Abstract
Understanding the intended meaning of a remark beyond what is explicitly stated is an integral part of successful social interactions. Here, we examined the neural circuitry underlying the interpretation of communicative intent in children and adults using irony comprehension as a test case. Participants viewed cartoon drawings while listening to short scenarios ending with a potentially ironic remark and were asked to decide whether the speaker was being sincere or ironic. In both children and adults, instructions to attend to the cues provided by the speaker's facial expression or tone of voice modulated the activity in visual and language cortices, respectively. Overall, children engaged the medial prefrontal cortex and left inferior frontal gyrus more strongly than adults, whereas adults recruited the fusiform gyrus, extrastriate areas and the amygdala more strongly than children. Greater involvement of prefrontal regions in children may subserve the integration of multiple cues to reconcile the discrepancy between the literal and intended meaning of an ironic remark. This developmental shift from a reliance on frontal regions to posterior occipitotemporal regions may reflect the automatization of basic reasoning about mental states. This study is the first to examine developmental changes in the neural circuitry underlying natural language pragmatics.
Keywords: development, functional Magnetic Resonance Imaging (fMRI), irony, language, theory of mind
In everyday conversations, the intended meaning of a remark is often different from what is explicitly stated. Successfully navigating social interactions requires not only an understanding of the literal meaning of an utterance, but also an appreciation for the paralinguistic cues conveyed by the speaker's facial expression and tone of voice—i.e. pragmatics. Early on, children show a preference for the human face and voice as well as an awareness of the emotional signals they convey (see Walker-Andrews, 1997 for a review). By 18 months, infants demonstrate sensitivity to the communicative intentions of others. Upon hearing a novel word, infants attend to the speaker's direction of gaze, gestures and facial expression in order to infer what the referent is and what the speaker's attitude toward it might be (Baldwin, 2000; Moses et al., 2001). In this way, infants use their interpersonal understanding to aid in language acquisition. However, as formal linguistic skills are mastered, limited attentional resources may lead children to focus more on the propositional content of utterances than on accompanying intentional cues (Friend and Bryant, 2000; Morton and Trehub, 2001), which may, in turn, lead to difficulties in appreciating non-literal language. Indeed, it is widely reported that children under 6–8 years of age have difficulty understanding the communicative intent behind an ironic remark (e.g. Ackerman, 1981; Demorest et al., 1984; Winner and Leekam, 1991; Hancock et al., 2000). Nevertheless, some studies suggest that the presence of intonational cues assists children as young as 6 years old in interpreting a potentially ironic comment (Ackerman, 1986; Capelli et al., 1990; de Groot et al., 1995; Milosky and Ford, 1997; Keenan and Quigley, 1999).
Since the advent of neuroimaging techniques, significant strides have been made in delineating the neural networks supporting language in the mature brain. However, much of this work has focused on formal linguistic skills including phonological, syntactic and semantic processing (Bookheimer, 2002). Thus, relatively little is known about the neural underpinnings of the higher-level pragmatic aspects of language including the comprehension of non-literal speech such as irony and sarcasm in the adult brain. Even less is known about the neural events that accompany the development of pragmatic language skills in the typically developing brain. Most of the existing research comes from neuropsychological studies of patients with brain lesions and suggests an important role for both the right hemisphere (RH) and the prefrontal cortex. Several researchers have found that patients with unilateral RH brain damage are significantly impaired in irony comprehension relative to healthy control subjects (Tompkins and Mateer, 1985; Kaplan et al., 1990; Winner et al., 1998). Giora et al. (2000) extended this work by including a comparison group of patients with left hemisphere (LH) brain damage and found that patients with RH lesions performed significantly worse than those with LH lesions after controlling for the effects of aphasia. With regard to the role of the prefrontal cortex, Giora et al., observed that the performance scores of patients with LH lesions decreased reliably as the extent of damage in the inferior and middle frontal gyri increased (Zaidel et al., 2002). Furthermore, Shamay and colleagues (2002) found that patients with lesions in the prefrontal cortex, regardless of which hemisphere was affected, performed more poorly on a measure of sarcasm understanding than patients with posterior lesions. Recently, the same group showed that, more specifically, patients with prefrontal lesions in ventromedial (but not dorsolateral) regions were impaired in irony comprehension relative to both patients with posterior lesions and healthy control subjects (Shamay-Tsoory et al., 2005a, b).
Although no neuroimaging studies to date have examined the interpretation of irony, closely related is the rapidly growing literature on the neural basis of ‘theory of mind’ or the ability to attribute beliefs, attitudes and desires to others. Just as understanding others' mental states requires the ability to decouple belief from reality (Frith and Frith, 2003; Gallagher and Frith, 2003), understanding irony requires separating the literal from the intended meaning of a comment. Using a very diverse set of mentalizing tasks including inferring mental states from characters in stories and cartoons (Fletcher et al., 1995; Brunet et al., 2000; Gallagher et al., 2000; Vogeley et al., 2001), attributing mental states to moving geometric forms (Castelli et al., 2000; Schultz et al., 2003), detecting intentional violations of social norms (Berthoz et al., 2002) and evaluating emotional states in the self or others (Ochsner et al., 2004; Ruby and Decety, 2004), researchers have converged on a consistent network of brain regions implicated in understanding the mental states of others. This circuitry includes the medial prefrontal cortex (MPFC), the temporal poles and the posterior superior temporal sulcus (STS).
Perhaps the most reliably activated region across studies has been the MPFC, although peaks of activity in various studies have fallen in both dorsal and ventral aspects of this region (Ochsner et al., 2004). Some investigators have argued that the MPFC plays a specific role in reasoning about the beliefs or communicative intentions of others, whereas other regions frequently activated (i.e. the temporal poles and STS) are important for ‘decoding’ the available information, such as facial expression or tone of voice, to enable reasoning about mental states, while not taking part in the reasoning per se (Frith and Frith, 2003; Sabbagh et al., 2004; Walter et al., 2004). Interestingly, although the neuroimaging literature as a whole does not point to a special role for the RH in theory of mind (see Brunet et al., 2000), Happe et al. (1999) found that stroke patients with acquired RH brain damage were significantly impaired on comprehending stories and cartoons that required mental state reasoning relative to both normal adults and patients with LH damage. Although the development of theory of mind has been well studied in children behaviorally, only one neuroimaging study to date has examined mentalizing in children. Using the same animated shape paradigm employed by Castelli et al. (2000), Ohnishi et al. (2004) found that a sample of children aged 7–13 recruited regions very similar to those previously observed in adults including the MPFC, STS, temporoparietal junction and temporal poles. They did not, however, directly compare children with adults and, hence, could not assess whether any of these regions showed differential involvement as a function of age.
The goal of the present study was to examine developmental differences in the neural circuitry underlying the interpretation of communicative intent. Here, children and adults listened to short scenarios involving two characters in a conversational setting, where one character makes a remark that is potentially ironic. Scenarios were accompanied by cartoon drawings in which the facial expression of the speaker matched the intonation of the utterance. Irony is defined in the Merriam-Webster online dictionary as ‘the use of words to express something other than and especially the opposite of the literal meaning’. Although not all instances of irony involve sarcasm, which implies a mocking or critical attitude, the two often overlap in everyday usage. For the present purposes, the terms irony and sarcasm are used interchangeably. Because the ability to appreciate the subtleties of irony is still developing in school-age children (Demorest et al., 1984; Capelli et al., 1990; Dews et al., 1996; Dews and Winner, 1997), we chose straightforward, child-friendly stories that were paired with comments often used with both ironic and sincere intent (e.g. ‘Thanks a lot!’). Using irony as a test case allowed us to add to the limited literature on the neural basis of both non-literal/pragmatic language and the development of theory of mind.
This study attempts to address two main questions. First, broadly, what are the neural networks involved in inferring the intent beyond the literal meaning of a remark? Given the neuropsychological literature on patients with RH and frontal lesions, we hypothesized that scenarios involving irony comprehension would elicit greater activity in RH and prefrontal regions than scenarios containing only literal speech. Based on the neuroimaging research reviewed above, we expected to see brain activity in regions previously associated with reasoning about the mental states of others, particularly the MPFC, since understanding irony requires the ability to decouple the speaker's intended meaning from the literal meaning. Second, how do patterns of brain activity change with age? Given highly conventional scenarios, if the ability to infer ironic intent is still developing in children but relatively more automatic in adults, children might rely on prefrontal regions, particularly the MPFC, to a greater extent than adults. Furthermore, would the children's tendency to pay more attention to the propositional content of speech be reflected in the neural networks engaged by this task with less activity shown in areas involved in processing facial affect and prosodic cues? Since facial expression and tone of voice can be important cues for interpreting communicative intent, can we elicit a more adult-like activation pattern in children by specifically directing their attention to these cues? Increased attention to facial expressions should result in a modulation of activity in temporal-occipital regions associated with face and emotion processing including the fusiform gyrus (FG) (Wojciulik et al., 1998; Vuilleumier et al., 2001; Pessoa et al., 2002) and possibly the amygdala (Pessoa et al., 2002). Based on previous research on the influence of emotional intonation, attention to tone of voice is likely to engage frontal and temporal networks, particularly in the RH (Mitchell et al., 2003; Wildgruber et al., 2004; Hesling et al., 2005; Wildgruber et al., 2005).
MATERIALS AND METHODS
Participants
Twelve healthy adult volunteers (6 male, 6 female; mean age, 26.9 ± 3.5 years, range, 23–33) and twelve typically developing children (6 males, 6 females; mean age, 1.9 ± 1.8 years, range = 9–14) were recruited through flyers posted at UCLA and the Los Angeles area. All participants were native English speakers with no reported history of neurological or psychiatric disorders. All were right-handed, with the exception of one left-handed boy. We performed all analyses both with and without the left-handed participant; as the pattern of results did not change when this subject was excluded and his inclusion allows for complete counterbalancing, we report all results with the full sample of 12 adults and 12 children. Written informed consent was obtained from each subject in accordance with the procedures of the UCLA Institutional Review Board.
