Abstract
Several recent studies suggest that autism may result from abnormal communication between brain regions. We directly assessed this hypothesis by testing the presence of abnormalities in a model of the functional cerebral network engaged during explicit emotion processing in adults with high functioning autism or Asperger syndrome. Comparison of structural equation models revealed abnormal patterns of effective connectivity, with the prefrontal cortex as a key site of dysfunction. These findings provide evidence that abnormal long-range connectivity between structures of the ‘social brain’ could explain the socio-emotional troubles that characterize the autistic pathology.
Keywords: emotion, autism, prefrontal, effective connectivity, fMRI
INTRODUCTION
The multifaceted non-verbal information communicated by faces is crucial to social and communicative competence and contributes to our ability to regulate social interactions. Autism is a pervasive developmental disorder with a unique profile of impairments in social communication and interactions (Hobson, 1986), leading to a dramatic inability for adaptive social behaviour. Despite close scientific scrutiny, few robust findings have been observed and the pathobiology of such aberrant social behaviour remains elusive. Recent data however suggest that the problem in autism may be more due to abnormal connectivity patterns in the brain rather than to local deficits in a specific region (Belmonte et al., 2004; Courchesne and Pierce, 2005; Wickelgren, 2005). This hypothesis appeared as early as 1988 (Horwitz et al., 1988) but has received support only recently. Abnormal correlation of activation between extrastriate and superior temporal cortices was observed during attribution of mental states from movements of animated shapes (Castelli et al., 2002). Similarly, decreased functional connectivity between Wernicke's and Broca's areas during language processing (Just et al., 2004) and reduced functional connectivity between V1 and inferior frontal cortex in a visuomotor task (Villalobos et al., 2005) have been described. Other examples of reduced functional connectivity have been observed, e.g. in an executive functioning task (Just et al., 2007), an imagery task (Kana et al., 2007), an inhibition task (Kana et al., 2007) and in a fixation resting state (Cherkassky et al., 2006). Thus, a number of functional neuroimaging studies suggest that there is a lower level of coordination among brain areas in autism. Several interpretations have been proposed: a bottom-up failure of feedforward visual signals reaching the superior temporal sulcus (STS) from extrastriate cortices such as the fusiform gyrus; or a top-down failure of feedback signals reaching STS from the anterior components of the mentalizing system i.e. dorso-medial and lateral prefrontal cortices (DMPFC and LPFC). In addition, several recent studies reported evidence of disordered cortico–cortical connections in the autistic brain (Barnea-Goraly et al., 2004; Herbert et al., 2004; Keller et al., 2007). However, functional connectivity only refers to temporal correlations between remote neurophysiological events and is therefore simply a statement about observed correlations, which does not provide any direct insight into the causality of these correlations. Here, we directly addressed the idea of abnormal connectivity in the autistic brain by modelling effective connectivity, which is defined as the influence one system exerts over another with respect to a given experimental context (Büchel and Friston, 2000).
Although several studies have focused on face processing in autism (see Schultz et al., 2003, for review), few have explored the neural system involved in interpreting information communicated through facial movements such as emotional expression, or in understanding the social signals they convey (Dalton et al., 2005; Dapretto et al., 2006; Ashwin et al., 2007). Furthermore, the distributed network of brain regions thought to decode social signals has been empirically defined using static, usually photographic, displays of emotional expressions. However, static displays of facial emotional expression may represent non-canonical stimuli that are processed for emotional content by mental strategies and neural events, distinct from their more ecologically relevant dynamic counterparts (Kilts et al., 2003). In the present study typical control subjects and subjects with autism spectrum disorders (ASD) were scanned while viewing video sequences displaying an actor's face expressing either anger or happiness (Supplementary Movie 1 and 2). Such dynamic stimuli provide visual perceptual inputs that are as lifelike as possible and are thus more sensitive for the study of cerebral activity associated with everyday emotional processing. To investigate brain activity related to explicit perception and labelling of facial expressions, we examined the neural substrates of processing dynamic angry and happy faces, of young or older actors, under two different task conditions that employed the same stimuli. In the ‘Emotion’ condition the task was to judge whether the actor was angry or happy, whereas in the ‘Age’ condition the task was to judge whether the actor was old or young. The ‘Emotion’ condition thus forced the subject to pay attention to emotional features of the actor's face. The ‘Age’ condition led to an incidental perception of facial emotional expressions while still requiring subjects to carefully examine the stimuli.
METHOD
Subjects
The group of individuals with autism included 12 right-handed ASD adults, 11 male and 1 female (mean age = 27, s.d. = 11), who were diagnosed according to DSM-IV (American Psychiatric Association, 1994) criteria for autism or Asperger syndrome. They were recruited through associations of parents in France and Belgium. In order to ensure the diagnosis, parents of all the subjects were asked to answer the screening questionnaire for autistic spectrum disorders (Ehlers et al., 1999) during a semi-directive interview. All the subjects with ASD were also assessed with WAIS-III (Wechsler, 1981). Level of functioning of subjects with ASD was reflected by their education, total or partial social independence and employment. Characteristics of the subjects with ASD are presented in Table 1.
Table 1.
