] [PubMed] [Google Scholar]
- Muakkassa AF, Strick PL. Frontal lobe inputs to primate motor cortex: evidence for four somatotopically organized ‘premotor’ areas. Brain Research. 1979;177:176–82. doi: 10.1016/0006-8993(79)90928-4. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Johnson BW. Primary motor cortex activation during action observation revealed by wavelet analysis of the EEG. Clinical Neurophysiology. 2004;115:1760–6. doi: 10.1016/j.clinph.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Johnson BW, McNair NA. Mu rhythm modulation during observation of an object-directed grasp. Cognitive Brain Research. 2004;19:195–201. doi: 10.1016/j.cogbrainres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Nishitani N, Avikainen S, Hari R. Abnormal imitation-related cortical activation sequences in Asperger's syndrome. Annuals of Neurology. 2004;55:558–62. doi: 10.1002/ana.20031. [DOI] [PubMed] [Google Scholar]
- Nishitani N, Hari R. Temporal dynamics of cortical representation for action. PNAS. 2000;97(2):913–8. doi: 10.1073/pnas.97.2.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman LM, Hubbard EM, McCleery JP, Altschuler EL, Ramachandran VS, Pineda JA. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cognitive Brain Research. 2005;24:190–8. doi: 10.1016/j.cogbrainres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Parsons LM, Fox PT, Downs JH, et al. Use of implicit motor imagery for visual shape discrimination as revealed by PET. Nature. 1995;375:54–8. doi: 10.1038/375054a0. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Andrew C, Edlinger G. Foot and hand area mu rhythms. International Journal of Psychophysiology. 1997;26:121–35. doi: 10.1016/s0167-8760(97)00760-5. [DOI] [PubMed] [Google Scholar]
- Pineda JA. The functional significance of mu rhythms: Translating “seeing” and “hearing” into “doing”. Brain Research Reviews. 2005;50:57–68. doi: 10.1016/j.brainresrev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Pineda JA, Allison BZ, Vankov A. The effects of self-movement, observation, and imagination on mu rhythms and readiness potentials: toward a brain-computer interface. IEEE Transactions on Rehabilitation Engineering. 2000;8:219–22. doi: 10.1109/86.847822. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS. Mirror neurons and imitation learning as the driving force behind “the great leap forward” in human evolution. 2000 http://www.edge.org/documents/archive/edge69.html. [Google Scholar]
- Rizzolatti G, Arbib MA. Language within our grasp. Trends Neuroscience. 1998;21(5):188–94. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L. Grasping objects and grasping action meanings: the dual role of monkey rostroventral premotor cortex (area F5) In: Bock GR, Goode JA, editors. Sensory Guidance of Movement. Vol. 218. London, UK: John Wiley & Sons; 1998. pp. 81–103. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nature Reviews Neuroscience. 2001;2:661–70. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Shimazu H, Maier MA, Cerri G, Kirkwood PA, Lemon RN. Macaque ventral premotor cortex exerts powerful facilitation of motor cortex outputs to upper limb motoneurons. The Journal of Neuroscience. 2004;24:1200–11. doi: 10.1523/JNEUROSCI.4731-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Theoret H, Halligan E, Kobayashi M, Fregni F, Tager-Flusberg H, Pascual-Leone A. Impaired motor facilitation during action observation in individuals with autism spectrum disorder. Current Biology. 2005;15:R84–5. doi: 10.1016/j.cub.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Tokuno H, Nambu A. Organization of nonprimary motor cortical inputs on pyramidal and nonpyramidal tract neurons of primary motor cortex: an electrophysiological study in the macaque monkey. Cerebral Cortex. 2000;10:56–68. doi: 10.1093/cercor/10.1.58. [DOI] [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Muller RA. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage. 2005;25:916–25. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–64. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]