Stimuli
Participants viewed cartoon drawings of children in a conversational setting while listening to a short story that ended with a potentially ironic remark. Each scenario had a sarcastic version and a sincere version that shared the same neutral setup (for example, ‘Tom and Mary are building a tall tower’). The sarcastic ending consisted of an undesirable outcome and a final remark uttered in a clearly sarcastic tone of voice (‘When Mary accidentally knocks it down, Tom says, “Way to go!” ’). The sincere ending had a positive outcome with a final comment made in a sincere, complimentary tone of voice (‘When Mary finally finishes it up, Tom says, “Way to go!” ’). Example drawings are shown in Figure 1. Following the sincere or sarcastic comment, participants were asked to decide whether the speaker really meant what he or she said. Yes/no judgments were indicated by pressing a button with the index or middle finger, respectively. Instructions were clear that a ‘yes’ response should be given if the comment was sincere and should be taken literally, while a ‘no’ response should indicate that the final remark was sarcastic and the speaker meant the opposite of what he or she said. Participants were shown examples of both sincere and sarcastic scenarios not used during the scan and all answered correctly.
Fig. 1.
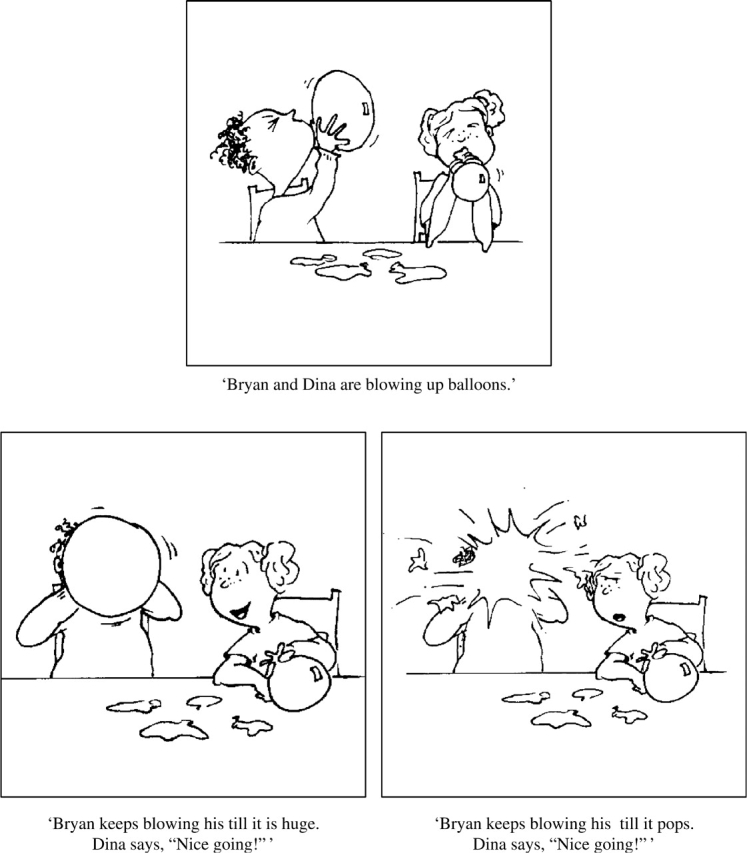
Example scenario. The setup (top panel) is shared by both the sincere and ironic versions of the scenario. The sincere ending is shown in the bottom left panel and the ironic ending is displayed in the bottom right panel. The text below the drawings represents the accompanying auditory stimuli. Participants first view the setup, then either the sincere or ironic version of a scenario followed by a blank screen and the question, ‘Did Dina mean what she said?’
To verify that the final comments sounded sincere or sarcastic as intended, twelve adult volunteers who did not take part in the fMRI study listened to the remarks, which were presented in isolation (i.e. without the surrounding context) and rated them on a scale of 1–7, with 1 as the anchor for sarcastic and 7 for sincere/complimentary. Sarcastic remarks received a mean rating of 1.4 ± 0.7, while sincere comments received a mean rating of 6.6 ± 0.7. Sincere and ironic versions of each scenario were matched in terms of syntactic structure, semantic complexity and length.
In order to examine the neural circuitry underlying the interpretation of irony per se, we also included a No Irony Control condition comprised of scenarios (different from but carefully matched with those used in the Irony condition) that ended in straightforward remarks where the speaker's intent was unambiguous. For example, ‘Ashley and Zack are riding their bikes. When it starts to get dark out, Ashley says, “Let's go home.” ’ As per the Irony conditions, participants were asked to decide whether the speaker meant what he or she said, though these remarks were not easily interpretable in a non-literal light and were made in a neutral tone of voice.
Ironic remarks are often marked by slower tempo, greater intensity and lower pitch level than are non-ironic remarks (Rockwell, 2000). These speech characteristics are integral to irony and are likely to impact the pattern of activity observed when comparing ironic with non-ironic statements. However, because the neural networks involved in processing prosody are somewhat well characterized, we can still draw inferences as to which components of the networks subserving irony may reflect these speech characteristics vs other interpretative processes, as well as examine developmental changes occurring in the neural correlates of irony.
Activation paradigm
Four activation blocks were interspersed with rest periods lasting 21 s. The first three blocks each consisted of six scenarios (three ironic, three sincere, 15 s per scenario) that ended in a potentially ironic remark (e.g. ‘Thanks a lot!’). While instructions given before the first block were simply to pay attention (Neutral Instructions condition), before the second and third blocks, the participants were told to pay attention to the facial expression (Attend Face condition) or to the tone of voice (Attend Prosody condition). We chose to put the Neutral Instructions condition first in order to be able to examine how participants would naturally process scenarios that potentially involve irony without any carryover effect of instructions to attend specifically to the facial expression or tone of voice of the speaker. The order of instructions to attend to a particular cue in the second and third blocks was counterbalanced across subjects. In order to avoid any specific item effects, scenarios were used equally often in each of the three Irony conditions across subjects. Each participant saw only one version (sincere vs sarcastic) of each scenario. Finally, the fourth and last activation block consisted of six scenarios that did not involve any irony (No Irony Control condition), which ended in an unambiguous statement (e.g. ‘Please pass the crayons’).
In designing the scenarios, we sought to move toward a more naturalistic, ecologically valid paradigm by incorporating multiple elements of the rich environmental context within which most interpretation of others’ communicative intentions takes place (i.e. facial expression, prosody and event context). The paradigm employed is suboptimal in some ways, in that the length of each scenario necessitates activation blocks that are relatively long and precludes an event-related design that would allow for the isolation of sincere vs sarcastic scenarios. However, these compromises were made in the framework of examining the neural processes involved in interpreting a speaker's intent as a whole, within a more naturalistic communicative context, rather than examining the ambiguous remark in isolation and assuming that inferring sincerity and irony are independent functional processes (Small and Nusbaum, 2004).
Data acquisition
Images were acquired on a Siemens Allegra 3T Scanner. A T2-weighted sagittal scout was used to prescribe the planes of the functional images and to ensure that no structural abnormalities were present. For each subject, the functional data consisted of 155 whole-brain volumes collected in the axial plane parallel to the anterior commissure–posterior commissure (AC-PC) line using an EPI gradient-echo sequence (TR = 3000 ms, TE = 25 ms, 3 mm slice thickness/1 mm gap, 64 × 64 matrix size, FOV = 20 cm). A high-resolution EPI structural volume (TR = 5000 ms, TE = 33 ms, 128 × 128 matrix size, FOV = 20 cm) was also acquired coplanar with the functional images.
Data Analysis
We analyzed the imaging data using SPM99 (Wellcome Department of Cognitive Neurology; http://www.fil.ion.ucl.ac.uk/spm/). Functional images were first realigned to correct for head motion with AIR (Woods et al., 1998a) using a linear rigid-body registration algorithm. In order to allow for inter-subject averaging, all images were then transformed into a Talairach-compatible standard space (Woods et al., 1999) using polynomial non-linear warping (Woods et al., 1998b). Functional volumes were smoothed using a 6 mm full width-half maximum Gaussian kernel to increase signal to noise ratio.
For each subject, condition effects were estimated according to the general linear model using a box-car reference function with a 6 s delay to compensate for the lag in hemodynamic response. Response time and accuracy scores collected during scanning were entered as regressors to ensure that any differences observed in activation patterns between conditions or groups were not due to differences in task difficulty. The resulting contrast images were entered into group analyses using a random-effect model to allow for inferences at the population level (Friston et al., 1999). For each group, one-sample t-tests were conducted to identify clusters of significant activity for each activation condition. Between-group differences were examined using two-sample t-tests; these comparisons were made within regions where reliable activation was detected in either group across all conditions. For children, a simple regression analysis was used to identify regions of activity associated with chronological age. Based on previous research, we predicted activity in regions implicated in reasoning about the intentions of others (i.e. MPFC, STS and temporal poles), as well as processing facial expression (i.e. FG, amygdala and STS) and tone of voice (frontotemporal networks). Results were initially explored using liberal thresholds of P < 0.05, uncorrected for multiple comparisons, for both magnitude and spatial extent; however, we consider reliable and discuss only activity that survived considerably more stringent thresholds of P < 0.01 (t > 2.72) at the voxel level and P < 0.05 corrected for multiple comparisons at the cluster level (corresponding to a minimum cluster size of at least 79 voxels). Within the regions mentioned above for which we had a priori hypotheses, we employed a small volume correction (corresponding to a minimum cluster size of at least 37 voxels) to test for significance (Worsley et al., 1996).
RESULTS
Behavioral results
Behavioral performance during scanning is shown in Table 1. Across conditions, while no reliable between-group differences were observed in response times, children were slightly, but not significantly, less accurate than adults overall (F1,22 = 3.40, P = 0.08). Planned comparisons revealed that adults and children did not differ reliably in response time or accuracy in any of the tasks, although there was a trend toward less accurate performance in children in the Neutral Instructions condition (F1,22 = 3.14, P = 0.09), when participants were not instructed to attend to a particular cue.
Table 1.