Characteristics of subjects with high functioning autism (HFA) or Asperger syndrome (ASD)
| ASD Subjects | Age | Sex | Diagnosis | Verbal IQ | Non-verbal IQ | Total IQ | Score ASSQ |
|---|---|---|---|---|---|---|---|
| 1 | 53 | M | Asperger | 117 | 127 | 124 | – |
| 2 | 43 | M | Autism | 63 | 67 | 63 | 42 |
| 3 | 20 | M | Autism | 57 | 64 | 59 | 30 |
| 4 | 19 | F | Asperger | 97 | 77 | 87 | 43 |
| 5 | 20 | M | Autism | 62 | 95 | 76 | 40 |
| 6 | 19 | M | Autism | 79 | 74 | 76 | 35 |
| 7 | 18 | M | Autism | 69 | 63 | 64 | 35 |
| 8 | 22 | M | Asperger | 104 | 98 | 102 | 24 |
| 9 | 24 | M | Asperger | 113 | 99 | 107 | 33 |
| 10 | 19 | M | Autism | 74 | 82 | 76 | 28 |
| 11 | 33 | M | Autism | 81 | 66 | 75 | 39 |
| 12 | 34 | M | Autism | 82 | 67 | 73 | 40 |
Control participants were 14 healthy, right-handed male adults (mean age = 23.4, s.d. = 10) selected to match the subjects with ASD as closely as possible on the basis of age. A screening procedure was conducted to rule out any history of psychiatric or neurological disorder among the control participants. IQ level of the sample of typical subjects was assessed. However, groups were not matched on IQ level on an individual basis and these data are therefore not reported because IQ level is not a critical matching parameter in this study. Instead, because we aimed at comparing neural activity between ASD and control subjects during an experimental task of explicit emotion processing, the main concern was the level of behavioural performance at the task. This level had to be equal in both populations to avoid possible confounding effects (Table 2). Moreover, there was no significant correlation between performance at the emotional task and IQ in the ASD group (Spearman Rank Correlation test: Rho = 0.01, P = 0.94). Only three subjects with ASD have an IQ score <70. At the group level, mean IQ is over 70 and may thus be considered as within the normal range of intelligence (WAIS III manual). Several studies have shown that performance in an emotional task was not related to IQ level (e.g. Moore, 2001; Bar-On et al., 2003). In the same trend, it should be noted that both Asperger syndrome with high IQ and autistic patients with lower IQ share deficits in non-verbal communication such as facial expression (DSM-IV). Nevertheless, we have also tested whether brain activity correlates with IQ in the group with ASD. To do so, we extracted brain activity in main regions of interest for the contrast ‘Emotion vs Age’. Results showed no correlation between the level of activity within those regions and IQ score (Table 2). The absolute value of correlation coefficient varies between 0.0059 and 0.3454. This 0.3454 value corresponds to a t equal to 1.14 (with 10 degrees of freedom), i.e. a 0.3 risk if one assumes that there is a correlation. Therefore, we can consider that brain activation differences cannot be explained in terms of difference in IQ between groups.
Table 2.
Percentages of correct responses (± s.d.) in the ASD and control groups for each condition
| Group | Conditions |
|
|---|---|---|
| Age | Emotion | |
| Controls | 97.29 ± 8.5% | 96.51 ± 9.1% |
| ASD | 97.03 ± 14.3% | 91.28 ± 10.8% |
An ANOVA (2 Groups × 2 Conditions) was also performed on the percentage of correct answers. This ANOVA yielded no significant effect of group [F(1–22) = 2.6, P < 0.10], nor of condition [F(1–22) = 1.6, P > 0.10]. Moreover the Group × Condition was also not significant [F(1–22) = 0.001, P > 0.10]. Pairwise t-tests were also computed. Theses analyses failed to reveal any significant difference between conditions in both the ASD group [t(1–11) = 0.88, P > 0.10] and the Control group [t(1–11) = 0.92, P > 0.10]. There was also no group difference in the Emotion [t(1–22) = 1.2, P > 0.10) nor in the Age [t(1–22) = 1.3, P > 0.10) condition.
Handedness was assessed by means of the Edinburgh questionnaire (Oldfield, 1971). Written consent was obtained after the procedure had been fully explained. The study was approved by the local Ethics Committee and was conducted in accordance with the Declaration of Helsinki. Control subjects were paid for their participation.
Experimental design
To identify brain regions involved in explicit recognition of facial expressions, we examined neural activation during the processing of dynamic angry and happy faces of young or older actors, under two different task conditions. The experiment used a blocked paradigm and was designed as a 2 × 2 factorial. One factor was the task: subjects were asked to perform either explicit expression recognition (angry/happy) or age decision (young/old). The other factor was the dynamic shift of actor's gaze direction in the video stimuli (averted to direct or averted to averted; see Supplementary Movie 1 and 2). Four experimental conditions were thus created.
Direct gaze emotion (DGE)
The actor switched gaze from averted (0.5 s., 50% right, 50% left) to eye contact with the camera (2 s) and then expressed either an angry or friendly expression (50% friendly, 50% angry expression). The subjects were asked to make a decision about the emotional expression. This condition required subjects to assess emotional attitudes of others towards themselves, during direct eye contact.
Averted gaze emotion (AGE)
The actor switched gaze from right (0.5 s) to left (2 s), or from left (0.5 s) to right (2 s), focused either at a lateral cylinder or at a distance equal to that of the camera, and then expressed friendly or hostile emotion (50% friendly, 50% angry expression, 50% switch from right to left, 50% switch from left to right). The subjects were asked to make a decision about the emotional expression. This condition required subjects to assess emotional attitudes of others whose attention was directed away from them.
Direct gaze age (DGA)
Video stimuli were identical to those used in condition DGE. Subjects were asked to decide whether the actor was young or old.
Averted gaze age (AGA)
Video stimuli were identical to those used in condition AGE. Subjects were asked to decide whether the actor was young or old.
