Abstract
Recent studies of autism have identified functional abnormalities of the default network during a passive resting state. Since the default network is also typically engaged during social, emotional and introspective processing, dysfunction of this network may underlie some of the difficulties individuals with autism exhibit in these broad domains. In the present experiment, we attempted to further delineate the nature of default network abnormality in autism using experimentally constrained social and introspective tasks. Thirteen autism and 12 control participants were scanned while making true/false judgments for various statements about themselves (SELF condition) or a close other person (OTHER), and pertaining to either psychological personality traits (INTERNAL) or observable characteristics and behaviors (EXTERNAL). In the ventral medial prefrontal cortex/ventral anterior cingulate cortex, activity was reduced in the autism group across all judgment conditions and also during a resting condition, suggestive of task-independent dysfunction of this region. In other default network regions, overall levels of activity were not different between groups. Furthermore, in several of these regions, we found group by condition interactions only for INTERNAL/EXTERNAL judgments, and not SELF/OTHER judgments, suggestive of task-specific dysfunction. Overall, these results provide a more detailed view of default network functionality and abnormality in autism.
Keywords: autism spectrum disorders, retrosplenial cortex, posterior cingulate cortex, default mode, rest
INTRODUCTION
Several recent studies of autism have identified functional abnormalities of the default network (Kennedy et al., 2006; Cherkassky et al., 2006; Kennedy and Courchesne, 2008). This network, comprised of the medial prefrontal cortex (MPFC)/ventral anterior cingulate cortex (vACC), retrosplenial cortex/posterior cingulate cortex (RSC/PCC) and angular gyrus (ANG), among other regions, is so named because it exhibits high levels of metabolic activity at rest, in the absence of an externally imposed cognitively demanding task (Raichle et al., 2001). In other words, the brain defaults to this pattern of activity when allowed to rest. Interestingly, similarly high activity of this network is also seen when typical subjects engage in tasks of a social, emotional or introspective nature (Fletcher et al., 1995; Maddock, 1999; Gusnard et al., 2001; Maddock et al., 2001; Iacoboni et al., 2004; Ochsner et al., 2004, 2005; D’Argembeau et al., 2005; Cavanna and Trimble, 2006; Northoff et al., 2006)—the very tasks which are most difficult for individuals with autism (Kanner, 1943; Hurlburt et al., 1994).
Along with the few studies that have explicitly examined resting functionality or resting functional connectivity of the default network in autism (Kennedy et al., 2006; Cherkassky et al., 2006; Kennedy and Courchesne, 2008), other studies have also found abnormalities in regions of the default network (and, in particular, the MPFC) during a variety of socioemotional tasks. For instance, such abnormalities have been noted during viewing of personally familiar faces (Pierce et al., 2004), reading of negatively valenced emotional words (Kennedy et al., 2006) and in a mentalizing task, where subjects observed geometric objects moving in particular ways to imply intentionality (Castelli et al., 2002).
Importantly, there are at least two different explanations for the pervasiveness of functional abnormality in default network regions across both socioemotional tasks and no-task resting conditions. First, perhaps regions of this network are simply unable to function properly in individuals with autism, regardless of the task being performed—in other words, a task-independent dysfunction. Alternatively, however, such abnormalities may simply reflect the known impairments of individuals with autism to automatically engage in socioemotional and introspective processes, in the absence of explicit instructions (Klin et al., 2003). In fact, for the above described studies, attending to and processing the social, emotional or mentalizing aspects of the stimuli were not explicit requirements of the task. For instance, in Pierce et al. (2004), subjects were required simply to identify female faces, regardless of whether they were familiar or not. In Kennedy et al. (2006), subjects were asked only to count the number of emotional or neutral words displayed on the screen, rather than explicitly process the meaning of the words. Lastly, in Castelli et al. (2002), subjects were asked to describe what they observed, and were free to interpret the meaning of the movements as either reflecting intentionality or not (and, in fact, the autism group provided significantly lower intentionality ratings than the control group). Thus, it is possible that given explicit instructions and explicit performance requirements regarding the social, emotional or mentalizing aspects of such tasks, regions of the default network may exhibit more typical patterns of activity in autism.
In the current experiment, we used explicitly defined social and introspective tasks to determine whether abnormality of default network regions reflects task-specific or task-independent dysfunction. To do so, we used a self- and other-reflection task, which has been shown previously to robustly activate regions of the default network, including the MPFC, RSC/PCC and ANG (Fletcher et al., 1995; Gusnard et al., 2001; Johnson et al., 2002; Kelley et al., 2002; Gallagher and Frith, 2003; Ochsner et al., 2005). While being scanned, 13 autism and 12 control subjects read particular statements about themselves or about a close other person (i.e. their mother), and made judgments as to whether the statements were true or false. Thus, the subjects’ task (i.e. making true/false judgments about themselves or others) was directly relevant to the experimental conditions of interest (i.e. reflection on oneself and others), reducing the likelihood of non-engagement in the mental processes of interest. We also included two different types of self- and other-reflection conditions—(i) those regarding psychological personality traits (which we term INTERNAL) and (ii) those regarding observable external characteristics and behaviors (which we term EXTERNAL)—which allowed us to examine whether there may be a selective impairment in one or the other type of judgment. All person judgment conditions were compared to a cognitively demanding MATH condition, which served as an experimental baseline task. Finally, we included a resting fixation condition (REST) to compare resting default network activity between groups, to examine the overlap between regions of the default network and brain regions involved in self- and other-reflection, and to functionally define the default network for use in region-of-interest analyses.
METHODS
Participants
Fourteen male autism spectrum disorder (ASD) and 13 male control subjects were scanned. Due to excessive movement during scanning, one ASD subject and one control subject were removed from the analysis, resulting in a final sample size of 13 ASD (six autism, six Asperger's, one PDD-NOS) and 12 control subjects. With the exception of the one subject (A10, Table 1), this sample of control and ASD subjects completely overlapped with those from a separate imaging study that examined resting functional connectivity in autism (Kennedy and Courchesne, 2008). Informed written consent was obtained from all participants or, when appropriate, their legal guardians, and all participants received monetary compensation for their time. The protocol was approved by the Institutional Review Board of UCSD and Children's Hospital at San Diego. ASD participants were diagnosed by a clinical psychologist using the Autism Diagnostic Interview—Revised (ADI-R) (Lord et al., 1994) and the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2000). Individuals meeting the criteria for an ASD diagnosis but without early spoken language delay and with average to above-average IQ scores received the diagnosis of Asperger's Syndrome. The PDD-NOS subject did not meet the combined social and communication cutoff score of 10 to warrant a diagnosis of autism on the ADOS, nor did he meet the above criteria for Asperger's Syndrome. With the exception of one control subject, IQ scores were obtained from all participants using the Wechsler Adult Intelligence Scale (WAIS) or WAIS-R (Revised). The mean age of the autism participants (26.9 years) and the control participants (27.5 years) was not significantly different [t(23) = 0.129, P > 0.85]. Subject groups did not differ significantly in verbal, performance or full-scale IQ [verbal: t(22) = 1.641, P = 0.115; performance: t(22) = 1.512, P = 0.145; full-scale: t(22) = 1.959, P = 0.063]. See Table 1 for detailed clinical information.
Table 1.