Task performancea
| Accuracy (correct %) |
Response time (s) |
|||
|---|---|---|---|---|
| Adults | Children | Adults | Children | |
| Neutral instructions | 100 (0) | 94.4 (10.8) | 2.4 (.26) | 2.4 (.32) |
| Attend face | 100 (0) | 98.6 (4.8) | 2.4 (.34) | 2.3 (.28) |
| Attend voice | 98.6 (4.8) | 95.6 (8.1) | 2.4 (.21) | 2.4 (.38) |
| Control | 100 (0) | 100 (0) | 2.5 (.28) | 2.5 (.16) |
aValues are presented as mean (s.d.).
fMRI results
Effects of irony
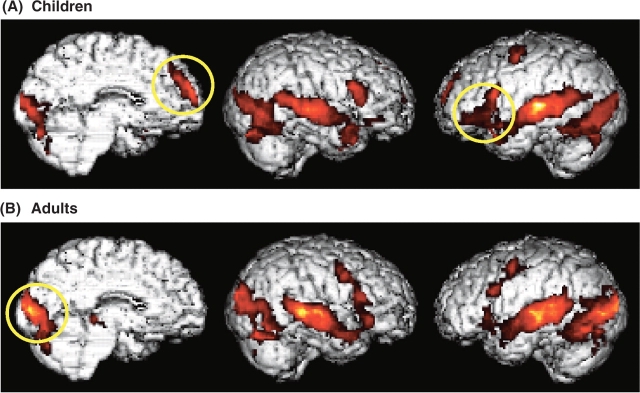
Across all irony conditions (as compared with rest), children and adults engaged similar overall networks including frontal, temporal and occipital cortices bilaterally, as expected (Figure 2). However, a preliminary inspection of the group profiles revealed some noteworthy differences. Specifically, children recruited left inferior frontal regions more strongly than adults and showed reliable activity in the MPFC (Figure 2A), whereas adults did not. In contrast, adults activated posterior occipitotemporal regions more strongly than children (Figure 2B). In the No Irony condition, where the final remark was an unambiguous direct request, both groups again showed significant activity in frontotemporal and occipital regions relative to rest. However, adults exhibited the left-lateralized activation pattern typical of basic language processing, whereas children recruited a more bilateral network similar to that activated in the Irony conditions. Furthermore, despite the lack of ambiguity in the intent of the speaker in this condition, activity was also detected in the MPFC in children, albeit at a less stringent spatial extent threshold (P < 0.001, uncorrected).
Fig. 2.
Brain activity averaged across all irony conditions compared with rest in children (A) and adults. (B) Figures are thresholded at t > 1.80, corrected for multiple comparisons at the cluster level, P < 0.05.
To examine the networks specific to processing irony, we compared the activity summed across the three irony conditions (Neutral Instructions + Attend to Face + Attend to Prosody) with the No Irony Control condition within each group. As expected, adults showed reliable activity bilaterally in the superior temporal gyrus with stronger peaks in the RH. Children also showed greater activity during the Irony tasks bilaterally in the STG as well as in the left temporal pole. Both groups activated the MPFC more strongly in the Irony conditions than in the No Irony condition, with adults also recruiting an additional, slightly more dorsal region within this area (Table 2).
Table 2.
Peaks of activation for the Irony vs No Irony comparison
| All irony – No irony |
||||||
|---|---|---|---|---|---|---|
| Region | BA | x | y | z | t | |
| Adults | ||||||
| Superior temporal gyrus | 22 | L | −48 | −38 | 12 | 5.30 |
| 22 | R | 60 | −22 | −2 | 6.93 | |
| Medial prefrontal cortex | 10 | 2 | 62 | 2 | 4.79a | |
| 11 | L | −12 | 50 | −10 | 5.53a | |
| Children | ||||||
| Superior temporal gyrus | 22 | L | −58 | −22 | 4 | 5.22a |
| 22 | R | 58 | −34 | 10 | 3.19a | |
| Temporal pole | 38 | L | −44 | 14 | −16 | 3.91a |
| Medial prefrontal cortex | 11 | −2 | 44 | −10 | 4.51a | |
Only clusters surviving a threshold of P < 0.05, corrected for multiple comparisons at the cluster level (k ≥ 79 voxels) and P < 0.01, uncorrected, for peak magnitude, are reported. BA refers to Brodmann's areas. L and R refer to the left and right hemispheres. x, y, and z reflect positions in Talairach coordinate space corresponding to the left-right, anterior–posterior and superior–inferior dimensions, respectively. t refers to the highest t-score. asurvive a small volume correction for multiple comparisons at P < 0.05, k ≥ 37 voxels.
Effects of Age
To examine developmental differences in the neural circuitry involved in inferring communicative intent, we compared adults and children using two sample two-tests. For each irony condition, differences between children and adults were remarkably similar. For this reason, we focus below on reliable between-group differences summed across all three Irony conditions. For the sake of completeness, coordinates for areas differentially activated by children and adults for each condition (relative to rest) are presented in Table 3.
Table 3.
Peaks of differential activity between adults and children
| Neutral instructions |
Attend face |
Attend voice |
No irony |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | BA | x | y | z | t | x | y | z | t | x | y | z | t | x | y | z | t | |
| Adults > Children | ||||||||||||||||||
| Superior temporal gyrus | 42 | L | −36 | −26 | 8 | 2.89 | ||||||||||||
| 22 | L | −42 | −36 | 8 | 2.75 | −46 | −36 | 10 | 3.73 | −66 | −40 | 8 | 3.15 | −44 | −32 | 14 | 3.24 | |
| Superior temporal sulcus | R | 58 | −38 | 4 | 4.13 | |||||||||||||
| Middle temporal gyrus | 21 | L | −66 | −44 | 6 | 3.17 | ||||||||||||
| 21 | R | 58 | −28 | −8 | 2.68 | 60 | −36 | 0 | 4.31a | |||||||||
| Fusiform gyrus | 19 | L | −26 | −64 | −10 | 3.54 | −26 | −62 | −6 | 2.85 | ||||||||
| 19 | R | 32 | −64 | −12 | 3.14 | 22 | −62 | −10 | 4.59 | |||||||||
| Lingual gyrus | 18 | L | −20 | −76 | −8 | 3.38 | −6 | −82 | 4 | 3.49 | −14 | −82 | −2 | 3.59 | ||||
| 18 | R | |||||||||||||||||
| 19 | L | −16 | −74 | −4 | 2.95 | |||||||||||||
| Cuneus | 17 | L | −10 | −90 | 2 | 3.66 | −10 | −90 | 6 | 3.72 | ||||||||
| 18 | L | −16 | −90 | 14 | 4.31 | |||||||||||||
| Occipital gyrus | 18 | L | −34 | −92 | 2 | 2.85 | ||||||||||||
| Middle occipital gyrus | 19 | L | −50 | −74 | 2 | 3.40 | ||||||||||||
| Amygdala | L | −22 | −2 | −12 | 3.48a | |||||||||||||
| Children > Adults | ||||||||||||||||||
| Superior temporal gyrus | 42 | R | 36 | −38 | 10 | 3.43 | 60 | −14 | 12 | 3.49 | ||||||||
| 22 | R | 38 | −36 | 8 | 3.57a | |||||||||||||
| Superior temporal sulcus | R | 38 | −46 | 18 | 3.61 | 42 | −44 | 16 | 4.23 | 44 | −46 | 16 | 3.85 | |||||
| Inferior frontal gyrus | 44 | L | −48 | 8 | 32 | 3.11 | −50 | 16 | 12 | 3.70 | ||||||||
| 44 | R | |||||||||||||||||
| 45 | L | −58 | 12 | 4 | 2.86 | |||||||||||||
| 45 | R | 36 | 14 | 20 | 2.97 | |||||||||||||
| 47 | L | |||||||||||||||||
| Middle frontal gyrus | 9 | R | 28 | 16 | 28 | 3.20 | ||||||||||||
| Medial prefrontal cortex | 9 | L | −4 | 56 | 22 | 3.18 | −8 | 52 | 26 | 3.24 | −10 | 52 | 16 | 3.41 | ||||
| 10 | L | −4 | 60 | 16 | 2.93a | −14 | 58 | 16 | 4.77 | |||||||||
Only clusters surviving a threshold of P < 0.05, corrected for multiple comparisons at the cluster level (k ≥ 79 voxels) and P < 0.01, uncorrected, for peak magnitude, are reported. BA refers to Brodmann's areas. L and R refer to the left and right hemispheres. x, y, and z reflect positions in Talairach coordinate space corresponding to the left-right, anterior–posterior and superior–inferior dimensions, respectively. t refers to the highest t score. asurvive a small volume correction for multiple comparisons at P < 0.05, k ≥ 37 voxels.