The ‘Emotion’ condition forced the subject to pay attention to emotional features of the actor's face. The ‘Age’ condition led to an incidental perception of facial emotional expressions while still requiring subjects to carefully examine the stimuli. The simplicity of the task and design ensured that participants with autism would perform as well as controls, removing confounding effects due to difference in behavioural performance between groups.
In all conditions, subjects were presented with video-clips. Each block comprised 10 short video sequences of 3 s. showing faces of four young and four older semi-professional male actors. At the beginning of each block a screen was presented to the subject to inform about which button correspond to which answer. A blank screen of 1 s. was presented between each 3 s. video. The same set of video stimuli was used in the emotional and age judgment tasks. Presentation order of videos within a block was randomized, and blocks were randomized within and between subjects. Responses were given at any time during the video clip using a two-position button. Response accuracy was collected during the scanning sessions. Video stimuli were projected onto a screen positioned in the back of the scanner using a video projector. Subjects could see the video reflected in a mirror (15 × 9 cm) suspended 10 cm in front of their face and subtending visual angles of 42° horizontally and 32° vertically.
Eye movements related to gaze direction shifts, and social saliency associated with the presence or absence of an eye contact are important features in our stimuli, and different gaze directions associated with emotional expressions are known to elicit distinct cerebral activations (Wicker et al., 2003). However, we decided to focus data analysis on explicit emotional processing as a general cognitive mechanism, and the distinction between gaze directions was not addressed in the present article for the following reasons:
Gaze direction turned out to have no effect on performance level in either group (t-tests, ps > 0.05). This factor thus did not influence the ability to recognize facial emotional expression in this study.
As the focus of this article is on effective connectivity modelling of interactions between regions of the social brain, it has been considered that the two emotional gaze conditions could be pooled to give more power to group comparison and reveal activations of brain areas that were of interest for this study.
In everyday life situations, expressions of emotion are associated to various gaze directions. In this respect, pooling the two emotion conditions somehow adds ecological validity to the experimental design.
Finally, in the context of our specific purpose, the expression of emotions associated with eye contact or averted gaze possibly have a similar social saliency. Although an angry eye contact and an angry averted gaze do not have the same social meaning, they both necessitate appropriate perception and understanding in social terms in order to trigger adequate adaptative behaviour.
Image acquisition and analysis
Images were acquired using a 3-T whole-body imager equipped with a circular polarized head coil. For each participant, we first acquired a high-resolution structural T1-weighted anatomical image (inversion-recovery sequence, 1 × 0.75 × 1.22 mm) parallel to the AC-PC plane, covering the whole brain. For functional imaging, we used a T2*-weighted echo-planar sequence at 36 interleaved 3.5 mm-thick axial slices with 1 mm gap (TR = 3000 ms, TE = 35 ms, flip angle = 80°, FOV = 19.2 × 19.2 cm, 64 × 64 matrix of 3 × 3 mm voxels).
Image processing and analysis of fMRI data were conducted with SPM99 software (http://www.fil.ion.ucl.ac.uk). All functional images for each subject were slicetime corrected to a slice acquired half-way through image acquisition in order to correct for temporal differences (up to 3 s) between slices acquired early, and those acquired late, in the image volume. All volumes were realigned to the first volume to correct for head movement between scans. A mean image was created using the realigned volumes. This mean image was spatially normalized to the standard EPI template given in the SPM software. Finally all images were spatially normalized using the normalization parameters determined during the normalization of mean image to EPI template. Data were then smoothed using 8 mm full width at half maximum isotropic Gaussian kernel to accommodate inter-subject differences in anatomy.
Data analysis was performed by modelling the different conditions as reference waveforms in the context of the general linear model. Specific effects were tested with appropriate linear contrasts of the parameter estimates for each condition, resulting in a t-statistic for each voxel. These t-statistics (transformed to Z-statistics) constitute a statistical parametric map (SPM) calculated for each subject individually. In order to make inferences about the population, we used a mixed effect model taking into account inter-subject variance (‘random effect analysis’ in SPM software).
Explicit emotional processing was estimated by comparing regional changes in brain activation in the contrast ‘Emotion’ and ‘Age’ conditions for each subject. The significance of the effect for each group was determined by comparing the mean of the contrast across the subjects to 0 using a one sample Student's t. The difference between the two groups was determined by comparing the means of the contrast relative to the two groups using a two samples Student's t.
Structural equation modelling
Structural equation modelling identifies connection strengths that best predict the observed variance-covariance structure of the data, with respect to a specified structural model. Effective connectivity analyses are hypothesis-driven rather than data-driven and are most applicable when one can specify the relevant functional areas. In practice, a structural model is posited which specifies the number and direction of connections between the observed variables. The aim of the analysis is to produce the key elements of the association structure among observed measures using the minimum (a parsimoniously small) number of parameters. The results are the path coefficients by which the implied covariance estimates best match the observed variance-covariance structure of the empirical data. A path coefficient quantifies the influence that one variable exerts upon another, scaling the magnitude of the influence in the range −1 to +1. A path coefficient of +1 indicates a unit change in activation (in units of s.d.) of a target area (the dependent variable) to a unit change in activation of a source area (the explanatory variable).