Clinical information for autism and control participants
| IQ | ADI-R | ADOS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Diagnosis | Age | Sex | handedness | Verbal | Performance | Full-scale | Social (cutoff = 10) | Communication (cutoff = 8) | Stereotypy (cutoff = 3) | Social (cutoff = 4) | Communication (cutoff = 2) | Stereotypy |
| A1 | Autism | 15.7 | M | Right | 73 | 66 | 67 | 10 | 21 | 11 | 10 | 3 | 3 |
| A2 | Asperger's | 16.2 | M | Right | 120 | 124 | 125 | 13 | 17 | 3 | 11 | 6 | 1 |
| A3 | Asperger's | 17.4 | M | Right | 99 | 93 | 96 | 23 | 18 | 9 | 9 | 5 | 1 |
| A4 | Autism | 17.7 | M | Right | 101 | 118 | 109 | 26 | 19 | 6 | 7 | 5 | 1 |
| A5 | Asperger's | 18.3 | M | Right | 108 | 107 | 109 | 14 | 8 | 6 | 5 | 3 | 1 |
| A6 | Autism | 18.8 | M | Right | 55 | 109 | 80 | 28 | 20 | 4 | 9 | 5 | 0 |
| A7 | Asperger's | 22.9 | M | Right | 97 | 105 | 101 | 13 | 12 | 3 | 6 | 3 | 0 |
| A8 | Asperger's | 24.0 | M | Right | 116 | 109 | 114 | 7 | 11 | 10 | 8 | 2 | 2 |
| A9 | Asperger's | 27.7 | M | Right | 111 | 99 | 106 | 21 | 20 | 7 | 11 | 6 | 0 |
| A10 | PDD-NOS | 31.4 | M | Right | 90 | 126 | 107 | 14 | 14 | 3 | 6 | 3 | 2 |
| A11 | Autism | 41.3 | M | Left | 98 | 114 | 104 | 21 | 22 | 10 | 11 | 5 | 2 |
| A12 | Autism | 46.4 | M | Right | 86 | 115 | 100 | 22 | 19 | 6 | 7 | 5 | 1 |
| A13 | Autism | 52.0 | M | Right | 102 | 105 | 104 | 26 | 17 | 6 | 9 | 4 | 1 |
| Mean (s.d.) | 26.9 (12.3) | 96.6 (17.7) | 106.9 (15.4) | 101.7 (14.6) | |||||||||
| C1 | Control | 15.9 | M | Left | 95 | 99 | 97 | ||||||
| C2 | Control | 16.2 | M | Right | N/A | N/A | N/A | ||||||
| C3 | Control | 17.8 | M | Right | 107 | 119 | 114 | ||||||
| C4 | Control | 19.0 | M | Right | 106 | 118 | 113 | ||||||
| C5 | Control | 20.6 | M | Left | 99 | 106 | 103 | ||||||
| C6 | Control | 22.9 | M | Right | 107 | 93 | 100 | ||||||
| C7 | Control | 25.3 | M | Right | 109 | 116 | 114 | ||||||
| C8 | Control | 29.4 | M | Right | 109 | 125 | 118 | ||||||
| C9 | Control | 32.3 | M | Right | 108 | 128 | 119 | ||||||
| C10 | Control | 40.7 | M | Right | 108 | 132 | 121 | ||||||
| C11 | Control | 44.6 | M | Right | 106 | 109 | 108 | ||||||
| C12 | Control | 45.4 | M | Right | 108 | 128 | 119 | ||||||
| Mean (s.d.) | 27.5 (10.9) | 105.6 (4.5) | 115.7 (12.7) | 111.5 (8.3) | |||||||||
Cutoff scores for an ASD diagnosis are shown.
Stimuli
While in the scanner, subjects made true/false judgments for various statements about themselves (SELF condition) or a close other person (OTHER condition). These SELF and OTHER statements either referred to psychological personality traits (INTERNAL condition) or to observable external characteristics and behaviors (EXTERNAL condition). In all cases, the close other was their mother, with the exception of one control subject who read statements about a close friend rather than his mother, as his parents were deceased. Thus, there were four person judgment (i.e. MENTAL) conditions in total: INTERNAL-SELF (e.g. ‘I am polite’), INTERNAL-OTHER (e.g. ‘My mother is generous’), EXTERNAL-SELF (e.g. ‘I drink coffee’) and EXTERNAL-OTHER (e.g. ‘My mother drives a car’) (see Appendix A for a complete list of statements). A MATH condition served as an experimental baseline condition, wherein subjects were shown math equations [in the form of a two-digit number plus a one-digit number equaling either a correct or incorrect answer (e.g. ‘45 + 8 = 53’)], and again instructed to respond via button presses as to whether the equation was true or false. Finally, there was a REST condition where subjects passively viewed a fixation cross that appeared on the screen. The functional scans also included an episodic memory judgment condition, but this condition was not examined in the current analysis.
Each trial consisted of a statement, equation or fixation cross shown for 2500 ms, followed by a blank screen for 500 m. Conditions were presented in a counterbalanced block design manner, with six trials per block, eight blocks per condition, and each block lasting 18 s. The specific statements or equations that appeared within each block were randomized for each subject. The total time of the experiment was 17 min, 28 s, which was divided into two shorter functional runs lasting 8 min, 44 s each.
Behavioral data acquisition and analysis
Stimuli were presented using the Presentation software package (Neurobehavioral Systems, Albany, CA, USA). Subject response (true/false) and reaction time (from stimulus onset until subject response) were recorded during scanning. Responses that occurred any time within the 3000 ms trial were recorded.
After scanning was complete (∼1 h later), subjects were asked to again provide true/false judgments for each statement. This procedure allowed us to calculate the reliability of each participant's responses, ensuring they made deliberate choices, rather than simply guessing while in the scanner. Due to a computer problem, this second set of true/false responses was not recorded from one ASD subject and one control subject.
All behavioral analyses were conducted with SPSS 12.0 statistical software package (SPSS, Chicago, IL, USA). To compare performance (RT and percent concordance) between groups across the four MENTAL judgments, we ran two separate three-way repeated measures ANOVAs (SELF/OTHER × INTERNAL/EXTERNAL × group). Follow-up t-tests were run for all significant main effects of group and group by condition interactions. For the MATH condition, group differences in RT and accuracy were examined with independent sample t-tests.
Functional imaging data acquisition and analysis
Functional and anatomical images were acquired using a 3 Tesla GE Signa EXCITE scanner. Whole brain axial slices were collected with a gradient-recalled echo-planar imaging pulse sequence with the following parameters: TR (repetition time) = 2000 ms; TE (echo time) = 30 ms; flip angle = 90°; field of view (FOV) = 220 mm; matrix = 64 × 64 (3.44 mm2 in-plane resolution); slice thickness = 4 mm; no. of axial slices = 32; no. of volumes = 262 (for each of the two runs). T1-weighted anatomical images were collected for co-registration with the functional images [FOV = 256 mm; matrix = 256 × 256 (1 mm2 in-plane resolution); slice thickness = 1 mm; no. of axial slices = 124].