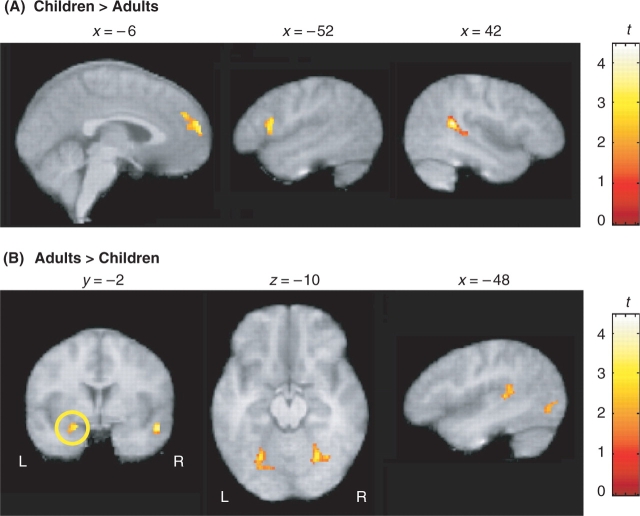
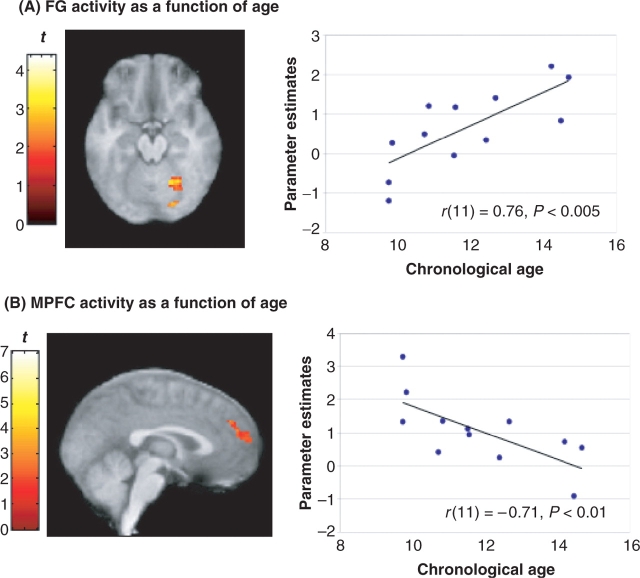
As predicted, across all irony tasks, children recruited frontal regions significantly more than adults did, particularly the MPFC and left inferior frontal gyrus. The right posterior STS was also more strongly activated in children than in adults (Figure 3A). In contrast, adults showed reliably greater activity than children in the left amygdala, bilateral fusiform gyrus (FG), left STG (Figure 3B), as well as extrastriate cortices. In order to further examine changes in brain activity with age, we conducted a simple regression analysis in children. Consistent with the between-group results, across all irony tasks relative to rest, activity in the right FG increased reliably with chronological age (Figure 4A) and activity in prefrontal regions, the MPFC and the left IFG in particular, decreased with age (Figure 4B). Bilateral temporal activity, specifically in the left STG and right MTG, also decreased reliably with age.
Fig. 3.
Brain regions differentially activated in children and adults across all irony tasks. (A). Children showed significantly greater activity than adults in the MPFC (x = −8, y = 58, z = 20; P < 0.05), the left inferior frontal gyrus (x = −48, y = 16, z = 14; P = 0.05) and the right posterior STS (x = 42, y = −44, z = 16; P < 0.05). (B) Adults showed reliably greater activity than children in posterior temporal-occipital regions, including the left amygdala (x = −22, y = −2, z = −12; P < 0.05, SVC), bilateral FG (L: x = −28, y = −64, z = −8; R: x = 30, y = −66, z = −14; P < 0.05) and left STG (x = −36, y = −26, z = 8; P < 0.05). All regions shown survive a threshold of t > 1.80 and whole-volume correction for multiple comparisons at the cluster level, P < 0.05, or small volume correction (SVC) where noted.
Fig. 4.
Age-related brain activity in children across all irony tasks. (A) Increasing chronological age was reliably associated with increased activation of the right FG (x = 24, y = −58, z = −14; P < 0.05). (B) Decreased activation of the MPFC (peak in dorsal MPFC, x = −2, y = 44, z = 32; P < 0.05) was reliably associated with increasing age. Activity in the left IFG (x = −58, y = 20, z = 10; P < 0.05, SVC), left STG (x = −46, −22, 6; P < 0.05) and right MTG (x = 44, y = −36, z = 4; P < 0.05) also decreased as a function of age (not shown). All regions survive a threshold of t > 1.80 and whole-volume correction for multiple comparisons at the cluster level, P < 0.05, or small volume correction (SVC) where noted.
In the No Irony condition, the only region more strongly activated in adults than in children was the left STG. Children, on the other hand, engaged both RH superior temporal regions and the MPFC reliably more than adults did, even though the scenarios contained only straightforward remarks, perhaps reflecting the ongoing development of basic discourse comprehension skills.
Direct between-group comparisons between children and adults for the contrast Irony (collapsed) vs No Irony conditions corroborate the findings presented earlier. More specifically, adults showed greater activity than children in the right FG (BA 37, Talairach coordinates: 24, −56, −14, t = 3.26, spatial extent threshold: P < 0.001, uncorrected), whereas children showed greater activity than adults in the left IFG (BA 45, Talairach coordinates: −48, 24, 2, t = 3.12, spatial extent threshold: P < 0.001, uncorrected). However, greater activity in MPFC was not observed when comparing children with adults for this contrast (i.e. Irony vs No Irony), likely reflecting the fact that children did activate this region also in the No Irony condition (though to a lesser extent than in the Irony conditions).
Effect of Attentional Modulation
Coordinates for peaks of activity in each condition vs rest are presented in Tables 4 and 5 for children and adults, respectively. To assess the effect of task instructions, we directly compared the Attend to Face condition with the Neutral Instructions condition within each group (Table 6). Consistent with our hypotheses, adults showed reliably greater activity in regions associated with facial emotion processing, particularly the left amygdala and the right fusiform and lingual gyri, when instructed to attend to the facial expression than when instructed simply to pay attention. Children also showed stronger activity in the right fusiform gyrus, as well as the right temporal pole and bilateral extrastriate cortices (middle occipital and inferior temporal gyri). The attentional modulation effect seen in the FG was comparable across the two groups as revealed by between-group comparisons for the Attend to Face vs Neutral Instruction contrast showing no differences between children and adults in this region.
Table 4.
Peaks of activation in children
| Neutral instructions |
Attend face |
Attend voice |
No irony |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | BA | x | y | z | t | x | y | z | t | x | y | z | t | x | y | z | t | |
| Superior temporal gyrus | 42 | L | −58 | −26 | 8 | 8.70 | −52 | −20 | 8 | 12.77 | −66 | −28 | 6 | 12.29 | −58 | −22 | 8 | 9.25 |
| 42 | R | 44 | −20 | 10 | 3.66 | 42 | −30 | 10 | 5.75 | 42 | −42 | 12 | 6.42 | 60 | −16 | 10 | 6.68 | |
| 22 | L | −62 | −12 | 0 | 7.87 | −58 | −22 | 2 | 12.31 | −62 | −18 | −2 | 10.57 | −60 | −38 | 6 | 6.84 | |
| 22 | R | 60 | −20 | 4 | 8.22 | 42 | −38 | 6 | 8.07 | 54 | −40 | 10 | 7.26 | 44 | −42 | 14 | 11.17 | |
| Superior temporal sulcus | L | −58 | −42 | 6 | 5.18 | −64 | −54 | 14 | 5.49 | −60 | −56 | 14 | 6.59 | |||||
| R | 54 | −38 | 4 | 5.06 | 58 | −28 | 4 | 6.37 | 56 | −24 | 2 | 6.52 | 48 | −30 | 2 | 7.07 | ||
| Middle temporal gyrus | 21 | L | −54 | −32 | 0 | 5.27 | −60 | −44 | 2 | 6.91 | −50 | −46 | 2 | 6.98 | −58 | 0 | −8 | 5.14 |
| 21 | R | 54 | −34 | 2 | 4.99 | 52 | −38 | 4 | 5.16 | 48 | −12 | −4 | 6.05 | |||||
| 39 | L | −56 | −62 | 16 | 6.02 | |||||||||||||
| 39 | R | 48 | −62 | 12 | 4.02 | |||||||||||||
| Inferior temporal gyrus | 37 | L | −42 | −66 | −2 | 3.19 | 44 | −68 | 0 | 9.15 | ||||||||
| 37 | R | 44 | −72 | 0 | 3.94 | 44 | −68 | 0 | 5.99 | 44 | −70 | 0 | 7.69 | −42 | −70 | 0 | 5.97 | |
| Temporal pole | 38 | L | −54 | 10 | −10 | 5.85 | −52 | 8 | −26 | 4.55 | −42 | 12 | −26 | 5.08 | ||||
| 38 | R | 46 | 10 | −12 | 4.68 | 30 | 12 | −26 | 4.66 | 44 | 6 | −22 | 5.05 | |||||
| Lingual gyrus | 18 | L | −12 | −80 | −10 | 2.78 | −24 | −86 | −8 | 4.79 | −22 | −84 | −6 | 4.05 | −18 | −84 | −6 | 6.86 |
| 18 | R | 20 | −80 | −8 | 3.30 | −16 | −90 | −2 | 5.53 | 20 | −80 | −4 | 4.65 | 4 | −82 | 4 | 4.55 | |
| Fusiform gyrus | 19 | L | −36 | −78 | −12 | 3.69 | −42 | −−50 | −10 | 5.09 | −34 | −60 | −6 | 4.69 | −32 | −78 | −10 | 5.47 |
| 19 | R | 42 | −48 | −8 | 6.74 | 38 | −48 | −8 | 5.02 | 34 | −60 | −6 | 6.02 | 40 | −60 | −6 | 6.16 | |
| 37 | L | −40 | −50 | −18 | 5.33 | −38 | −50 | −16 | 5.04 | −44 | −48 | −14 | 6.46 | −40 | −48 | −14 | 5.02 | |
| 37 | R | 42 | −54 | −12 | 4.77 | 38 | −52 | −10 | 4.94 | 38 | −46 | −10 | 5.42 | 36 | −50 | −10 | 6.82 | |
| Cuneus | 17 | L | −12 | −96 | 6 | 3.43 | −6 | −96 | 8 | 3.74 | −12 | −96 | 8 | 4.77 | −4 | −90 | 2 | 6.57 |
| 17 | R | 2 | −82 | 6 | 3.27 | 8 | −92 | 2 | 6.13 | 6 | −94 | 8 | 4.66 | 18 | −92 | 6 | 8.13 | |
| 18 | L | −16 | −98 | 18 | 2.86 | −20 | −98 | 8 | 4.41 | −20 | −98 | 12 | 3.49 | −10 | −98 | 14 | 4.44 | |
| 18 | R | 14 | −94 | 12 | 5.39 | 8 | −94 | 20 | 3.46 | 14 | −92 | 10 | 4.78 | 6 | −94 | 18 | 3.46 | |
| Middle occipital gyrus | 19 | L | −48 | −76 | 0 | 3.21 | −50 | −72 | −8 | 3.22 | −40 | −70 | −6 | 3.25 | ||||
| 19 | R | 38 | −78 | 8 | 8.07 | 38 | −74 | 4 | 5.41 | 28 | −76 | 8 | 6.35 | |||||
| Inferior frontal gyrus | 44 | L | −56 | −42 | 16 | 4.00 | −52 | 16 | 12 | 4.91 | ||||||||
| 44 | R | 44 | 14 | 26 | 5.48a | 52 | 18 | 14 | 4.59a | |||||||||
| 45 | L | −58 | 20 | 8 | 5.94 | −58 | 18 | 20 | 4.62 | −48 | 26 | 12 | 3.68a | |||||
| 45 | R | 52 | 24 | 16 | 3.51a | 48 | 24 | 14 | 6.17 | |||||||||
| 47 | L | −48 | 22 | 0 | 5.15 | −48 | 34 | −4 | 4.67 | |||||||||
| 47 | R | 54 | −12 | −4 | 4.67 | |||||||||||||
| Middle frontal gyrus | 9 | L | −54 | 4 | 38 | 5.56a | ||||||||||||
| 9 | R | 36 | 24 | 30 | 2.98a | |||||||||||||
| 46 | R | 46 | 28 | 24 | 2.83a | |||||||||||||
| Dorsomedial prefrontal cortex | 8 | R | 2 | 46 | 40 | 5.53 | ||||||||||||
| 9 | L | −6 | 46 | 32 | 5.74 | −6 | 46 | 32 | 4.89 | −6 | 48 | 30 | 5.28 | |||||
| Ventromedial prefrontal cortex | 10 | L | −10 | 62 | 20 | 4.93 | −8 | 60 | 22 | 7.52 | −10 | 58 | 20 | 4.53 | ||||
| 10 | R | 8 | 62 | 16 | 4.56 | |||||||||||||
Only clusters surviving a threshold of P < 0.05, corrected for multiple comparisons at the cluster level (k ≥ 79 voxels) and P < 0.01, uncorrected, for peak magnitude, are reported. BA refers to Brodmann's areas. L and R refer to the left and right hemispheres. x, y and z reflect positions in Talairach coordinate space corresponding to the left-right, anterior–posterior and superior–inferior dimensions, respectively. t refers to the highest t-score. asurvive a small volume correction for multiple comparisons at P < 0.05, k ≥ 37 voxels.