Structural model
The choice of an anatomical model is an important issue since modelling results are dependent on both the brain areas chosen for the analysis and the constraints imposed upon the relationships between these areas. Our model comprised a set of seven brain regions chosen on the basis of two main criteria: (i) Prior evidence of their functional involvement in emotional tasks, in autism, or evidence of correlations in activity between them, (ii) Evidence in our own fMRI study of an influence of the task on their activation. Because our fMRI results from ‘classic’ categorical comparisons using an output statistical image comparing ‘emotion’ and ‘age’ experimental conditions in the control group were consistent with previous neuroimaging studies on emotion processing, our model includes the primary visual areas, the fusiform gyrus, the superior temporal cortex, the amygdala, dorsal and ventral parts of the lateral prefrontal cortex and the dorsomedial prefrontal cortex (Supplementary Figure 1 and Supplementary Table 5). This set of brain areas includes most of those previously reported to be impaired in autism, such as the fusiform gyrus, the amygdala and the anterior dorsomedial prefrontal cortex (Baron-Cohen et al., 1999; Critchley et al., 2000; Pierce et al., 2001, 2004; Schultz et al., 2003; Ting et al., 2007).
Connections were defined on the basis of data from neuroanatomical tracer studies in primates (McDonald, 1998; Barbas, 2000; Ghashghaei et al., 2007), as well as on data from previous fMRI studies demonstrating functional correlations between brain regions (Castelli et al., 2002; Just et al., 2004; Villalobos et al., 2005). In addition to connections between primary visual areas, STS and fusiform gyrus, we included direct connections between anterior frontal areas known to have a top-down influence on posterior visual areas (Buchel and Friston, 1997; Chaminade and Fonlupt, 2003), and direct connections between the amygdala and the dorsomedial prefrontal cortex in light of their central role in the social and emotional colouring of information, empathy and theory of mind (Decety and Jackson, 2004).
We extracted mean adjusted signal from the seven clusters. For each single-subject analysis, local maxima were located within these predefined regions and time-series were extracted from the ensemble of voxels within an activated cluster (Supplementary Table 5). To assess effective connectivity in a group-specific fashion, we used time-series that comprised data collected during the ‘Emotion’ conditions in both groups (Supplementary note 1).
Path coefficient determination
The maximum likelihood approach finds path coefficients that minimize the discrepancy between the observed inter-regional correlation matrix and the matrix predicted by the model. Two approaches are then possible. The path coefficients can be deduced from a correlation matrix that is calculated by concatenating the time series of all subjects. This approach assumes that the pattern of connectivity estimated over subject is a good approximation of the underlying connectivity in all subjects studied. Thus, the inferences about the group of subjects are drawn under the assumption that the variations in connectivity from subject to subject are random and well behaved (Mechelli et al., 2002). However, this approach may be problematic when inter-subject variability is pronounced. An alternative approach is to deduce path coefficients separately for each subject using correlation matrices calculated from individual time series and then to submit individual path coefficients to a second level analysis (Chaminade and Fonlupt, 2003; Rowe et al., 2005). We used the latter approach in this study.
All path models were estimated using the maximum likelihood method implemented in Amos 4.01 software. We fitted the model separately to each inter-regional covariance matrix that had been constructed for each subject. After optimal model fit for each subject, the path coefficients were treated as dependent variables in one sample t-tests for each group (control or patients). This second-level analysis allowed us to investigate the consistency of each inter-regional coupling across each of the two groups, testing the null hypothesis that a path coefficient was zero. Two samples t-tests were used to investigate the modification of the path coefficients between the two groups, testing the null hypothesis that path coefficients were identical for the two groups.
RESULTS
Task performance
Behavioural data confirmed that both subject groups performed the tasks in the scanner with a very high level of accuracy, and that there was no difference in performance level between groups (Table 2).
Brain activations
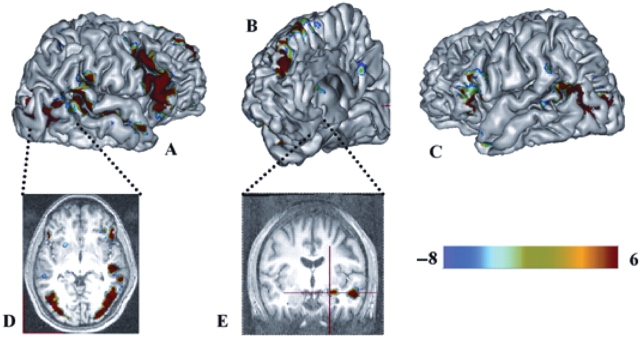
Neuroimaging data were analysed using statistical parametrical mapping (SPM99). First, we contrasted conditions requiring ‘emotion’ vs ‘age’ judgments to identify brain regions displaying increased activation during explicit perception and labelling of facial emotional expressions. In the control group, explicit processing of emotional expressions (‘Emotion’ minus ‘Age’ conditions) revealed regional BOLD signal increases in a distributed network of brain regions, as illustrated in Figure 1. This network includes bilateral fusiform gyri, superior temporal gyrus extending to the temporo-parietal junction in the right hemisphere, ventro-lateral prefrontal cortex (vLPFC, BA44/45) extending dorsally to the precentral sulcus in the right hemisphere, posterior cingulated cortex, right temporal pole, right amygdala and anterior dorso-medial prefrontal cortex (DMPFC, BA9). We then performed second level random effect analysis to compare cerebral activations in the control and ASD groups. Between-groups, whole-brain analysis revealed three foci, in the DMPFC, right vLPFC (BA 44/45) and right superior temporal gyrus at the temporo-parietal junction, which showed greater activation in the normal relative to ASD group (Table 3).
Fig. 1.
Brain activation clusters associated with explicit judgment of emotional expressions in the control group. Clusters are superimposed on a 3D anatomical image. (A) and (C), Lateral view; (B), Mid sagittal view; (D), Transverse view; (E), Coronal view. The clusters are colour-coded based on the Emotion-minus-Age t-statistic values (positive values indicate Emotion greater than Age). For graphical reporting significant activation effects are shown thresholded at an uncorrected threshold of P < 0.001.