Functional analyses were carried out using the Analyses of Functional NeuroImages (AFNI) statistical software package (version 2.56; http://afni.nimh.nih.gov/afni) (Cox, 1996). First, field maps, which were acquired during the scan sessions, were used to correct for field inhomogeneities. Next, the first 10 TRs (which consisted of 20 s of fixation) were removed from the beginning of each functional run. Motion correction and 3D registration of each participant's functional images were performed with AFNI's automated alignment program (3dVolReg), which co-registers each individual functional volume with a manually specified middle reference volume. Brief periods of subject movement, which were objectively defined from the output of this volume registration procedure, were removed from the analysis (for details, see Kennedy and Courchesne, 2008). Subjects with >20% of the entire run removed were excluded entirely from the study (one ASD, one control subject). There was no difference in the percent of the scans removed from the remaining participants [control = 2.46%; autism = 1.93%; t(23) = 0.492, P = 0.628]. Images were corrected for timing of slice acquisition, spatially smoothed with a Gaussian filter (full-width half-maximum = 6 mm), and linear trend was removed from the time series. Next, the data were converted to percent signal change values and the two separate functional runs were concatenated, producing a single time series.
Functional data were analyzed using AFNI's 3dDeconvolve. First, an impulse response function (IRF) was estimated based on the measured fMRI signal for each voxel and the input stimulus functions. These input functions included six experimental conditions (only five of which were examined in the present article—INTERNAL-SELF, INTERNAL-OTHER, EXTERNAL-SELF, EXTERNAL-OTHER and REST) and six motion parameters [i.e. rotational movement (roll, pitch, yaw) and translational movement (x, y, z)]. The MATH condition served as the baseline state. The estimated IRF was then convolved with the input stimulus time series, and multiple regressions were run to determine a goodness-of-fit coefficient (i.e. linear contrast weight) for 0, 2, 4 and 6 s after stimulus presentation. These four linear contrast weights were summed for each condition separately, yielding a single linear contrast weight for each of the five conditions at each voxel. Next, several a priori contrasts were carried out [SELF vs MATH, OTHER vs MATH, INTERNAL vs MATH, EXTERNAL vs MATH, SELF vs OTHER, INTERNAL vs EXTERNAL, MENTAL (all four person judgment conditions) vs MATH, and REST vs MATH] at every voxel.
For whole-brain analyses, images were spatially normalized to Talairach space (Talairach and Tournoux, 1988) using AFNI's 12 sub-volume piecewise linear transformation based on manually-defined landmarks. The t-tests were run for each group separately to determine, for each of the above contrasts, which voxels were significantly different from zero. Unless indicated otherwise, whole-brain functional maps are shown at a voxel threshold of P < 0.001, and a corresponding minimum cluster volume of 384 mm3. Minimum cluster volumes were calculated using an iterative Monte Carlo simulation using AFNI's AlphaSim program with a voxel-wise threshold of P < 0.05. Throughout the text, we use the term ‘volume-corrected’ to refer to analyses that were corrected for minimum cluster volume.
Finally, between-group comparisons were carried out using a functional region-of-interest (ROI) approach. The first three ROIs were defined as regions in the control group that were active in the MENTAL (all four conditions) vs MATH contrast (P < 0.001) and that also overlapped with regions with greater activity in the REST vs MATH contrast (P < 0.01). These regions of overlap were then volume-corrected at the more stringent volume threshold (minimum cluster volume = 1152 mm3) corresponding to the P < 0.01 threshold used in the REST vs MATH contrast. This created ROIs that were both part of a functionally defined default network and also involved in self- and other-reflection. A fourth region, the dorsal MPFC (dMPFC), was included as an ROI post hoc (see Results section).
For each ROI, percent signal change values were extracted from each subject, averaged across all voxels in the ROI, and analyzed using SPSS. Three-way repeated measures ANOVAs (SELF/OTHER × INTERNAL/EXTERNAL × group) were run for each ROI. Additionally, independent sample t-tests were run for the REST vs MATH contrast for each ROI. Follow-up t-tests were run for all significant main effects of group and group by condition interactions.
RESULTS
Behavioral results
In terms of reliability (percent concordance of a subject's responses) across all four MENTAL judgment conditions, there was no main effect of group [F(1,21) = 0.289, P = 0.596]. Though the three-way interaction was not significant [F(1,23) = 0.807, P > 0.80], there were significant group by condition interactions in reliability for both INTERNAL vs EXTERNAL judgments [F(1,21) = 9.821, P = 0.005] and SELF vs OTHER judgments [F(1,21) = 8.584, P = 0.005]. Follow-up t-tests revealed that the control group had greater reliability of responses for SELF compared to OTHER judgments [89.4% vs 84.1%, t(10) = 2.384, P = 0.0384] and EXTERNAL compared to INTERNAL judgments [88.8% vs 84.6%, t(10) = 2.701, P = 0.0223], while reliability between these conditions was not significantly different in the autism group (84.4% vs 86.1% and 83.4% vs 87.1%, respectively; both P-values >0.05).
In terms of reaction time across all four MENTAL judgment conditions, there was no main effect of group [F(1,23) = 3.211, P = 0.086]. There were also no significant group by condition interactions for either SELF vs OTHER or INTERNAL vs EXTERNAL judgments [F(1,23) = 0.275, P = 0.605; F(1,23) = 1.902, P = 0.181], nor a significant three-way interaction [F(1,21) = 0.908, P > 0.90]. Both groups responded faster to SELF compared to OTHER judgments [control: 1341.9 ms vs 1513.4 ms, t(11) = 8.519, P < 0.00001; autism: 1547.3 ms vs 1703.6 ms, t(12) = 11.223, P < 0.00001], and were faster to INTERNAL compared to EXTERNAL judgments [control: 1401.3 ms vs 1487.8 ms, t(11) = 4.588, P = 0.0008; autism: 1543.3 ms vs 1673.8 ms, t(12) = 5.183, P = 0.0002]. Although there was neither a main effect of group nor a group by condition interaction, the control group did respond significantly faster than the autism group for EXTERNAL judgments [t(23) = 2.140, P = 0.043, all other conditions, P > 0.05].
Finally, for the MATH baseline condition, there was no group difference in accuracy [control: 93.1%, autism: 91.8%, t(23) = 0.536, P = 0.60] or reaction time [control: 1573.7 ms, autism: 1703.5 ms, t(23) = 1.639, P = 0.12].
Functional imaging results – whole-brain analyses
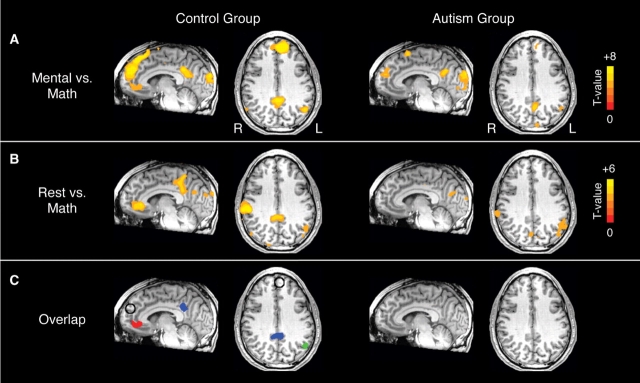
In the MENTAL vs MATH contrast, the control group had significantly greater activity in the MENTAL conditions in the dMPFC, vMPFC/vACC, RSC/PCC and ANG bilaterally, among other regions (for a complete list of regions, see Table 2). In the autism group, among these regions, activation was limited to the dMPFC, RSC/PCC and left ANG (Figure 1A, Table 2). In a voxelwise direct group comparison at a relatively conservative threshold (P < 0.001, volume-corrected), there were no significant differences between group in any of these regions. However, at a more liberal threshold (P < 0.01, uncorrected), there was significantly reduced activity in the vMPFC/vACC in the autism group.