Table 5.
Peaks of activation in adults
| Neutral instructions |
Attend face |
Attend voice |
No irony |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | BA | x | y | z | t | x | y | z | t | x | y | z | t | x | y | z | t | |
| Superior temporal gyrus | 42 | L | −56 | −18 | 6 | 7.01 | −54 | −22 | 6 | 10.85 | −54 | −20 | 6 | 8.12 | −62 | −18 | 8 | 8.61 |
| 42 | R | 54 | −22 | 6 | 10.22 | 44 | −24 | 6 | 6.90 | 44 | −24 | 8 | 6.95 | 40 | −28 | 8 | 3.31 | |
| 22 | L | −62 | −6 | −2 | 8.55 | −66 | −24 | 4 | 10.04 | −54 | −46 | 10 | 7.54 | −66 | −24 | 6 | 10.05 | |
| 22 | R | 50 | −24 | 4 | 8.44 | 46 | −42 | 8 | 8.77 | 50 | −18 | 4 | 10.93 | 50 | −22 | 4 | 4.98 | |
| Superior temporal sulcus | L | −66 | −26 | 2 | 6.91 | −56 | −30 | 6 | 7.06 | −64 | −42 | 4 | 10.46 | −64 | −26 | 0 | 8.09 | |
| R | 52 | −10 | −2 | 7.07 | 56 | −32 | 4 | 7.01 | 54 | −34 | 6 | 8.45 | 52 | −32 | 4 | 5.87 | ||
| Middle temporal gyrus | 21 | L | −62 | −22 | −6 | 6.64 | −60 | −24 | −4 | 12.30 | −58 | −28 | 0 | 14.47 | −62 | −44 | 2 | 6.75 |
| 21 | R | 54 | −10 | −6 | 7.72 | 48 | −14 | −4 | 7.06 | 60 | −36 | 2 | 10.89 | 42 | −36 | −2 | 6.30 | |
| Inferior temporal gyrus | 37 | L | −50 | −74 | 2 | 5.63 | −46 | −60 | −6 | 6.36 | −46 | −68 | 2 | 4.00 | −48 | −64 | 2 | 2.91 |
| 37 | R | 46 | −66 | 2 | 3.99 | 44 | −72 | 6 | 7.16 | 38 | −68 | 2 | 4.32 | |||||
| Temporal pole | 38 | L | −46 | 16 | −12 | 4.75 | −54 | 8 | −10 | 5.22 | −56 | 6 | −8 | 4.97 | −44 | 6 | −20 | 7.30 |
| 38 | R | 42 | 14 | −18 | 4.47 | 42 | 14 | −18 | 6.39 | 42 | 14 | −18 | 4.94 | 38 | 8 | −24 | 6.49 | |
| Lingual gyrus | 18 | L | −16 | −86 | −2 | 6.14 | −14 | −86 | −2 | 6.95 | −16 | −82 | −4 | 6.99 | −22 | −76 | −2 | 5.48 |
| 18 | R | 8 | −84 | −6 | 5.71 | 12 | −80 | −2 | 4.40 | 4 | −78 | −12 | 6.17 | 18 | −70 | −4 | 6.89 | |
| Fusiform gyrus | 19 | L | −22 | −78 | −10 | 5.94 | −26 | −62 | −8 | 3.31 | −26 | −78 | −10 | 4.37 | −24 | −84 | −10 | 5.29 |
| 19 | R | 32 | −64 | −12 | 7.37 | 38 | −64 | −8 | 6.98 | 42 | −54 | −10 | 6.32 | 28 | −76 | −12 | 3.73 | |
| 37 | L | −42 | −62 | −10 | 6.94 | −30 | −62 | −12 | 4.09 | −30 | −56 | −14 | 3.04 | −44 | −48 | −14 | 4.61 | |
| 37 | R | 42 | −62 | −16 | 4.66 | 42 | −54 | −12 | 4.57 | 46 | −60 | −10 | 4.58 | 42 | −54 | −12 | 5.10 | |
| Amygdala | L | −16 | −6 | −10 | 4.68a | −26 | −2 | −16 | 4.49 | |||||||||
| R | 22 | −2 | −18 | 4.12 | ||||||||||||||
| Cuneus | 17 | L | −14 | −92 | 8 | 8.52 | −12 | −94 | 6 | 10.71 | −14 | −94 | 4 | 9.65 | −6 | −90 | 4 | 7.22 |
| 17 | R | 10 | −90 | 4 | 8.26 | 8 | −90 | 2 | 10.99 | 8 | −90 | 2 | 11.97 | 8 | −90 | 6 | 12.39 | |
| 18 | L | −14 | −98 | 10 | 6.00 | −26 | −98 | 10 | 19.30 | −22 | −96 | 12 | 9.37 | −26 | −96 | 8 | 11.17 | |
| 18 | R | 8 | −96 | 16 | 8.28 | 22 | −94 | 12 | 7.00 | |||||||||
| Middle occipital gyrus | 19 | L | −24 | −98 | 16 | 6.90 | −36 | −86 | 8 | 9.85 | −44 | −82 | 4 | 7.87 | −42 | −86 | 4 | 6.91 |
| 19 | R | 24 | −92 | 22 | 5.11 | 30 | −82 | 10 | 6.59 | 22 | −90 | 26 | 6.60 | 28 | −82 | 8 | 5.75 | |
| Inferior frontal gyrus | 44 | L | −52 | 16 | 26 | 5.32a | ||||||||||||
| 44 | R | 46 | 18 | 24 | 3.85a | 48 | 18 | 16 | 3.49 | |||||||||
| 45 | R | 52 | 16 | 4 | 2.98 | 42 | 24 | 18 | 5.66 | 52 | 20 | 6 | 4.38a | |||||
| 47 | L | −46 | 22 | −6 | 4.43 | |||||||||||||
| 47 | R | 46 | 22 | 0 | 5.38a | 44 | 28 | −4 | 4.39 | 38 | 30 | −10 | 3.85a | |||||
| Middle frontal gyrus | 9 | L | −52 | 20 | 28 | 5.75 | ||||||||||||
| 9 | R | 50 | 4 | 38 | 3.94 | 48 | 6 | 36 | 7.02 | 50 | 2 | 38 | 4.62a | |||||
| Dorsomedial prefrontal cortex | 8 | L | −2 | 38 | 40 | 5.75a | ||||||||||||
| 9 | L | −6 | 50 | 34 | 3.80a | |||||||||||||
Only clusters surviving a threshold of P < 0.05, corrected for multiple comparisons at the cluster level (k ≥ 79 voxels) and P < 0.01, uncorrected, for peak magnitude, are reported. BA refers to Brodmann's areas. L and R refer to the left and right hemispheres. x, y and z reflect positions in Talairach coordinate space corresponding to the left-right, anterior–posterior and superior–inferior dimensions, respectively. t refers to the highest t-score. asurvive a small volume correction for multiple comparisons at P < 0.05, k ≥ 37 voxels.
Table 6.