Table 3.
Coordinates of clusters of activation when comparing control and ASD populations in the contrast ‘emotion’ vs ‘age’ judgement conditions
| Anatomical region | Control group > ASD group |
||||
|---|---|---|---|---|---|
| Talairach coordinates |
Cluster size | Z-score | |||
| x | y | z | |||
| Right temporo-parietal junction | 67 | -34 | 23 | 24 | 5.12 |
| Right inferior frontal gyrus BA 45 | 53 | 21 | 8 | 18 | 4.10 |
| Medial superior frontal gyrus BA 9/10 | 12 | 56 | 28 | 35 | 4.15 |
BA: Brodmann areas. Significant regional effects discussed in the text and reported in the tables survived a correction at P = 0.05. This correction used a search volume on the differences between the groups that was derived from the orthogonal contrast testing for main effects of task. No significant clusters were found in the comparison ASD group > Control group.
Effective connectivity modelling
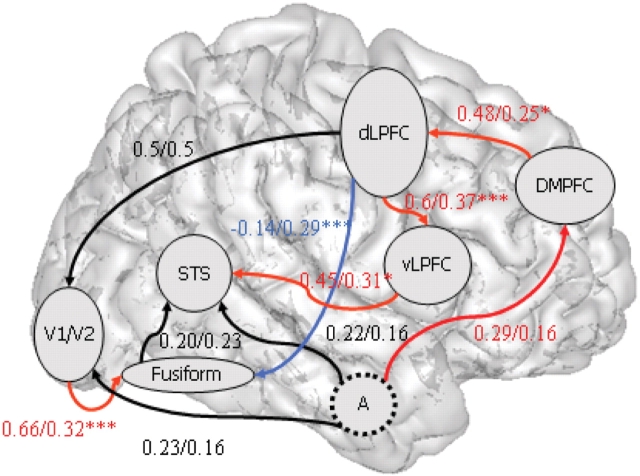
Although connections between regions are generally reciprocal, for simplicity and in order to maintain a stable model solution, we only modelled unidirectional paths. Reciprocal pathways probably subserve the interactions under investigation but the current approach specifically aimed to analyse the information-flow from posterior to frontal association cortex, and the modulation of activation of posterior areas by frontal brain regions. The comparison between control and ASD groups revealed the following significant results (Table 4 and Figure 2). First, the positive influence of amygdala activation on activity in the DMPFC observed in the control group was apparently absent in the autistic group. Second, the strong influence of DMPFC activation on the dLPFC in controls is absent in autism. Third, we observed an abnormally weak influence of activation of the dLPFC on the ventral part of the PFC in the ASD group. Fourth, activation of the vLPFC has an abnormally weak influence on STS activity in the ASD group. Fifth, the activation in the LPFC has a strong influence on activation in the fusiform gyrus in the autistic group but not in the control group. Finally, our findings indicate that activation of occipital cortex has an abnormally weak influence on the activation of the fusiform gyrus when subjects with autism are explicitly engaged in emotional expression recognition.
Table 4.
Standardized path coefficients are presented. Levels of significance are Bonferroni corrected
| Connections | Path coefficients |
||||
|---|---|---|---|---|---|
| Controls | ASD | Controls/ASD | |||
| Amygdala | → | DMPFC | 0, 29* | 0, 16 | 0, 29/0, 16 Trend |
| DMPFC | → | dLPFC | 0, 48** | 0, 25 | 0, 48/0, 25* |
| dLPFC | → | Occipital cortex | 0, 50*** | 0, 50*** | NS |
| Amygdala | → | Occipital cortex | 0, 23* | 0, 16 | NS |
| Occipital cortex | → | Fusiform gyrus | 0, 66*** | 0, 32** | 0, 66/0, 32** |
| dLPFC | → | Fusiform gyrus | −0, 14 | 0, 29** | −0, 14/0, 29*** |
| Fusiform gyrus | → | STS | 0, 20 | 0, 23 | NS |
| Amygdala | → | STS | 0, 11 | 0, 15 | NS |
| vLPFC | → | STS | 0, 45** | 0, 31* | 0, 45/0, 31 Trend |
| dLPFC | → | vLPFC | 0, 60*** | 0, 37* | 0, 6/0, 37*** |
*P < 0.05; **P < 0.01; ***P < 0.001.
Trend: significant at P < 0.01 uncorrected; NS: non significant. DMPFC, Dorso Medial PreFrontal Cortex; dLPFC, DorsoLateral PreFrontal Cortex; vLPFC, VentroLateral PreFrontal Cortex; STS, Superior Temporal Sulcus.
Fig. 2.
Path diagrams from the causal analysis using structural equation modelling. Values of the path coefficients in the control/autism groups are indicated. Significant differences in these values between groups are shown in red for Control > ASD and in blue for ASD > Control.