Table 2.
Regions significantly active in the MENTAL vs MATH contrast (P <0.001, volume-corrected)
| Control | Autism | |||
|---|---|---|---|---|
| Region | (X, Y, Z) | t-value | (X, Y, Z) | t-value |
| L superior frontal gyrus | (−6, 39, 48) | 12.24 | (−6, 7, 60) | 6.85 |
| Dorsal medial prefrontal cortex | (−2, 51, 28) | 11.94 | (−6, 51, 24) | 5.82 |
| Posterior cingulate/ retrosplenial cortexa | (2, −49, 28) | 8.80 | (−6, −61, 32) | 7.51 |
| L inferior frontal gyrus | (−42, 19, 16) | 8.53 | (−38, 19, 8) | 5.99 |
| Cuneus (bilateral) | (−3, −97, 24) | 8.31 | (−6, −97, 24) | 7.62 |
| L middle frontal gyrus | (−42, 3, 48) | 8.25 | (−42, 3, 44) | 5.06 |
| L angular gyrusa | (−50, −65, 36) | 8.06 | (−58, −61, 24) | 6.67 |
| vMPFC/vACCa | (−2, 31, 0) | 7.07 | – | – |
| L temporal pole | (−42, 3, −28) | 6.97 | – | – |
| R angular gyrus | (58, −65, 28) | 6.26 | – | – |
| L superior temporal gyrus | (−50, −33, 4) | 4.80 | (−50, −21, −4) | 5.65 |
aBrain regions that also had significant activity in the REST vs MATH contrast in control subjects. Talairach coordinates and t-values correspond to the most significant voxel within each cluster. Only regions with greater activity during MENTAL judgments relative to MATH are included. At the whole-brain level, there were no significant differences between groups in any of the regions listed above (P <0.001, volume-corrected).
Fig. 1.
Functional activity in control and autism groups for (A) the MENTAL vs MATH contrast (P < 0.001, uncorrected) and (B) the REST vs MATH contrast (P < 0.01, uncorrected); and (C) the regions of overlap between these two contrasts (minimum cluster volume = 1152 mm3). These regions of overlap in the control group were used as ROIs for further analysis (red cluster = vMPFC/vACC; blue cluster = RSC/PCC; green cluster = left ANG). The dMPFC ROI (open circle) is also shown. In the autism group, the left ANG just missed the minimum cluster volume threshold (1139 mm3).
Similarly, in the REST vs MATH contrast, the control group had significant greater activity in the REST condition in the vMPFC/vACC, RSC/PCC and left ANG, among other regions (for a complete list of regions, see Table 3). In the autism group, among these regions, significantly greater activity was only seen in the left ANG (Figure 1B, Table 3). Again, activity in these regions was not significant between groups (P < 0.01, corrected), though at a more liberal threshold (P < 0.05, uncorrected), there was significantly reduced activity in the vMPFC/vACC in the autism group.
Table 3.
Regions significantly active in the REST vs MATH contrast (P < 0.01, volume-corrected)
| Control | Autism | |||
|---|---|---|---|---|
| Region | (X, Y, Z) | t-value | (X, Y, Z) | t-value |
| R inferior parietal lobule | (55, −32, 20) | 7.92 | (46, −32, 20) | 6.31 |
| vMPFC/vACCa | (7, 39, −1) | 7.26 | – | – |
| R superior parietal lobule | (26, −44, 59) | 6.84 | – | – |
| R lateral precentral gyrus | (51, −8, 12) | 5.82 | (54, −9, 15) | 5.97 |
| Posterior cingulate/retrosplenial cortex, extending into precuneusa | (6, −45, 35) | 5.30 | – | – |
| Mid-cingulate | (10, −28, 44) | 5.05 | (10, −25, 40) | 5.16 |
| L fusiform gyrus | – | – | (−17, −37, −9) | 4.88 |
| L angular gyrusa | (−53, −61, 24) | 4.66 | (−53, −60, 20) | 5.00 |
| L insula | (−37, −12, 0) | 4.47 | (−41, −8, −5) | 5.98 |
| Cuneus (bilateral) | (−2, −89, 20) | 4.30 | (2, −73, 27) | 5.09 |
aBrain regions that also had significant activity in the MENTAL vs. MATH contrast in control subjects. Talairach coordinates and t-values correspond to the most significant voxel within each cluster. Only regions with greater activity during REST relative to MATH are included. At the whole-brain level, there were no significant differences between groups in any of the regions listed above (P < 0.01, volume-corrected).
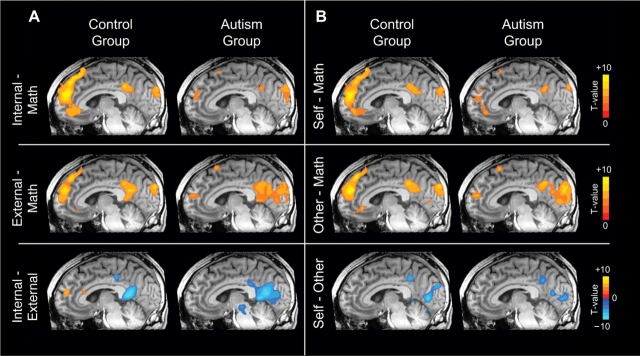
With the exception of the vMPFC/vACC, both the groups recruited largely similar regions during SELF, OTHER, INTERNAL and EXTERNAL judgments (relative to the MATH baseline condition) (Figure 2). Interestingly, both groups engaged specific regions of RSC/PCC to a greater extent for EXTERNAL (vs INTERNAL) judgments and OTHER (vs SELF) judgments (Figure 2, bottom panel; Tables 4 and 5), perhaps reflecting differences in the degree of mental imagery associated with these different types of judgments (Fletcher et al., 1995; Cavanna and Trimble, 2006).
Table 5.
Regions significantly active in the SELF vs OTHER contrast (P <0.001, volume-corrected)
| Control | Autism | Interaction | ||||
|---|---|---|---|---|---|---|
| Region | (X, Y, Z) | t-value | (X, Y, Z) | t-value | (X, Y, Z) | t-value |
| Self > Other | ||||||
| NONE | – | – | – | – | – | – |
| Other > Self | ||||||
| L lingual gyrus | (−6, −73, 0) | 10.27 | (−25, −76, −4) | 6.41 | – | – |
| R lingual gyrus | (22, −68, 4) | 9.16 | (11, −80, 0) | 7.78 | – | – |
| Posterior cingulate/retrosplenial cortex | (7, −57, 27) | 8.22 | (3, −60, 32) | 9.09 | – | – |
| L posterior parahippocampal gyrus | (−18, −45, 4) | 6.22 | – | – | – | – |
| R posterior parahippocampal gyrus | (22, −45, 7) | 5.81 | – | – | – | – |
| R caudate (tail) | (18, −25, 17) | 5.42 | (14, −25, 20) | 4.65 | – | – |
Talairach coordinates and t-values correspond to the most significant voxel within each cluster. In the regions with significant effects of condition in either the autism or control group, there were no significant group by condition interactions at the whole-brain level (P <0.001, volume-corrected).
Fig. 2.
