Abstract
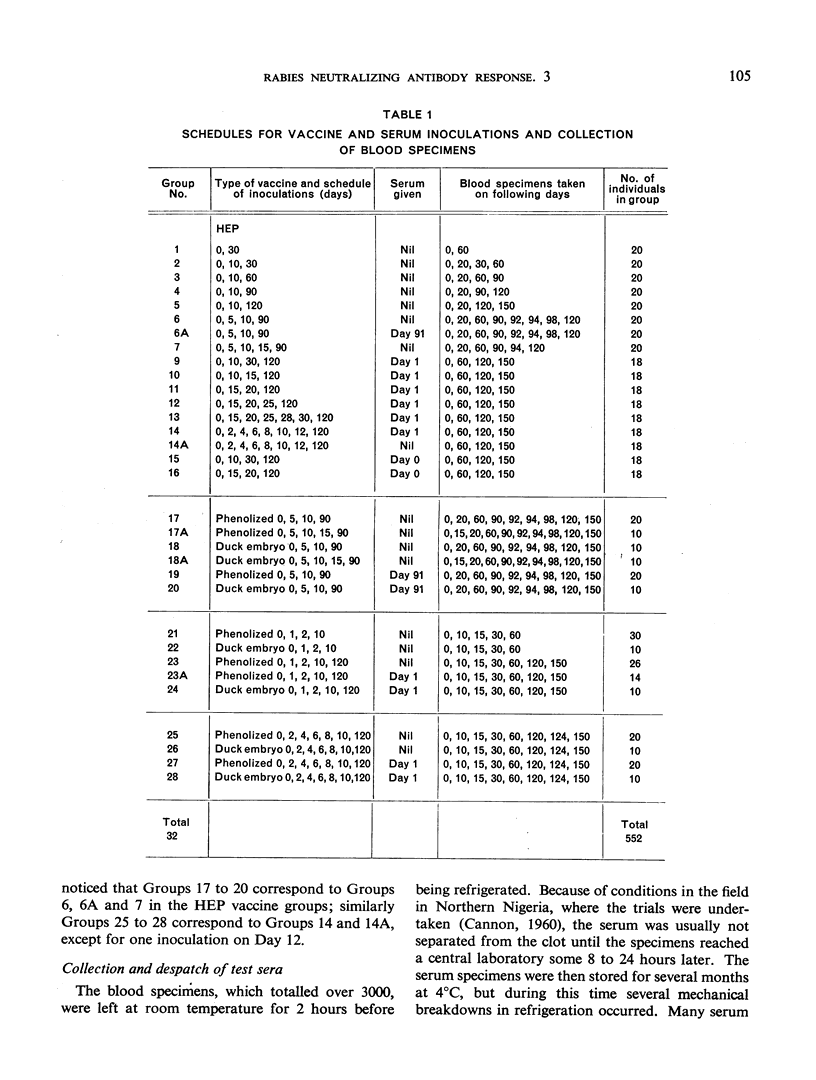
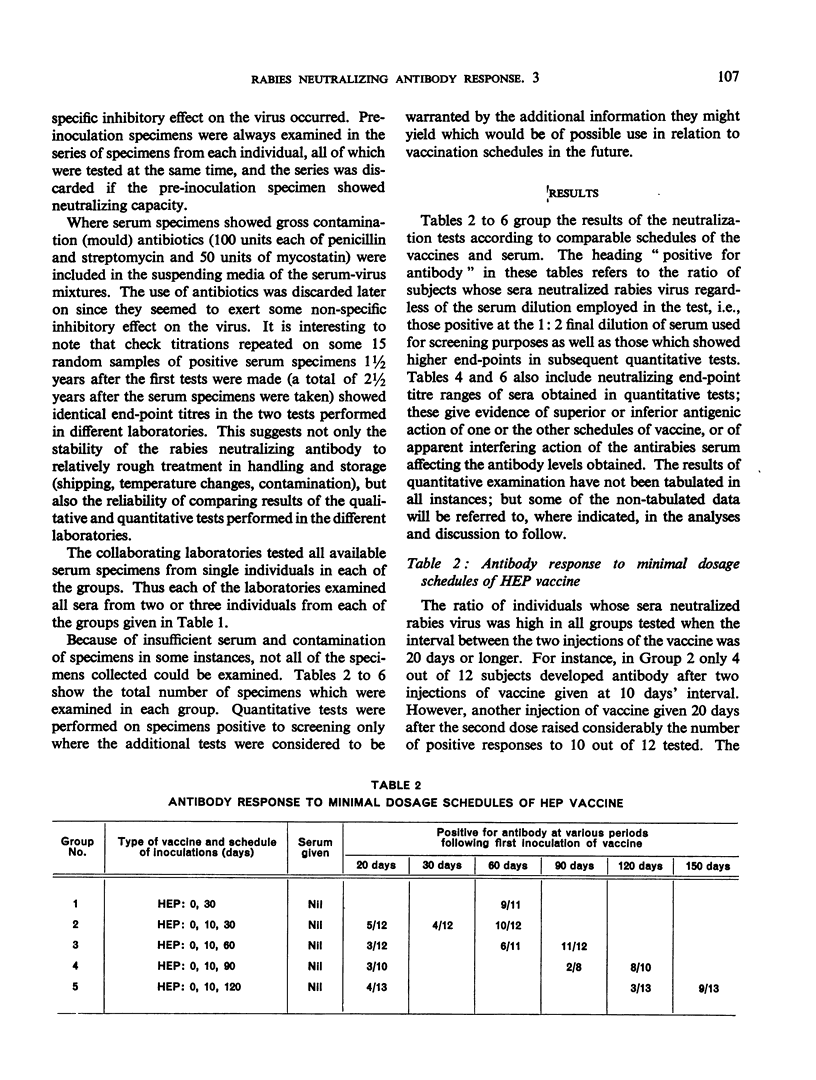
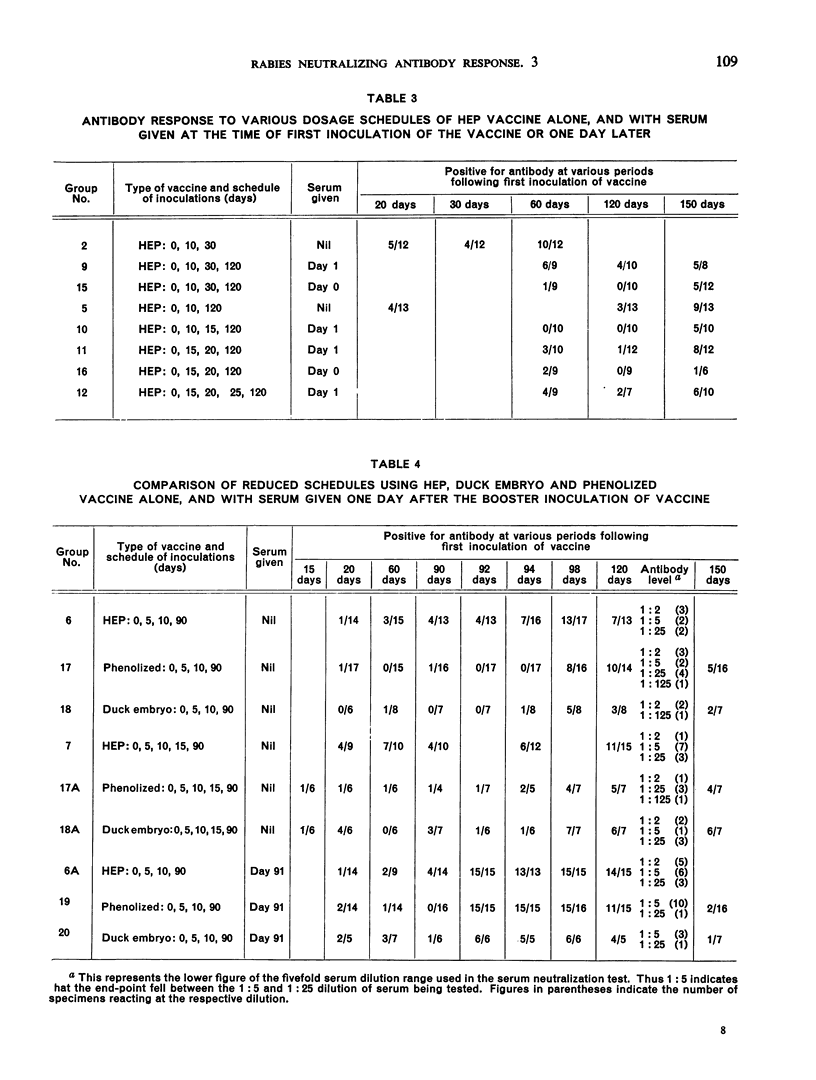
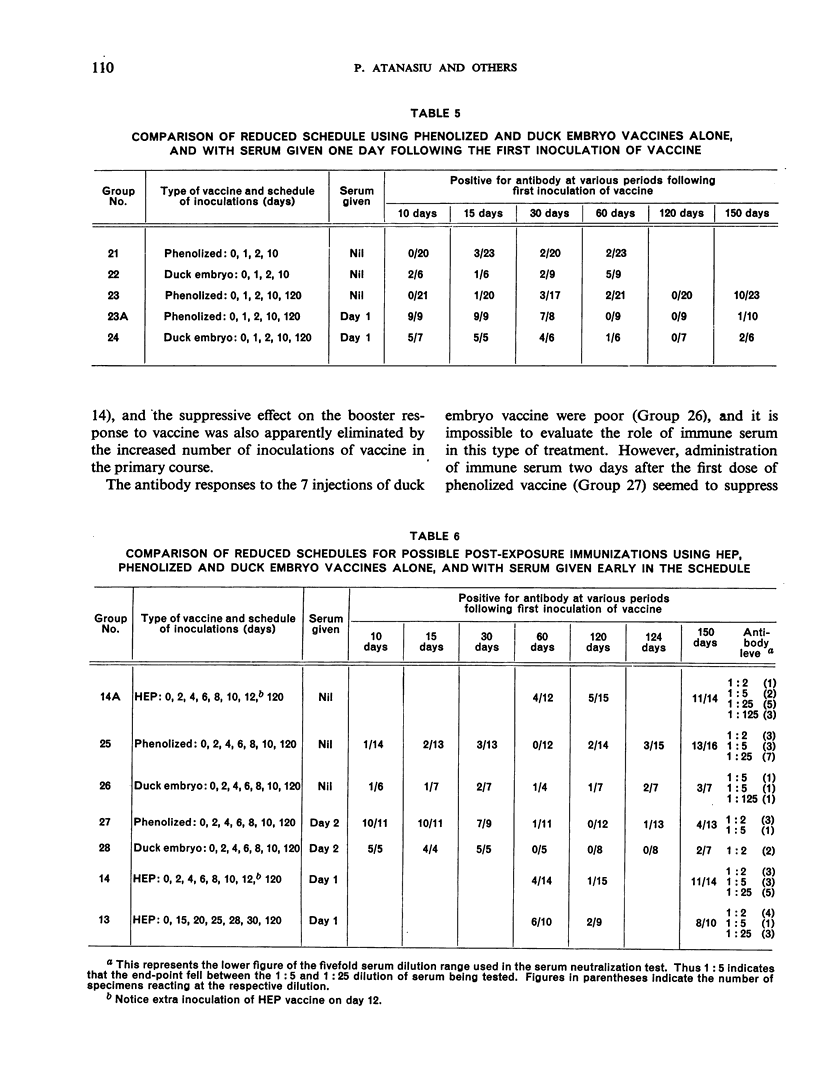
This study is the third in a series on virus-neutralizing antibody response to different schedules of antirabies serum and vaccines in previously non-exposed persons. Three types of vaccine were studied—phenolized (Semple), duck embryo and high-egg-passage (HEP) chicken embryo. Reduced schedules of vaccine, consisting of 2-7 inoculations given at various intervals, did not give results comparable in efficacy (time of appearance, level and persistence of antibody) with schedules comprising at least 14 daily inoculations of vaccine as determined in previous trials. The effectiveness of a booster dose in previously sensitized individuals was confirmed with a demonstration that a rise in serum antibody appears between 4 and 8 days after the booster inoculation. Effective sensitization appears to be as much a function of spacing of inoculations as of total dosage of vaccine antigen. Interference by immune serum with the