DISCUSSION
Cerebral areas of differential activation identified in the control subjects during explicit processing of emotional expressions (Figure 1) are coherent with the circumscribed neural network that has consistently been found activated in imaging studies of emotional processing (Phan et al., 2002 for review) and other aspects of social cognition (e.g. Pelphrey et al., 2004). Contradictory to previous studies (Pierce et al., 2001; Schultz et al., 2003; but see Pierce et al., 2004; Hadjikhani et al., 2004), regions classically involved in the perceptual analysis of facial features and expression, such as STS and fusiform gyrus, are normally engaged in the autistic group. This suggests that subjects with ASD might perceive and categorize adequately emotional expression of anger and happiness, as further reflected by their normal behavioural performance. In contrast, a lack of activation is observed in the autistic group in brain regions involved in higher order processing of perceived emotional information such as the DMPFC and the right ventrolateral PFC. Considering the role of the DMPFC (Ridderinkhof et al., 2004) and the recent report of an abnormal activation of the mid-ventrolateral prefrontal cortex in a study on emotion processing in children with autism (Dapretto et al., 2006), this suggests that processing strategies adopted by controls and ASD subjects are quite different and may reflect a failure to interpret and associate emotional features of the face correctly with their social value. This first main finding would in itself deserve in depth discussion, however the scope of the present article is to study how the abnormal activation of these brain regions may be related to abnormal connectivity in the brain of ASD subjects.
Five sites of abnormal functional interactions in the ASD group have been revealed by the modelling of effective connectivity (Figure 2 and Table 4). Evidence from studies on emotional modulation of sensory and associative cortical activation point to the amygdala as the generator of a boost signal, triggered by emotional salience and directed at representational sites of emotional stimuli or events, thus suggesting one likely mechanism of emotionally-guided attentional amplification. On the other hand, the medial prefrontal region is important in cognitive and affective functions and receives and integrates information from widespread cerebral and subcortical systems (Barbas, 2000; Ghashghaei and Barbas, 2002; Ghashghaei et al., 2007). This intricate network may thus be recruited in cognitive tasks that are inextricably linked with emotional associations. The reduced activation of pathways connecting the amygdala with prefrontal limbic cortices observed in the ASD group could therefore disrupt a circuit that is likely to have a crucial role in the emotional colouring of events (McDonald, 1998; Ridderinkhof et al., 2004). This may be a first account for the flattening of emotions and inappropriate affect processing typically observed in autism.
The prefrontal cortex is heavily interconnected and so any emotional information registered in the DMPFC could influence executive priorities coded in the dorsolateral PFC (dLPFC) and ultimately alter the direction of attention or, more generally, modify the distribution of processing resources in a given context (Barcelo et al., 2000). The DMPFC can thus be seen as the bridge that conveys emotional information from subcortical limbic regions such as the amygdala to cortical executive centres of the lateral prefrontal cortex. Anatomical studies on connectivity in the non-human primate brain suggest that feedback projections from limbic cortices may serve to compare the input and output necessary for the interpretation of events, be they emotional or not (Ghashghaei and Barbas, 2002; Ghashghaei et al., 2007). The breakdown of massive feedback—originating in limbic areas—observed in the autism group could be related to its role in the integration of distributed pathways associated with sensory perception, associative mnemonic and emotional processes (Barbas, 1995). The fact that these regions are both phylogenetically and ontogenically late-developing regions (Giedd, 2004) suggests that they may retain some developmental features to a greater extent than other cortices, which would further support the hypothesis of their preferential vulnerability in several neurological and psychiatric disorders such as autism, schizophrenia and epilepsy.
There is considerable evidence by now that the lateral prefrontal cortex plays a major role in high-order control processes that exercise a top-down regulation of cognition and behaviour (e.g. Dove et al., 2008). Various lateral prefrontal areas have structural differences and distinct connections with other cortical and subcortical brain structures, which suggest that these areas are involved in distinct aspects of the higher-level control of cognitive processing and behaviour. The dorsal lateral PFC (dLPFC) has been suggested to be a specialized region where stimuli or events that have been first interpreted and maintained in posterior association cortical areas can be re-coded in an abstract form. The mid-ventrolateral prefrontal cortex (vLPFC), in interaction with posterior cortical association areas, would subserve the expression of various first order executive processes, such as active selection, comparison and judgment of stimuli held in short-term and long-term memory (Petridès, 2005). Interactions between these regions of the LPFC are necessary for the active encoding and active retrieval of information, i.e. processing initiated under conscious effort by the subject and guided by the subject's plan and intentions, but not necessarily for automatic stimulus-driven or context driven encoding and retrieval of information (Petridès, 2005). In the present study, the task was to explicitly identify emotional faces, thus necessitated manipulating cognitive representations of emotional stimuli to enable conscious active and control of planned behaviour and cognition. Functional interactions between the dorsal and ventral parts of the LPFC are likely to subserve this function, together with functional interactions with posterior associative areas such as the STS and the fusiform gyrus. Results of effective connectivity analyses consistently reveal that the control group performed the task via functional interactions between prefrontal and posterior temporal areas. In contrast, abnormal activation of the vLPFC region and its abnormal pattern of effective connectivity with the dLPFC and with posterior temporal region such as the multimodal upper bank of the STS suggest that these processes are not adequately performed in ASD. We also observed an abnormally strong influence of activity in the right dLPFC on activity in the fusiform gyrus in the ASD group. dLPFC activation plays an important role in sustaining emotional representations of stimuli so that attention can be effectively directed in order to achieve task goals (Taylor and Fragopanagos, 2005). A recent study showed clear differences in how individuals with autism scan and process facial images and suggests that these differences may be the proximal cause of the commonly reported fusiform gyrus hypoactivation in autism during face processing (Dalton et al., 2005). This lack of ability to use and search out the right information might explain our own results of abnormal interactions between dLPFC and fusiform gyrus. However, it is also plausible that an inability to attribute emotional states to others causes an abnormal seeking strategy that drives a need to look at the expressions in different ways to the control group. This latter process could be supported by the heavy fusiform activation control by the dLPFC observed in our study. An abnormally strong influence of right dLPFC on fusiform gyrus could thus represent the neural instantiation of a compensatory cognitive mechanism that would explain the similarly high levels of performance in the explicit emotional task in both groups (Supplementary Table 1). By normalizing behaviour in explicit contexts, such compensatory processing strategies may mask primary dysfunction in the emotional information-processing network that normally involves the amygdala-DMPFC axis. Under unconstrained everyday socio/emotional conditions, the compensatory dLPFC → Fusiform activation might be less pronounced or unable to be updated as quickly. Consequently, the lack of boosting signals from amygdala to DMPFC in subjects with autism could then result in uncompensated impairment of automatic face processing, thus generating the typically observed behavioural deficits. Coherent with this, studies using functional connectivity analysis have recently begun to reveal evidence of underconnectivity between frontal and posterior temporal region in autism in various tasks such as memory for faces (Just et al., 2007; Koshino et al., 2008). The autism group may thus not use an affective or socially oriented strategy to approach the task. Nevertheless, adults with ASD may be able to learn how to explicitly decode social and emotional information, providing an encouraging opportunity for therapy.