Functional activity in control and autism groups for (A) INTERNAL vs MATH, EXTERNAL vs MATH, and INTERNAL vs EXTERNAL contrasts; and (B) SELF vs MATH, OTHER vs MATH, and SELF vs OTHER contrasts (all P < 0.001, volume-corrected). The same mid-sagittal slice location is shown for each image. Regions with greater activity in the MATH condition relative to the other conditions are not shown. In the bottom panel, red/yellow represents regions with greater activity in the INTERNAL (A) or SELF (B) conditions, while blue represents regions with greater activity in the EXTERNAL (A) or OTHER (B) conditions.
Table 4.
Regions significantly active in the INTERNAL vs EXTERNAL contrast (P <0.001, volume-corrected)
| Control | Autism | Interaction | ||||
|---|---|---|---|---|---|---|
| Region | (X, Y, Z) | t-value | (X, Y, Z) | t-value | (X, Y, Z) | t-value |
| Internal > External | ||||||
| L inferior frontal gyrus | (−38, 28, 0) | 7.97 | – | – | – | – |
| Mid-cingulate | (2, −16, 32) | 7.54 | – | – | – | – |
| Dorsal MPFC | (−1, 51, 24) | 7.00 | – | – | * | * |
| Dorsal anterior cingulate | (−2, 20, 20) | 5.59 | – | – | – | – |
| External > Internal | ||||||
| Retrosplenial cortex | (−5, −48, 8) | 11.31 | (11, −49, 4) | 11.66 | – | – |
| L superior frontal gyrus | (−21, 20, 48) | 8.47 | (−21, 23, 47) | 9.18 | – | – |
| Posterior cingulate | (3, −33, 39) | 8.36 | (−1, −40, 27) | 5.43 | – | – |
| L parahippocampal gyrus | (−18, −33, −12) | 7.96 | (−25, −25, −9) | 9.18 | – | – |
| L middle temporal gyrus | (−38, −73, 29) | 6.25 | (−30, −73, 20) | 10.12 | (−38, −77, 23) | 4.53 |
| R superior frontal gyrus | (38, 20, 52) | 5.85 | (23, 23, 47) | 8.01 | (18, 16, 52) | 5.28 |
| R middle temporal gyrus | (47, −68, 24) | 5.40 | (42, −76, 35) | 5.94 | – | – |
| Midbrain | – | – | (−1, −20, −9) | 5.70 | (−1, −21, −9) | 5.43 |
| R thalamus | – | – | (23, −24, 16) | 5.39 | (22, −13, 8) | 4.58 |
| R cuneus | – | – | (14, −77, 11) | 9.26 | – | – |
| R parahippocampal gyrus | – | – | (26, −29, −5) | 8.13 | – | – |
| L cuneus | – | – | (−5, −77, 7) | 6.28 | – | – |
*A dMPFC cluster [Talairach location = (2, 40, 12); t-value = 5.12; volume = 300 mm3] just missed the cluster volume threshold of 384 mm3. Talairach coordinates and t-values correspond to the most significant voxel within each cluster. Group by condition interactions (P <0.001, volume-corrected) are listed for all regions that exhibited significant effects of condition in either the autism or control group. For all regions with significant interactions, the autism group had a greater difference in activity between EXTERNAL and INTERNAL conditions than the control group.
Functional imaging results – ROI analyses
In total, four regions were included in the ROI analysis (Figure 1C). Three of these ROIs, the ventral MPFC (vMPFC)/vACC, RSC/PCC and lANG, were defined by functional overlap between MENTAL vs MATH and REST vs MATH contrasts in control subjects. A fourth ROI, the dorsal MPFC (dMPFC), was included as a ROI post hoc. This region demonstrated significant activity in the MENTAL vs MATH contrast in both groups (P < 0.001, volume-corrected), but not in the REST vs MATH contrast. However, given that this region is typically found to be part of the default network, we refer to all ROIs (including the dMPFC) as default network regions. The dMPFC ROI was created by placing a sphere with 8 mm radius at the point of peak significance in the MENTAL vs MATH contrast for the control group. This sphere also encompassed the point of peak significance for the autism group. Percent signal change values from these four regions were extracted from each individual and analyzed using SPSS. One ASD subject was a large outlier in their vMPFC/vACC activity (>3 SD from the mean of both the autism and control groups) and was therefore excluded from all analyses involving this region.
Main effects of group
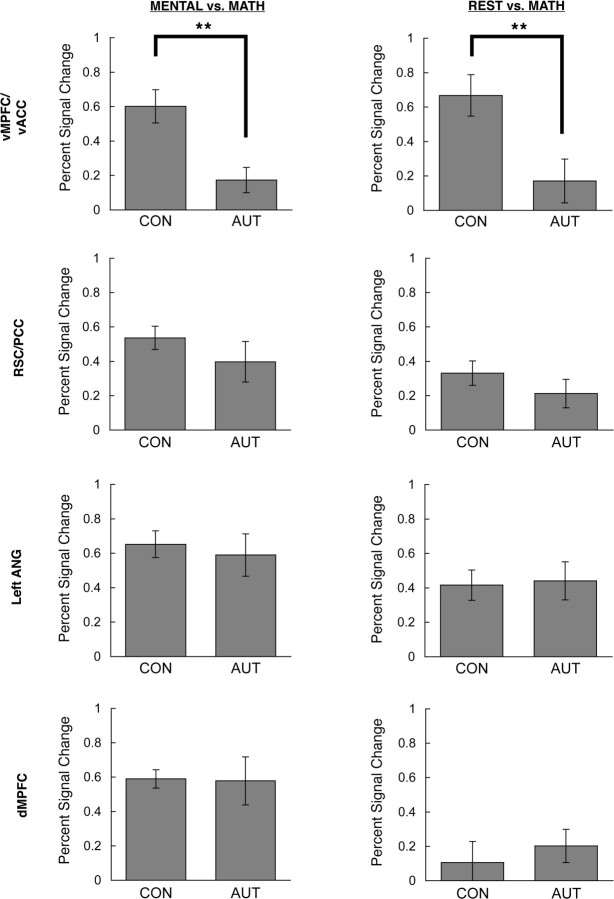
Of the four ROIs, only the vMPFC/vACC demonstrated a main effect of group for the MENTAL (all four person judgment conditions) vs MATH contrast [F(1,22) = 12.48, P = 0.002; all other regions: P > 0.30]. In this region, activity was significantly lower in the autism group compared to the control group (percent signal change of 0.17% vs 0.60%, respectively) (Figure 3, left panel). Follow-up t-tests revealed that this reduction of vMPFC/vACC activity was significant in each of the types of person judgment conditions [INTERNAL: t(22) = 4.50, P = 0.0002; EXTERNAL: t(22) = 2.38, P = 0.026; SELF: t(22) = 3.21, P = 0.004; OTHER: t(22) = 3.47, P = 0.002].
Fig. 3.
Bar graphs depicting percent signal change in control and autism groups in MENTAL vs MATH and REST vs MATH contrasts, shown separately for each ROI. **P ≤ 0.01.
In the REST vs MATH contrast, a significant group difference was also found only in the vMPFC/vACC [t(23) = 2.838, P = 0.01; all other regions, P > 0.25], with a smaller difference in activity between these conditions in the autism group relative to the control group (0.17% vs 0.67%, respectively) (Figure 3, right panel), replicating previous findings of abnormal resting activity in autism (Kennedy et al., 2006).