antigenicity of subsequently administered vaccine, noted previously by the present authors and by other workers, was again confirmed. This interference could be overcome by the administration of a sufficient amount of vaccine.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON G. R., SCHNURRENBERGER P. R., MASTERSON R. A., WENTWORTH F. H. Avian embryo rabies immunization I. Duck-embryo vaccine administered intradermally in man. Am J Hyg. 1960 Mar;71:158–167. doi: 10.1093/oxfordjournals.aje.a120099. [DOI] [PubMed] [Google Scholar]
- BALTAZARD M., BAHMANYAR M. Essai pratique du sérum antirabique chez les mordus par loups enragés. Bull World Health Organ. 1955;13(5):747–772. [PMC free article] [PubMed] [Google Scholar]
- CANNON D. A. Rabies vaccine trials in Nigeria. West Afr Med J. 1960 Feb;9:12–20. [PubMed] [Google Scholar]
- CULBERTSON C. G., PECK F. B., Jr, POWELL H. M. Duck-embryo rabies vaccine; study of fixed virus vaccine grown in embryonated duck eggs and killed with beta-propiolactone (BPL). J Am Med Assoc. 1956 Dec 8;162(15):1373–1376. [PubMed] [Google Scholar]
- FOX J. P., KOPROWSKI H., CONWELL D. P., BLACK J., GELFAND H. M. Study of antirabies immunization of man; observations with HEP Flury and other vaccines, with and without hyperimmune serum, in primary and recall immunizations. Bull World Health Organ. 1957;17(6):869–904. [PMC free article] [PubMed] [Google Scholar]
- GREENBERG M., CHILDRESS J. Vaccination against rabies with duck-embryo and Semple vaccines. J Am Med Assoc. 1960 May 28;173:333–337. doi: 10.1001/jama.1960.03020220007002. [DOI] [PubMed] [Google Scholar]
- HABEL K. Antiserum in the prophylaxis of rabies. Bull World Health Organ. 1954;10(5):781–788. [PMC free article] [PubMed] [Google Scholar]
- HABEL K., KOPROWSKI H. Laboratory data supporting the clinical trial of anti-rabies serum in persons bitten by a rabid wolf. Bull World Health Organ. 1955;13(5):773–779. [PMC free article] [PubMed] [Google Scholar]
- KOPROWSKI H., COX H. R. Studies on chick embryo adapted rabies virus; culture characteristics and pathogenicity. J Immunol. 1948 Dec;60(4):533–554. [PubMed] [Google Scholar]