Although described here in the case of explicit emotional processing, this abnormal pattern of connectivity between prefrontal and posterior associative areas could be generalized to other forms of monitoring and the pattern of abnormal interactions observed in the present study might be found in other studies that do not focus particularly on emotion processing per se.
As stated in the ‘Results’ section, our model is incomplete because connections between prefrontal and other cortical areas are most likely to be bidirectional. However, the fact that unidirectional connections are already abnormal in ASD strongly support the hypothesis of abnormal cortico–cortical interactions involved in high level processing such as emotion processing.
Our findings also indicate that activity of occipital cortex has an abnormally weak influence on the activity of the fusiform gyrus when subjects with autism are explicitly engaged in emotional expression recognition. This can be interpreted by considering the well-known functional role of the fusiform gyrus and its intricate connectivity with other components of the social/emotional perceptual network. A dysfunction at such an early stage may contribute to the general impairment in social communicative deficits that characterize autism.
By incorporating new data that reveal distinct patterns of effective connectivity, our results provide direct evidence of abnormal long-range connectivity between the brain structures implicated in the socio-emotional network in autism. This is in line with the idea of reduced long-distance anterior to posterior cortico–cortical connectivity (Courchesne et al., 2004), which impairs the fundamental frontal function of integrating information from widespread and diverse systems (emotional, language, sensory, autonomic, …) and providing complex context-rich feedback, guidance and control to lower-level systems (Taylor and Fragopanagos, 2005). Furthermore, our data suggest that this abnormal modulation most likely has its origins in abnormal activation, and effective connectivity, of the medial and lateral prefrontal cortices. This adds a functional relevance and value to recent data from histopathological, voxel-based morphometry, MRI volumetric analysis and diffusion tensor imaging studies suggesting abnormal development, and abnormal local connectivity in the medial part of the prefrontal cortex (Courchesne et al., 2004; Waiter et al., 2004, 2005). Abnormalities in effective connectivity could actually result from—as opposed to give rise to—the socio/emotional/communicative deficits seen in autism. Indeed, plenty of evidence suggest that the establishment and maintenance of neural connections is shaped by experience (Blakemore et al., 2007), especially in the socio-cognitive domain in which abnormalities actually define autism. Future research will benefit from the study of model including more brain areas known to be involved in social cognition, such as the temporal poles and the right temporo-parietal junction.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
This research has been supported by the Fondation France Telecom, the Fondation Lejeune, the Fondation de France and Fondation EDF. With thanks to M. Roth, B. Nazarian and J.L. Anton for help with fMRI scanning. We are grateful to all ASD persons who kindly volunteered to participate in this study.
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. (DSM-IV), Washington DC: APA; 1994. [Google Scholar]
- Ashwin C, Baron-Cohen S, Wheelwright S, O’Riordan M, Bullmore ET. Differential activation of the amygdala and the ‘social brain’ during fearful face-processing in Asperger syndrome. Neuropsychologia. 2007;45:2–14. doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Barbas H. Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neuroscience and Biobehavioral Reviews. 1995;19:499–510. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Research Bulletin. 2000;15:319–30. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Suwazono S, Knight R. Prefrontal modulation of visual processing in humans. Nature Neuroscience. 2000;3:399–403. doi: 10.1038/73975. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biological Psychiatry. 2004;55:323–6. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Bar-On R, Tranel D, Denburg NL, Bechara A. Exploring the neurological substrate of emotional and social intelligence. Brain. 2003;126:1790–800. doi: 10.1093/brain/awg177. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, et al. Social intelligence in the normal and autistic brain: an fMRI study. European Journal of Neuroscience. 1999;11:1891–8. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Belmonte M, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. Journal of Neuroscience. 2004;24:9228–31. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, den Ouden H, Choudhury S, Froth C. Adolescent development of the neural circuitry for thinking about intentions. Social Cognitive and Affective Neuroscience. 2007;2:130–9. doi: 10.1093/scan/nsm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C, Friston K. Assessing interactions among neuronal systems using functional neuroimaging. Neural Networks. 2000;13:871–82. doi: 10.1016/s0893-6080(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Buchel C, Friston KJ. Modulation of connectivity in visual pathways by attention: cortical interactions evaluated with structural equation modelling and fMRI. Cerebral Cortex. 1997;7:768–78. doi: 10.1093/cercor/7.8.768. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–49. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Chaminade T, Fonlupt P. Changes of effective connectivity between the lateral and medial parts of the prefrontal cortex during a visual task. European Journal of Neuroscience. 2003;18:675–9. doi: 10.1046/j.1460-9568.2003.02787.x. [DOI] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–90. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Current Opinion in Neurobiology. 2005;15:225–30. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Redcay E, Kennedy D. The autistic brain: birth through adulthood. Current Opinion in Neurology. 2004;17:489–96. doi: 10.1097/01.wco.0000137542.14610.b4. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, et al. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123:2203–12. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, et al. Gaze fixation and the neural circuitry of face processing in autism. Natural Neuroscience. 2005;4:519–26. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Natural Neuroscience. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Jackson PL. The functional architecture of human empathy. Behavioral and Cognitive Neuroscience Reviews. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Dove A, Manly T, Epstein R, Owen AM. The engagement of mid-ventrolateral prefrontal cortex and posterior brain regions in intentional cognitive activity. Human Brain Mapping. 2008;29:107–19. doi: 10.1002/hbm.20378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers S, Gillberg C, Wing L. A screening questionnaire for Asperger syndrome and other high-functioning autism spectrum disorders in school age children. Journal of Autism and Developmental Disorders. 1999;29:129–41. doi: 10.1023/a:1023040610384. [DOI] [PubMed] [Google Scholar]