Finally, we should emphasize that the above analyses utilized a MATH baseline condition, rather than REST, to avoid the problem of group differences in resting activity affecting the interpretation of the functional results. However, to facilitate comparison between this and other studies of control subjects that utilize a REST baseline, we ran the MENTAL vs REST contrast for the control group alone. The control group had significantly greater activity during MENTAL judgments vs REST in the dMPFC [0.59% vs 0.11%; t(11) = 5.375, P < 0.001], the RSC/PCC [0.54% vs 0.33%; t(11) = 3.497, P = 0.005] and the lANG [0.65% vs 0.42%; t(11) = 3.217, P = 0.008], but no significant difference in the vMPFC/vACC [0.60% vs 0.67%; t(11) = 0.716, P = 0.489].
Group by condition interactions
Because there were no significant interactions between condition type (INTERNAL/EXTERNAL × SELF/OTHER) and no significant three-way interactions (INTERNAL/EXTERNAL × SELF/OTHER × group) in any of the four regions of interest (all P > 0.10), the results from INTERNAL/EXTERNAL analyses and the results from the SELF/OTHER analyses are described separately (Figure 3).
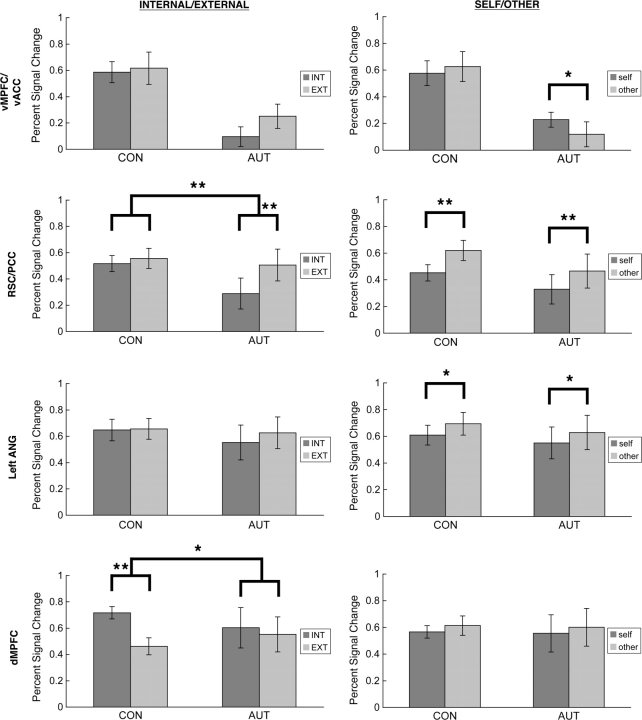
INTERNAL/EXTERNAL judgments
In the dMPFC, although there was no main effect of group, there was a significant group by condition (INTERNAL/EXTERNAL) interaction [F(1,23) = 7.17, P = 0.013]. Follow-up t-tests revealed that the control group had greater activity during INTERNAL compared to EXTERNAL judgments [0.72% vs 0.46%, respectively; F(1,11) = 51.90, P = 0.00002], while there was no difference between these conditions in the autism group [0.60% vs 0.55%; F(1,12) = 0.568, P = 0.47].
There was also a significant group by condition interaction in the RSC/PCC [F(1,23) = 12.88, P = 0.002]. While there was no difference in the level of activity between INTERNAL and EXTERNAL conditions in the control group [F(1,11) = 1.72, P = 0.22], there was a significant difference in the autism group [F(1,12) = 32.27, P = 0.0001], with greater activity for EXTERNAL compared to INTERNAL judgments. This difference between the groups was driven largely by reduced activity in the autism group during INTERNAL judgments (autism = 0.29%, control = 0.52%) rather than differences in activity during EXTERNAL judgments (autism = 0.51%, control = 0.56%).
There were no group by condition interactions for the vMPFC/vACC or lANG (both P > 0.25). Both groups showed the same pattern of activity for INTERNAL and EXTERNAL judgments—namely, no difference in activity between these conditions in either the vMPFC/vACC [autism: F(1,11) = 3.628, P = 0.083; control: F(1,11) = 0.169, P = 0.689] or lANG [autism: F(1,12) = 1.484, P = 0.247; control: F(1,11) = 0.044, P = 0.837] (Figure 4, left panel).
Fig. 4.
Bar graphs depicting percent signal change in control and autism groups during INTERNAL and EXTERNAL judgments and SELF and OTHER judgments (each relative to the MATH baseline condition), shown separately for each ROI. For main effects of group collapsed across all person judgment conditions, see Figure 3. *P ≤ 0.05; **P ≤ 0.01.
Finally, because there was a significant interaction of group and reliability of INTERNAL/EXTERNAL judgments, the above group by condition interaction analyses were repeated using reliability of INTERNAL and EXTERNAL judgments as covariates. The results remained unchanged [dMPFC: F(1,19) = 10.40, P = 0.004; RSC/PCC: F(1,19) = 15.766, P = 0.001; vMPFC/vACC and lANG, both P > 0.10].
SELF/OTHER judgments
There were no significant group by condition (SELF/OTHER) interactions for any of the four ROIs (all P > 0.05). Both groups showed largely similar patterns of activity for SELF and OTHER judgments—greater activity during OTHER compared to SELF judgments in the lANG [autism: F(1,12) = 7.20, P = 0.02; control: F(1,11) = 6.285, P = 0.029] and RSC/PCC [autism: F(1,12) = 23.816, P < 0.0004; control: F(1,11) = 31.66, P = 0.0002]. Furthermore, with the exception of marginally significantly greater activity in the vMPFC/vACC during OTHER judgments compared to SELF judgments in the autism group [F(1,11) = 5.129, P = 0.045], there were no other differences in activity between these conditions in the vMPFC/vACC [control: F(1,11) = 0.465, P = 0.509] or dMPFC [autism: F(1,12) = 1.931, P = 0.190; control: F(1,11) = 0.661, P = 0.433] (Figure 4, right panel).
The group by condition interactions remained non-significant after including covariates for reliability of SELF and OTHER judgments (all P > 0.05).
Additional analyses
To directly examine the relationship between task-constrained and task-unconstrained activity of the default network, we examined correlations between activity in the MENTAL and REST conditions for each ROI and each group separately. In the control group, there were significant correlations between MENTAL and REST conditions in all ROIs [vMPFC/vACC: r(12) = 0.650, P = 0.022; dMPFC: r(12) = 0.738, P = 0.006; RSC/PCC: r(12) = 0.641, P = 0.025; lANG: r(12) = 0.617, P = 0.033]. However, such correlations were absent in the autism group [vMPFC/vACC: r(12) = −0.016, P = 0.961; dMPFC: r(13) = 0.480, P = 0.097; RSC/PCC: r(13) = 0.260, P = 0.391; lANG: r(13) = 0.407, P = 0.168].
In an exploratory analysis, we examined whether there were differences between the autism subjects (n = 6) and Asperger's subjects (n = 6) within the ASD group. There was a weak trend toward greater activation of the dMPFC in the MENTAL vs MATH contrast in the Asperger's sample [Asperger's = 0.85%; Autism = 0.33%; F(1,10) = 3.614, P = 0.086]. All other main effects of subgroup and all subgroup by condition type (i.e. INTERNAL/EXTERNAL or SELF/OTHER) interactions were non-significant (all P > 0.20). Similarly, there were no differences between subgroups in the REST vs MATH contrast for any of the ROIs (all P > 0.15).