Peaks of activation for specific instructions vs neutral instructions
| Attend face – neutral instructions |
Attend prosody – neutral instructions |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | BA | x | y | z | t | x | y | z | t | |
| Adults | ||||||||||
| Superior temporal sulcus | L | −52 | −38 | 6 | 6.48a | |||||
| Lingual gyrus | 18 | R | 20 | −64 | 0 | 3.59 | ||||
| Fusiform gyrus | 37 | R | 24 | −64 | −10 | 2.96 | ||||
| Amygdala | L | −20 | −2 | −12 | 3.27 | |||||
| Inferior frontal gyrus | 47 | L | −46 | 22 | −4 | 3.10a | ||||
| 45 | R | 44 | 24 | 18 | 5.36a | |||||
| Children | ||||||||||
| Middle temporal gyrus | 21 | L | −48 | −54 | 6 | 3.78 | ||||
| 21 | R | 40 | 0 | −24 | 6.03 | |||||
| Inferior temporal gyrus | 37 | L | −40 | −60 | −8 | 3.30 | ||||
| 20 | R | 32 | −6 | −30 | 4.38a | 44 | −70 | 0 | 3.94 | |
| Temporal pole | 38 | R | 32 | −8 | −28 | 2.91a | ||||
| Lingual gyrus | 18 | R | 18 | −86 | 2 | 3.72 | ||||
| Fusiform gyrus | 19 | R | 26 | −74 | −8 | 3.06a | ||||
| Cuneus | 17 | R | 8 | −92 | 8 | 3.96 | ||||
| Occipital gyrus | 18 | L | −28 | −80 | 2 | 5.49 | ||||
| Middle occipital gyrus | 19 | L | −46 | −80 | 4 | 3.72 | −34 | −90 | 4 | 5.29 |
| 19 | R | 38 | −64 | −4 | 5.84 | 38 | −62 | −4 | 4.07 | |
Only clusters surviving a threshold of P < 0.05, corrected for multiple comparisons at the cluster level (k ≥ 79 voxels) and P < .01, uncorrected, for peak magnitude, are reported. BA refers to Brodmann's areas. L corresponding to the left-right, anterior–posterior and superior–inferior dimensions, respectively. t refers to the highest t-score. asurvive a small volume correction for multiple comparisons at P < 0.05, k ≥ 37 voxels.
As expected, for the Attend to Prosody vs Neutral Instructions comparison (Table 6), adults showed reliable activity in frontal and temporal networks including the inferior frontal gyrus bilaterally and the left STS. In contrast, children showed significant bilateral activity in the MTG as well as posterior extrastriate regions similar to those observed in the Attend to Face vs Neutral Instructions comparison (including the middle occipital gyrus, as well as the right inferior temporal and lingual gyri). Unlike what was observed in adults, instructions to attend to the tone of voice did not modulate activity in the inferior frontal gyrus in children, perhaps reflecting the high level of activity in this region even when instructions were neutral. Results from between-group comparisons for the Attend to Prosody vs Neutral Instructions contrast are consistent with the differences observed when comparing the Attend to Prosody vs Neutral Instructions within each group. More specifically, adults showed significantly greater activity than children in the left IFG (BA 47, Talairach coordinates: −46, 20, −4, t = 5.25, spatial extent threshold: P < 0.05, corrected), whereas children showed significantly greater activity than adults in posterior regions including the left occipital and lingual gyri (BA 18/19, Talairach coordinates: −30, −82, 0, t = 2.84, spatial extent threshold: P < 0.05, corrected).
DISCUSSION
Brain activity during irony comprehension
This is the first neuroimaging study to use irony comprehension to examine the neural circuitry underlying the interpretation of communicative intent. Based on previous neuroimaging and neuropsychological research, we expected to see greater activity in medial prefrontal and RH regions during ironic scenarios as compared with stories not involving irony. With respect to the role of the prefrontal cortex, both children and adults showed selective activity in the MPFC (extending well into ventral MPFC) in response to vignettes involving ironic remarks. This finding is consistent with studies showing that patients with lesions in the ventral MPFC have difficulty with social reasoning tasks including the detection of irony, deception and faux pas (Stone et al., 1998; Stuss et al., 2001; Shamay-Tsoory et al., 2005a, b). Most prior neuroimaging studies, however, have reported selective dorsal MPFC activity during tasks requiring mental state inferences (Frith and Frith, 2003; Saxe et al., 2004). Several researchers have proposed that the MPFC may be functionally organized such that the dorsal regions are engaged when the cognitive monitoring of mental states is required, while the ventral MPFC is associated with representing the affective meaning of stimuli (Gusnard et al., 2001; Adolphs, 2003; Mitchell et al., 2004; Ochsner et al., 2004; Hynes et al., 2006). Interestingly, the patients with ventral MPFC lesions examined by Stone et al. (1998) and Shamay-Tsoory et al. (2005b) were able to pass simple first- and second-order theory of mind tasks, yet failed to correctly identify when someone had made a faux pas or detect when a speaker was being ironic, suggesting that theory of mind may be a necessary, but not sufficient, component for appreciating mental states involving an affective component. Both Stone and colleagues (1998) and Shamay-Tsoory and colleagues (2005a, b) posit that the ventral MPFC may play a role in integrating a cognitive understanding of theory of mind with an emotional understanding of the significance of the affect displayed. In the present study, both children and adults activated ventral MPFC more strongly during Irony conditions relative to the No Irony conditions, perhaps reflecting the integration of the affect conveyed through facial expression and tone of voice with a cognitive appreciation for the intent of the speaker.
With regard to a RH contribution, directly comparing the Irony conditions with the No Irony condition yielded RH-lateralized activity in temporal regions in adults, suggesting an important role for the RH in interpreting non-literal language. This finding is consistent with prior research demonstrating that patients with RH brain damage are impaired in understanding irony relative to both patients with LH lesions and healthy controls (Tompkins and Mateer, 1985; Kaplan et al., 1990; Winner et al., 1998; Giora et al., 2000). In light of previous neuroimaging findings that the RH is engaged when coherence-seeking and integration is required, particularly at the sentence and discourse level (St George et al., 1999, Caplan and Dapretto, 2001, Kircher et al., 2001, Rodd et al., 2005), additional recruitment of RH temporal regions when the literal and intended meaning of a comment differ may reflect the need to integrate the contextual cues provided with the speaker's remark for the sake of coherence. Although children did not show the same right-lateralized activation pattern for ironic scenarios over and above the straightforward scenarios not involving irony, this likely reflects strong bilateral activity during the No Irony task rather than a failure to recruit RH temporal regions during the Irony conditions. While the remarks used in the No Irony condition did not lend themselves to non-literal interpretation (e.g. ‘Please pass the crayons’), these utterances still need to be interpreted within the context of the scenarios. Greater RH and frontal activity in children may reflect the ongoing development of discourse skills.
Developmental changes in brain activity
This study is the first investigation into developmental differences in the neural basis of inferring communicative intent. Although children and adults did not differ reliably on behavioral measures of task performance (i.e. response time and accuracy scores), patterns of brain activity did differ between the two groups in several ways. First, across all irony tasks, adults showed reliably greater activity than children in the right fusiform gyrus (FG), a region traditionally associated with processing faces and emotional expressions (Haxby et al., 2000, 2002), as well as surrounding extrastriate cortices. As previous studies have established that activity in the FG increases strongly with attention to faces and facial emotions (Wojciulik et al., 1998; Vuilleumier et al., 2001), greater activity in this region in adults likely reflects increased attentional resources directed toward the facial expression of the speaker. Greater recruitment of this region even when instructions were neutral may be a result of learning through experience that attending to facial expression can be the most efficient strategy for interpreting a speaker's intended meaning. Indeed, facial expressions may be discriminated more reliably than prosodic cues. There is evidence to suggest that basic facial expressions are universal across cultures and some species (Ekman, 1992; Izard, 1994), yet intonational cues, particularly those used to convey sarcasm, may be more variable. Although some research has shown that lower pitch level, slower tempo and greater intensity are vocal cues typical of sarcasm (Rockwell, 2000), other work suggests that there is no ‘sarcastic intonation’ per se, although pitch may serve as a contrastive marker for sarcasm (Attardo et al., 2003). The idea that experience and expertise in face processing increase with age is supported by a recent fMRI study showing that older children activated the right FG more strongly than younger children while processing faces (Aylward et al., 2005). Furthermore, Gauthier and colleagues (1999, 2000) have also shown that activation in the FG increases as a function of expertise. Our finding that activity in this region increased reliably with chronological age in children supports the interpretation that greater recruitment of the right FG in adults across all irony conditions reflects more experience with attending to faces, which, in turn, aids in inferring communicative intent.
With regard to another key region expected to play a role in understanding the communicative intent of others, children reliably activated medial prefrontal regions more strongly than did adults. Activity in the MPFC has been nearly ubiquitously reported in theory of mind studies (Frith and Frith, 2003; Saxe et al., 2004). Indeed, even when task instructions do not explicitly require understanding the intentions of others, MPFC activity has been elicited in previous language studies as long as reasoning about communicative intent is merely possible (Ferstl and von Cramon, 2002). Here, we explicitly asked participants to decide whether or not the speaker meant what he or she said, so why was so little activity observed in the MPFC in adults?
Some researchers have suggested that posterior regions commonly activated during mentalizing tasks (e.g. the STS, amygdala and FG) extract mental state information from relevant cues, but the role of the MPFC is to use that information to reason about intentions (Frith and Frith, 2003; Sabbagh et al., 2004). It is then possible that with increased experience attending to and decoding the important information conveyed by facial expressions, posterior regions might become more efficient and effective at obtaining the necessary information and basic reasoning about mental states might gradually become automatic enough to engage the MPFC less. This notion is supported by the finding that activity in the MPFC decreased as chronological age increased. Also consistent with this view, Bird et al. (2004) recently examined a patient with extensive damage to the MPFC resulting from a rare form of stroke. Despite the fact that the lesion encompassed foci of activity identified in numerous theory of mind neuroimaging studies, the patient performed normally on several mentalizing tasks including those shown to be associated with MPFC activity in previous imaging work (e.g. advanced theory of mind stories involving double bluff, mistake or white lies). As suggested by Bird et al., it is possible that the MPFC is important for normal development of theory of mind, yet not necessary for mental state reasoning in adulthood, at least as measured by the tasks utilized within a laboratory setting. Along these lines, our stimuli involved simple scenarios to ensure that children could perform the task successfully in order to rule out that any differences in brain activation patterns would be primarily attributable to task difficulty. The limited MPFC activity in adults may, therefore, reflect the ease and perhaps greater efficiency with which they performed this task (e.g. Casey et al., 2005).
Lastly, differential activity in the MPFC could also be attributed to a difference in the self-relevance of our scenarios to children vs adults. The dorsal MPFC is a region associated not only with understanding the beliefs and intentions of others, but also with self-relevant attributions, feelings and memories (Gusnard et al., 2001; Vogeley et al., 2001; Kelley et al., 2002; Macrae et al., 2004; Ochsner et al., 2004). The characters in our scenarios were children; the interactions depicted were typical everyday situations for them, and intonation and facial expression were slightly exaggerated, as is common when irony is directed at children (Dews and Winner, 1997). Stronger MPFC activity in children than in adults could then reflect greater salience of the stimuli for children and perhaps the use of a simulation strategy for interpreting communicative intent (Siegal and Varley, 2002).