- Ghashghaei H, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–79. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Ghashghaei H, Hilgetag HH, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–23. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, et al. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage. 2004;22:1141–50. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, et al. Localization of white matter volume increase in autism and developmental language disorder. Annals of Neurology. 2004;55:530–40. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Hobson RJ. The autistic child's appraisal of expressions of emotion. Journal of Child Psychology and Psychiatry. 1986;27:321–42. doi: 10.1111/j.1469-7610.1986.tb01836.x. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Rumsey J, Grady C, Rapoport S. The cerebral metabolic landscape in autism. Intercorrelations of regional glucose utilization. Archives of Neurology. 1988;45:749–55. doi: 10.1001/archneur.1988.00520310055018. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky V, Keller T, Minshew N. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–21. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cerebral Cortex. 2007;17:951–61. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biological Psychiatry. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. Neuroreport. 2007;18:23–7. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Egan G, Gideon DA, Ely TD, Hoffman JM. Dissociable neural pathways are involved in the recognition of emotion in static and dynamic facial expressions. NeuroImage. 2003;18:156–68. doi: 10.1006/nimg.2002.1323. [DOI] [PubMed] [Google Scholar]
- Koshino H, Kana RK, Keller TA, Cherkassky VI, Minshew NJ, Just MA. fMRI in vestigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cerebral Cortex. 2008;18:278–88. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Progress in Neurobiology. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Penny WD, Price CJ, Gitelman DR, Friston KJ. Effective connectivity and intersubject variability: using a multisubject network to test differences and commonalities. Neuroimage. 2002;17:1459–69. doi: 10.1006/nimg.2002.1231. [DOI] [PubMed] [Google Scholar]
- Moore DG. Reassessing emotion recognition performance in people with mental retardation: a review. American Journal of Mental Retardation. 2001;106:481–502. doi: 10.1352/0895-8017(2001)106<0481:RERPIP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pelphrey K, Morris J, McCarthy G. Grasping the intentions of others: the perceived intentionality of an action influences activity in the superior temporal sulcus during social perception. Journal of Cognitive Neuroscience. 2004;16:1706–16. doi: 10.1162/0898929042947900. [DOI] [PubMed] [Google Scholar]
- Petridès M. Lateral prefrontal cortex: architectonic and functional organization. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2005;360:781–95. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K, Wager T, Taylor S, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Pierce K, Haist F, Sedaghat F, Courchesne E. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004;127:2703–16. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain. 2001;124:2059–73. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–7. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Stephan KE, Friston K, Frackowiak RS, Passingham RE. The prefrontal cortex shows context-specific changes in effective connectivity to motor or visual cortex during the selection of action or colour. Cerebral Cortex. 2005;15:85–95. doi: 10.1093/cercor/bhh111. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Grelotti DJ, Klin A, et al. The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Philosophical Transaction of the Royal Society of London Series B, Biological Sciences. 2003;358:415–27. doi: 10.1098/rstb.2002.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, Fragopanagos N. The interaction of attention and emotion. Neural Networks. 2005;18:353–69. doi: 10.1016/j.neunet.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Ting WA, Lee SS, Sigman MBA, Dapretto M. Reading affect in the face and voice: neural correlates of interpreting communicative intent in children and adolescents with autism spectrum disorders. Archives of General Psychiatry. 2007;64:698–708. doi: 10.1001/archpsyc.64.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Muller RA. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage. 2005;25:916–25. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waiter GD, Williams JH, Murray AD, Gilchrist A, Perrett DI, Whiten A. A voxel based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage. 2004;22:619–25. doi: 10.1016/j.neuroimage.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Waiter GD, Williams JH, Murray AD, Gilchrist A, Perrett DI, Whiten A. Structural white matter deficits in high-functioning individuals with autistic spectrum disorder: a voxel based investigation. Neuroimage. 2005;24:455–61. doi: 10.1016/j.neuroimage.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-R Manual. New York: Psychological Corporation; 1981. [Google Scholar]
- Wickelgren I. Autistic brains out of synch? Science. 2005;24:1856–8. doi: 10.1126/science.308.5730.1856. [DOI] [PubMed] [Google Scholar]
- Wicker B, Perrett DI, Baron-Cohen S, Decety J. Being the target of another's emotion: a PET study. Neuropsychologia. 2003;41:139–46. doi: 10.1016/s0028-3932(02)00144-6. [DOI] [PubMed] [Google Scholar]