Lastly, there was a marginally significant negative correlation in the autism group between vMPFC/vACC activity in the REST vs MATH contrast and ADI-R social subscore [r(12) = 0.578, P = 0.049]. In other words, those subjects with higher scores on a clinical measure of social abnormality had greater abnormality in vMPFC/vACC activity during REST vs MATH, consistent with an earlier report demonstrating this same effect but using a different baseline task (i.e. the Counting Stroop Task) (Kennedy et al., 2006). However, as five of the subjects were common across these two studies, this analysis should only be viewed as exploratory.
DISCUSSION
The present study examined the functioning of the default network during self and other-person reflection and at rest in autism. There were four primary findings. First, the autism group had reduced functional activity in the vMPFC/vACC at rest. Second, when collapsed across all person judgment conditions, reduced activity was again found in the vMPFC/vACC in autism, but there were no group differences in the dMPFC, RSC/PCC or lANG. Third, although overall levels of activity were similar between groups in the dMPFC and RSC/PCC, there were group by condition interactions across INTERNAL/EXTERNAL judgments in these regions. Finally, there were no group by condition interactions across SELF/OTHER judgments for any ROI. Together, these findings give a more detailed view of default network functionality and abnormality in autism. Below, we discuss the implications of these findings.
Findings of abnormal vMPFC/vACC activity at rest are consistent with a growing body of literature demonstrating resting functional abnormalities of the default network in autism (Kennedy et al., 2006; Cherkassky et al., 2006; Kennedy and Courchesne, 2008). As activity in default network regions correlates to ones’ propensity to daydream (Mason et al., 2007) and to the amount of task-unrelated thoughts (McKiernan et al., 2006) and self-referential thoughts (D’Argembeau et al., 2005), one interpretation is that these group differences in functional activity reflect group differences in resting cognitive processes (Kennedy et al., 2006). Preliminary support for this possibility comes from an interesting behavioral study that attempted to assess the self-reported inner experience of adults with Asperger's syndrome (Hurlburt et al., 1994). Remarkably, two of the three individuals tested had difficulty simply understanding what it meant to describe their inner experience and thoughts (though, importantly, their verbal IQ was in the normal range and both could describe observable features of their immediate environment). Thus, the reduced propensity or reduced ability to introspect may underlie the reduced vMPFC/vACC activity at rest.
However, an alternative explanation of reduced levels of resting activity in autism is that there might be a pervasive dysfunction of these regions—in other words, the abnormality might be task- or cognition-independent. Previous task-based studies have provided preliminary support for this possibility, but the implicit nature of the socioemotional tasks used previously (Castelli et al., 2002; Pierce et al., 2004; Kennedy et al., 2006) leaves open the possibility that the autism subjects simply did not engage the socioemotional processes of interest. In the present study, we ensured that subjects engaged in introspective and socially oriented processing, by requiring true/false responses to self- and other-relevant statements. Even with these explicit tasks, we observed abnormality in the vMPFC/vACC region of the default network, supporting the idea that such functional abnormality of this region might be task-independent and pervasive. Importantly, such pervasive dysfunction of the vMPFC/vACC could also explain the introspective difficulties described earlier.
Group differences were also observed in the dMPFC and RSC/PCC, although the nature of these abnormalities was quite different from the vMPFC/vACC abnormality described earlier. In these regions, group differences were found in the relative pattern of activity between INTERNAL and EXTERNAL conditions, rather than group differences in overall levels of activity when collapsed across these tasks (as found in the vMPFC/vACC). In other words, group differences in the dMPFC and RSC/PCC were task-specific (i.e. group by condition interactions), rather than reflecting a more general, non-specific and pervasive dysfunction (i.e. main effects of group). For both regions, this interaction seems to have been driven primarily by reduced activity during the INTERNAL condition in the autism group, while exhibiting similar or slightly increased activity during the EXTERNAL condition. Such functional differences between groups cannot be accounted for simply by differences in reliability of judgments, because using this performance measure as a covariate did not change the results. However, a number of other plausible cognitive and behavioral explanations may account for these abnormalities. One possibility is that individuals with autism may have a specific deficit in making judgments that rely on inference (e.g. INTERNAL judgments), but an intact ability in making judgments that rely on observation (e.g. EXTERNAL judgments). This suggestion is consistent with previous behavioral findings of autism. When asked to describe a scene composed of geometric shapes moving in such a way to imply intentionality, subjects with autism can accurately describe the physical, observable features of the stimuli, but are impaired in describing the non-observable, but readily inferable, intentions of the stimuli (Klin, 2000; Castelli et al., 2002). Second, and potentially relating to a bias toward the observable over the inferable, individuals with autism may have less experience and expertise in making inferential personality judgments, but more experience and expertise in making judgments of externally observable characteristics of people. Lastly, there may be group differences in the depth of processing (e.g. the richness, detail and completeness of person representations) across INTERNAL and EXTERNAL judgments. For instance, the autism group may have had less elaborate representations of themselves and others during INTERNAL judgments but more elaborate representations during EXTERNAL judgments. Regardless of the explanation, however, since overall levels of activity were similar between groups, these findings point toward task-specific dysfunction of the dMPFC and the RSC/PCC, rather than a more pervasive task-independent dysfunction.
Although the above described abnormalities of default regions were found, perhaps equally interesting are the functional similarities between groups. With the exception of the vMPFC/vACC, the responses of the dMPFC, RSC/PCC and lANG were indistinguishable between groups during SELF and OTHER judgments. In the dMPFC, both groups had similar levels of activity in the SELF and OTHER conditions, a finding consistent with several previous studies of self and close other person reflection in control subjects (Schmitz et al., 2004; Ochsner et al., 2005; however, also see Heatherton et al., 2006). Furthermore, in the RSC/PCC and lANG, both groups had greater activity in OTHER relative to the SELF condition. This typical pattern of RSC/PCC activity in autism, given the abnormal pattern of RSC/PCC activity across INTERNAL/EXTERNAL judgments, further underscores the task-specific, as opposed to task-independent, dysfunction of this region. These findings suggest that, at a neural level, high functioning individuals with autism and Asperger's syndrome are able to differentiate between judgments of themselves and others, and, with the exception of the vMPFC/vACC, recruit the same default network regions to do so.
While the importance of using explicitly defined and well-controlled experimental conditions is clear, we should emphasize the importance of also measuring brain activity during unconstrained or underspecified tasks, since each approach provides a unique perspective on brain functioning.1 On the one hand, using a non-task resting state or an underspecified experimental task can reveal what the autistic brain does naturally (i.e. what it defaults to) when unconstrained by often artificial, rigidly defined and externally imposed task demands. On the other hand, studies using experimentally constrained tasks with explicit instructions and explicit requirements can reveal what the autistic brain is capable of doing when challenged with a particular task or situation. Although it is possible that brain activity (and underlying mental processes) can be similar during both unconstrained and constrained contexts, this relationship cannot be assumed, especially in patient populations. For instance, in the present study, the control group had significant positive correlations between resting activity and task-evoked (MENTAL) activity in each ROI, while such correlations were absent in the autism group. A recent study by Wang and colleagues (2007) also explored the relationship between implicit and explicit task demands on brain activity in high-functioning individuals with autism. After viewing and listening to short cartoon vignettes, subjects were asked to determine whether or not a story character's utterance was sincere or sarcastic. When given vague instructions (i.e. ‘pay attention’), the autism group did not activate several brain regions that were active in control subjects (including the dMPFC). Remarkably, however, when given explicit directions to attend to faces or prosody of voices, the autism group had more normal levels of activity in the dMPFC, further underscoring the task-dependent nature of dMPFC abnormality. Furthermore, early reports on hypoactivation of the fusiform face area in autism (Schultz et al., 2000; Pierce et al., 2001) might be explained by differences in unconstrained processes [e.g. task engagement (Pierce et al., 2004); eye gaze patterns (Hadjikhani et al., 2004; Dalton et al., 2005)], as opposed to pervasive regional dysfunction.