The other prefrontal region more strongly activated in children than in adults across Irony tasks was the left IFG. In a recent fMRI study, Schirmer et al. (2004) asked subjects to listen to positive and negative words spoken with happy or angry prosody. They found that the left IFG responded more strongly when word valence and emotional prosody were incongruent rather than congruent. Similarly, our finding that children showed greater activity in the left IFG than adults did could indicate that children are more sensitive to the incongruity between the positive literal meaning and the negative affect within ironic comments. Morton and Trehub (2001) asked subjects to listen to utterances with conflicting propositional and paralinguistic cues (i.e. happy sentences spoken in a sad tone of voice or vice versa) and judge how the speaker was feeling. While all adults used the speaker's intonation to judge her affect, children were equally likely to use what the speaker said and how she said it as the basis for making the decision. In the present study, children also had facial expression and contextual cues available to them to help judge the speaker's intention. Although they were able to correctly ignore the positive literal content of sarcastic remarks, they may have been more aware of the incongruity.
Attention to facial and prosodic cues
Directing participants' attention to facial expression or tone of voice appeared to have a more specific effect on brain activity in adults than in children. Relative to activation elicited when instructions were neutral (‘Pay attention’), adults showed reliably greater activity in regions associated with face processing (i.e. amygdala, right FG) when instructed to attend to faces and in frontotemporal networks when instructed to attend to tone of voice (i.e. bilateral IFG and left STG). This was as per our hypotheses, given past research on the effect of attentional modulation on affectively salient stimuli in the domain of both facial (Wojciulik et al., 1998; Vuilleumier et al., 2001; Pessoa et al., 2002) and prosodic processing (Kotz et al., 2003; Mitchell et al., 2003; Hesling et al., 2005).
Based on research suggesting that children may attend to the propositional content of utterances over the accompanying paralinguistic cues (Friend and Bryant, 2000; Morton and Trehub, 2001), we wondered whether instructions to attend to the facial expression or tone of voice would induce a more adult-like pattern of brain activity in children. However, this did not appear to be the case. Instead, although children did show greater activity in some areas associated with facial emotion processing (i.e. right FG and right temporal pole) when directed to attend to facial expression, increased activity was also observed more generally and more extensively in bilateral extrastriate cortices, including the middle occipital and inferior temporal gyri. Likewise, when instructed to attend to the tone of voice, children exhibited reliably greater activity in temporal-occipital regions than when instructed only to pay attention. However, a lack of increase in frontal activity could be due to a high level of engagement of these regions when instructions were neutral, perhaps reflecting the fact that an appreciation for irony is still developing in school-age children.
General conclusions
To our knowledge, this is the first study to examine the neural correlates of interpreting communicative intent in adults and children using irony comprehension as a test case. We have shown that, despite having no trouble distinguishing irony from sincerity, children were far from adult-like in their patterns of brain activity. Perhaps most interesting is the developmental shift from a reliance on frontal regions, including the MPFC, to posterior occipitotemporal cortices, particularly the FG. This shift may be an indication of the automatization of basic reasoning about mental states. The greater overall involvement of prefrontal areas in children may subserve the integration of multiple cues to reconcile the discrepancy between the literal and intended meaning of an ironic remark. Conversely, in adults, greater engagement of regions important for processing facial emotions may reflect increased reliance on facial cues in particular. These findings are in line with the ‘progressive neural scaffolding’ model of developing functional neuroanatomy (Petersen et al., 1998; Brown et al., 2005), where frontal regions may be recruited to serve as higher-level control mechanisms to guide the activation of lower-level mechanisms in order to perform a particular task.
Since the MPFC and FG are not known to be involved in processing the prosodic aspects of speech, the observed age-related effects are unlikely to reflect changes in processing the speech cues that index irony rather than changes in interpreting communicative intent. Nor can the developmental differences we observed merely be attributed to differences in performance since children and adults were equally able to judge when a remark was ironic or sincere. Thus, our findings lend support to an interactive model of brain maturation in which significant changes in functional specialization continue to occur during development as a function of expertise within a domain (Johnson, 2001). The present study represents a first step toward delineating such developmental changes with regard to the neural circuitry underlying natural language pragmatics. A better understanding of the normative networks subserving language use within a socio-communicative context should prove critical to then qualifying neural dysfunction in developmental disorders (e.g. autism and childhood-onset schizophrenia) characterized by marked impairments in social communication.
Acknowledgments
Support for this work was provided by grants from the National Institute on Deafness and Other Communication Disorders (R03 DC005159), the National Alliance for Autism Research, the Cure Autism Now foundation, as well as the U.C. Davis M.I.N.D. Institute. For generous support, the authors also wish to thank the Brain Mapping Medical Research Organization, the Brain Mapping Support Foundation, the Pierson-Lovelace Foundation, the Ahmanson Foundation, the William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, the Tamkin Foundation, the Jennifer Jones-Simon Foundation, the Capital Group Companies the Charitable Foundation, the Robson Family, and the Northstar Fund. This project was also in part supported by Grant Numbers RR12169, RR13642 and RR00865 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCR or NIH.
REFERENCES
- Ackerman BP. Young children's understanding of a speaker's intentional use of a false utterance. Developmental Psychology. 1981;17:472–80. [Google Scholar]
- Ackerman BP. Children's sensitivity to comprehension failure in interpreting a nonliteral use of an utterance. Child Development. 1986;57:485–97. [Google Scholar]
- Adolphs R. Cognitive neuroscience of human social behaviour. Nature Review of Neuroscience. 2003;4:165–78. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Attardo S, Eisterhold J, Hay J, Poggi I. Multimodal markers of irony and sarcasm. Humor: International Journal of Humor Research. 2003;16:243–60. [Google Scholar]
- Aylward EH, Park JE, Field KM, et al. Brain activation during face perception: Evidence of a developmental change. Journal of cognitive neuroscience. 2005;17:308–19. doi: 10.1162/0898929053124884. [DOI] [PubMed] [Google Scholar]
- Baldwin DA. Interpersonal understanding fuels knowledge acquisition. Current Directions in Psychological Science. 2000;9:40–45. [Google Scholar]
- Bird CM, Castelli F, Malik O, Frith U, Husain M. The impact of extensive medial frontal lobe damage on ‘theory of mind’ and cognition. Brain. 2004;127:914–28. doi: 10.1093/brain/awh108. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional mri of language: New approaches to understanding the cortical organization of semantic processing. Annual Review of Neuroscience. 2002;25:151–88. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cerebral Cortex. 2005;15:275–90. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Brunet E, Sarfati Y, Hardy-Bayle MC, Decety J. A pet investigation of the attribution of intentions with a nonverbal task. Neuroimage. 2000;11:157–66. doi: 10.1006/nimg.1999.0525. [DOI] [PubMed] [Google Scholar]
- Capelli CA, Nakagawa N, Madden CM. How children understand sarcasm: The role of context and intonation. Child Development. 1990;61:1824–41. [Google Scholar]
- Caplan R, Dapretto M. Making sense during conversation: An fmri study. Neuroreport. 2001;12:3625–32. doi: 10.1097/00001756-200111160-00050. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: What have we learned about cognitive development? Trends in Cognition Science. 2005;9:104–10. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Castelli F, Happe F, Frith U, Frith C. Movement and mind: A functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12:314–25. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- de Groot A, Kaplan J, Rosenblatt E, Dews S, Winner E. Understanding versus discriminating nonliteral utterances: Evidence for a dissociation. Metaphor & Symbol. Special Issue: Developmental perspectives on metaphor. 1995;10:255–73. [Google Scholar]
- Demorest A, Meyer C, Phelps E, Gardner H, Winner E. Words speak louder than actions: Understanding deliberately false remarks. Child Development. 1984;55:1527–34. [Google Scholar]
- Dews S, Winner E. Attributing meaning to deliberate false utterances: The case of irony. In: Mandell C, McCabe A, editors. The problem of meaning: Behavioral and cognitive perspectives. Advances in psychology. Amsterdam, Netherlands: North-Holland/Elsevier Science Publishers; 1997. pp. 377–414. [Google Scholar]
- Dews S, Winner E, Kaplan J, et al. Children's understanding of the meaning and functions of verbal irony. Child Development. 1996;67:3071–85. [PubMed] [Google Scholar]
- Ekman P. Are there basic emotions? Psychological Review. 1992;99:550–53. doi: 10.1037/0033-295x.99.3.550. [DOI] [PubMed] [Google Scholar]