- PECK F. B., Jr, POWELL H. M., CULBERTSON C. G. A new antirabies vaccine for human use; clinical and laboratory results using rabies vaccine made from embryonated duck eggs. J Lab Clin Med. 1955 May;45(5):679–683. [PubMed] [Google Scholar]
- RUEGSEGGER J. M., BLACK J., SHARPLESS G. R. Primary antirabies immunization of man with HEP flury virus vaccine. Am J Public Health Nations Health. 1961 May;51:706–716. doi: 10.2105/ajph.51.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELIMOV M. A., BOLTUCHII L. G., SEMENOVA I. V. [Dynamics of virus-neutralizing antibodies in severely-bitten subjects and in gamma-globulin and anti-rabies immunized men]. J Hyg Epidemiol Microbiol Immunol. 1959;3:487–500. [PubMed] [Google Scholar]
- SELIMOV M., BOLTUCIJ L., SEMENOVA E., KOBRINSKIJ G., ZMUSKO L. [The use of antirabies gamma globulin in subjects severely bitten by rabid wolves or other animals]. J Hyg Epidemiol Microbiol Immunol. 1959;3:168–180. [PubMed] [Google Scholar]
- VEERARAGHAVAN N., SUBRAHMANYAN T. P. Value of antirabies vaccine with and without serum against severe challenges. Bull World Health Organ. 1960;22:381–391. [PMC free article] [PubMed] [Google Scholar]