Several limitations of the current study should also be addressed. First, the full extent of regional dysfunction during rest in autism is likely not entirely captured in the present study. Given the inherently unconstrained (and thus, varied) nature of rest, the sample size might be too small to detect less robust regional differences in resting activity. Furthermore, REST blocks were randomly interspersed between the various person judgment conditions, which may have led to carry-over processing of self- and other-reflection, thus reducing the power to detect group differences. Second, as default regions are responsive to the evaluation of emotionally salient stimuli (Maddock, 1999; Maddock et al., 2003; Moran et al., 2006), possible group differences in incidental affective processing associated with self- and other-reflection may have contributed in part to the group differences reported here. Additional studies will be necessary to further explore this possibility.
In sum, the present experiment gives further insight into the nature of default network functionality and abnormality in autism. We provide evidence for task-specific deficits within particular default network regions (dMPFC and RSC/PCC) as well as evidence for more pervasive task-independent abnormalities (vMPFC/vACC). Such distinctions between the type of functional abnormality in these and other brain regions involved in social, emotional and introspective processes will likely be important for understanding the nature of such difficulties in autism.
Acknowledgments
We thank Dr Cindy Carter for clinical assessment, Graham Wideman and Stephanie Carapetian for technical assistance with stimulus presentation, Doreen Nguyen for assistance with data collection, Elizabeth Redcay and Graham Wideman for helpful discussions and the researchers and staff at the UCSD Center for Functional MRI. We also thank the participants and their families for graciously giving their time to take part in this study. This research was supported by National Institutes of Health (RO1 MH36840 to E.C.).
Appendix
Appendix A.
Complete list of stimuli used in the present experiment. Statements for the OTHER condition were modified by replacing ‘I’ with ‘My mother’ and modifying the verb appropriately (e.g., I am …’ becomes ‘My mother is …’).
| SELF, INTERNAL | SELF, EXTERNAL |
|---|---|
| I am a quiet person | I usually wear white socks |
| I am an emotional person | I eat pizza often |
| I am a loving person | I use computers often |
| I am generous | I usually eat breakfast |
| I am a relaxed person | I often make my bed |
| I am a good listener | I drive on highways often |
| I am funny | I drive a car |
| I am talkative | I eat fruit often |
| I am polite | I read books often |
| I am honest | I eat at restaurants a lot |
| I am competitive | I eat chicken often |
| I am a patient person | I watch a lot of TV |
| I am a quick learner | I go shopping often |
| I am friendly | I drink coffee often |
| I am a moody person | I talk on the phone a lot |
| I am a happy person | I take showers in the morning |
| I am easily upset | I go to the movies often |
| I am easily stressed | I read the newspaper |
| I am a focused person | I spend a lot of money |
| I am easily distracted | I listen to music often |
| I am a demanding person | I wash dishes |
| I am very thoughtful | I talk to my family often |
| I am very observant | I wear jeans often |
| I am a confident person | I am a deep sleeper |
| I am a curious person | I usually wake up early |
| I am compassionate | I listen to the radio |
| I am a nurturing person | I usually go to bed early |
| I am creative | I take naps often |
| I am easily bored | I usually cook dinner |
| I am easily scared | I dance often |
| I am shy | I go to the beach sometimes |
| I am dependable | I read books often |
| I am kind | I watch sports games |
| I am outgoing | I swim sometimes |
| I am helpful | I check my email often |
| I am sensitive | I have a dog |
| I am hard-working | I buy a lot of CDs |
| I am easily frustrated | I eat burritos often |
| I am a silly person | I go to the mall a lot |
| I am a caring person | I drink juice often |
| I am lazy | I go on walks often |
| I am a selfish person | I rent a lot of movies |
| I am controlling | I read magazines |
| I am a rude person | I eat a lot of candy |
| I am respectful | I go to the bank often |
| I am smart | I play video games often |
| I am a serious person | I work out a lot |
| I am easily disappointed | I do my laundry often |
Footnotes
REFERENCES
- Buckner RL, Vincent JL. Unrest at rest: default activity and spontaneous network correlations. Neuroimage. 2007;37:1091–6. doi: 10.1016/j.neuroimage.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–49. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–90. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, et al. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8:519–26. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A, Collette F, Van der Linden M, et al. Self-referential reflective activity and its relationship with rest: a PET study. Neuroimage. 2005;25:616–24. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RS, Dolan RJ. The mind's eye—precuneus activation in memory-related imagery. Neuroimage. 1995;2:195–200. doi: 10.1006/nimg.1995.1025. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happe F, Frith U, et al. Other minds in the brain: a functional imaging study of ‘theory of mind’ in story comprehension. Cognition. 1995;57:109–28. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends in Cognitive Science. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, et al. Activation of the fusiform gyrus when individuals with autism spectrum disorders view faces. Neuroimage. 2004;22:1141–50. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. Social Cognitive and Affective Neuroscience. 2006;1:18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlburt RT, Happe F, Frith U. Sampling the form of inner experience in three adults with Asperger syndrome. Psychological Medicine. 1994;24:385–95. doi: 10.1017/s0033291700027367. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Lieberman MD, Knowlton BJ, et al. Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. Neuroimage. 2004;21:1167–73. doi: 10.1016/j.neuroimage.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–14. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–50. [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39:1877–85. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8275–80. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A. Attributing social meaning to ambiguous visual stimuli in higher-functioning autism and Asperger syndrome: the social attribution task. Journal of Child Psychology and Psychiatry. 2000;41:831–46. [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F. The enactive mind, or from actions to cognition: lessons from autism. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2003;358:345–60. doi: 10.1098/rstb.2002.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism Development and Disorders. 2000;30:205–23. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism Development and Disorders. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends in Neuroscience. 1999;22:310–6. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104:667–76. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Human Brain Mapping. 2003;18:30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–5. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, D’Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: an fMRI investigation. Neuroimage. 2006;29:1185–91. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience. 2006;18:1586–94. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Fletcher PC. Does the brain have a baseline? Why we should be resisting a rest. Neuroimage. 2007a;37:1073–82. doi: 10.1016/j.neuroimage.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Fletcher PC. Cognitive neuroscience: the case for design rather than default. Neuroimage. 2007b;37:1097–9. [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, et al. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Pierce K, Haist F, Sedaghat F, Courchesne E. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004;127:2703–16. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain. 2001;124:2059–73. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–90. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. Neuroimage. 2004;22:941–7. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry. 2000;57:331–40. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. 3-Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme; 1988. Co-planar stereotaxic Atlas of the Human Brain. [Google Scholar]
- Wang AT, Lee SS, Sigman M, Dapretto M. Reading affect in the face and voice: neural correlates of interpreting communicative intent in children and adolescents with autism spectrum disorders. Archives of General Psychiatry. 2007;64:698–708. doi: 10.1001/archpsyc.64.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]