- Ferstl EC, von Cramon DY. What does the frontomedian cortex contribute to language processing: Coherence or theory of mind? Neuroimage. 2002;17:1599–612. doi: 10.1006/nimg.2002.1247. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happe F, Frith U, et al. Other minds in the brain: A functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–28. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Friend M, Bryant JB. A developmental lexical bias in the interpretation of discrepant messages. Merrill-Palmer Quarterly. 2000;46:342–69. [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fmri studies and conjunction analyses. Neuroimage. 1999;10:385–96. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical transactions of the Royal Society of London. Series B: Biological sciences. 2003;358:459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends in Cognitive Science. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happe F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: An fmri study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Skudlarski P, Gore JC, Anderson AW. Expertise for cars and birds recruits brain areas involved in face recognition. Nature Neuroscience. 2000;3:191–97. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. Activation of the middle fusiform ‘face area’ increases with expertise in recognizing novel objects. Nature Neuroscience. 1999;2:568–73. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- Giora R, Zaidel E, Soroker N, Batori G, Kasher A. Differential effect of right- and left-hemisphere damage on understanding sarcasm and metaphor. Metaphor & Symbol. Special: The uses of processing of irony and sarcasm. 2000;15:63–83. [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function; Proceedings of the National Academy of Sciences of the United States of America, 98; 2001. pp. 4259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JT, Dunham PJ, Purdy K. Children's comprehension of critical and complimentary forms of verbal irony. Journal of Cognition & Development. 2000;1:227–48. [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognition Science. 2000;4:223–33. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biological Psychiatry. 2002;51:59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- Hesling I, Clement S, Bordessoules M, Allard M. Cerebral mechanisms of prosodic integration: Evidence from connected speech. Neuroimage. 2005;24:937–47. doi: 10.1016/j.neuroimage.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Hynes CA, Baird AA, Grafton ST. Differential role of the orbital frontal lobe in emotional versus cognitive perspective-taking. Neuropsychologia. 2006;44:374–83. doi: 10.1016/j.neuropsychologia.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Izard CE. Innate and universal facial expressions: Evidence from developmental and cross-cultural research. Psychological Bulletin. 1994;115:288–99. doi: 10.1037/0033-2909.115.2.288. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Functional brain development in humans. Nature Review of Neuroscience. 2001;2:475–83. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- Kaplan JA, Brownell HH, Jacobs JR, Gardner H. The effects of right hemisphere damage on the pragmatic interpretation of conversational remarks. Brain and Language. 1990;38:315–33. doi: 10.1016/0093-934x(90)90117-y. [DOI] [PubMed] [Google Scholar]
- Keenan TR, Quigley K. Do young children use echoic information in their comprehension of sarcastic speech? A test of echoic mention theory. British Journal of Developmental Psychology. 1999;17:83–96. [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fmri study. Journal of cognitive neuroscience. 2002;14:785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Brammer M, Tous Andreu N, Williams SC, McGuire PK. Engagement of right temporal cortex during processing of linguistic context. Neuropsychologia. 2001;39:798–809. doi: 10.1016/s0028-3932(01)00014-8. [DOI] [PubMed] [Google Scholar]
- Kotz SA, Meyer M, Alter K, Besson M, von Cramon DY, Friederici AD. On the lateralization of emotional prosody: An event-related functional mr investigation. Brain and Language. 2003;86:366–76. doi: 10.1016/s0093-934x(02)00532-1. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cerebral Cortex. 2004;14:647–54. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Milosky LM, Ford JA. The role of prosody in children's inferences of ironic intent. Discourse Processes. 1997;23:47–61. [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Encoding-specific effects of social cognition on the neural correlates of subsequent memory. Journal of Neuroscience. 2004;24:4912–7. doi: 10.1523/JNEUROSCI.0481-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RLC, Elliott R, Barry M, Cruttenden A, Woodruff PWR. The neural response to emotional prosody, as revealed by functional magnetic resonance imaging. Neuropsychologia. 2003;41:1410–21. doi: 10.1016/s0028-3932(03)00017-4. [DOI] [PubMed] [Google Scholar]
- Morton JB, Trehub SE. Children's understanding of emotion in speech. Child development. 2001;72:834–43. doi: 10.1111/1467-8624.00318. [DOI] [PubMed] [Google Scholar]
- Moses LJ, Baldwin DA, Rosicky JG, Tidball G. Evidence for referential understanding in the emotions domain at twelve and eighteen months. Child development. 2001;72:718–35. doi: 10.1111/1467-8624.00311. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, et al. Reflecting upon feelings: An fmri study of neural systems supporting the attribution of emotion to self and other. Journal of cognitive neuroscience. 2004;16:1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Moriguchi Y, Matsuda H, et al. The neural network for the mirror system and mentalizing in normally developed children: An fmri study. Neuroreport. 2004;15:1483–7. doi: 10.1097/01.wnr.0000127464.17770.1f. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention; Proceedings of the National Academy of Sciences of the United States of America, 99; 2002. pp. 11458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME. The effects of practice on the functional anatomy of task performance; Proceedings of the National Academy of Sciences of the United States of America, 95; 1998. pp. 853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell P. Lower, slower, louder: Vocal cues of sarcasm. Journal of Psycholinguistic Research. 2000;29:483–95. [Google Scholar]
- Rodd JM, Davis MH, Johnsrude IS. The neural mechanisms of speech comprehension: Fmri studies of semantic ambiguity. Cerebral Cortex. 2005 doi: 10.1093/cercor/bhi009. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. Journal of cognitive neuroscience. 2004;16:988–99. doi: 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- Sabbagh MA, Moulson MC, Harkness KL. Neural correlates of mental state decoding in human adults: An event-related potential study. Journal of cognitive neuroscience. 2004;16:415–26. doi: 10.1162/089892904322926755. [DOI] [PubMed] [Google Scholar]
- Saxe R, Carey S, Kanwisher N. Understanding other minds: Linking developmental psychology and functional neuroimaging. Annual Review of Psychology. 2004;55:87–124. doi: 10.1146/annurev.psych.55.090902.142044. [DOI] [PubMed] [Google Scholar]
- Schirmer A, Zysset S, Kotz SA, Yves von Cramon D. Gender differences in the activation of inferior frontal cortex during emotional speech perception. Neuroimage. 2004;21:1114–23. doi: 10.1016/j.neuroimage.2003.10.048. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Grelotti DJ, Klin A, et al. The role of the fusiform face area in social cognition: Implications for the pathobiology of autism. Philosophical transactions of the Royal Society of London. Series B: Biological sciences. 2003;358:415–27. doi: 10.1098/rstb.2002.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay SG, Tomer R, Aharon-Peretz J. Deficit in understanding sarcasm in patients with prefronal lesion is related to impaired empathic ability. Brain Cognition. 2002;48:558–63. [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Aharon-Peretz J. The neuroanatomical basis of understanding sarcasm and its relationship to social cognition. Neuropsychology. 2005a;19:288–300. doi: 10.1037/0894-4105.19.3.288. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Goldsher D, Aharon-Peretz J. Impaired “affective theory of mind” is associated with right ventromedial prefrontal damage. Cogn Behav Neurol. 2005b;18:55–67. doi: 10.1097/01.wnn.0000152228.90129.99. [DOI] [PubMed] [Google Scholar]
- Siegal M, Varley R. Neural systems involved in “theory of mind”. Nature Review of Neuroscience. 2002;3:463–71. doi: 10.1038/nrn844. [DOI] [PubMed] [Google Scholar]
- Small SL, Nusbaum HC. On the neurobiological investigation of language understanding in context. Brain and Language. 2004;89:300–11. doi: 10.1016/S0093-934X(03)00344-4. [DOI] [PubMed] [Google Scholar]
- St George M, Kutas M, Martinez A, Sereno MI. Semantic integration in reading: Engagement of the right hemisphere during discourse processing. Brain. 1999;122:1317–25. doi: 10.1093/brain/122.7.1317. [DOI] [PubMed] [Google Scholar]
- Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. Journal of cognitive neuroscience. 1998;10:640–56. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Gallup, G.G., Alexander MP. The frontal lobes are necessary for ‘theory of mind’. Brain. 2001;124:279–86. doi: 10.1093/brain/124.2.279. [DOI] [PubMed] [Google Scholar]
- Tompkins CA, Mateer CA. Right hemisphere appreciation of prosodic and linguistic indications of implicit attitude. Brain & Language. 1985;24:185–203. doi: 10.1016/0093-934x(85)90130-0. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Bussfeld P, Newen A, et al. Mind reading: Neural mechanisms of theory of mind and self-perspective. Neuroimage. 2001;14:170–81. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and vemotion on face processing in the human brain: An event-related fmri study. Neuron. 2001;30:829–41. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Walker-Andrews AS. Infants' perception of expressive behaviors: Differentiation of multimodal information. Psychol Bull. 1997;121:437–56. doi: 10.1037/0033-2909.121.3.437. [DOI] [PubMed] [Google Scholar]
- Walter H, Adenzato M, Ciaramidaro A, Enrici I, Pia L, Bara BG. Understanding intentions in social interaction: The role of the anterior paracingulate cortex. Journal of cognitive neuroscience. 2004;16:1854–63. doi: 10.1162/0898929042947838. [DOI] [PubMed] [Google Scholar]
- Wildgruber D, Hertrich I, Riecker A, et al. Distinct frontal regions subserve evaluation of linguistic and emotional aspects of speech intonation. Cerebral Cortex. 2004;14:1384–9. doi: 10.1093/cercor/bhh099. [DOI] [PubMed] [Google Scholar]
- Wildgruber D, Riecker A, Hertrich I, et al. Identification of emotional intonation evaluated by fmri. Neuroimage. 2005;24:1233–41. doi: 10.1016/j.neuroimage.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Winner E, Leekam S. Distinguishing irony from deception: Understanding the speaker's second-order intention. Special Perspectives on the child's theory of mind: II. British Journal of Developmental Psychology. 1991;9:257–70. [Google Scholar]
- Wojciulik E, Kanwisher N, Driver J. Covert visual attention modulates face-specific activity in the human fusiform gyrus: Fmri study. Journal of Neurophysiolology. 1998;79:1574–8. doi: 10.1152/jn.1998.79.3.1574. [DOI] [PubMed] [Google Scholar]
- Wojciulik E, Kanwisher N, Driver J. Covert visual attention modulates face-specific activity in the human fusiform gyrus: Fmri study. Journal of Neurophysiolology. 1998;79:1574–8. doi: 10.1152/jn.1998.79.3.1574. [DOI] [PubMed] [Google Scholar]
- Woods RP, Dapretto M, Sicotte NL, Toga AW, Mazziotta JC. Creation and use of a talairach-compatible atlas for accurate, automated, nonlinear intersubject registration, and analysis of functional imaging data. Human Brain Mapping. 1999;8:73–9. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<73::AID-HBM1>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography. 1998a;22:139–52. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: Ii. Intersubject validation of linear and nonlinear models. Journal of Computer Assisted Tomography. 1998b;22:153–65. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Zaidel E, Kasher A, Soroker N, Batori G. Effects of right and left hemisphere damage on performance of the “right hemisphere communication battery”. Brain and Language. 2002;80:510–35. doi: 10.1006/brln.2001.2612. [DOI] [PubMed] [Google Scholar]