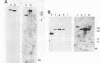Abstract
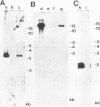
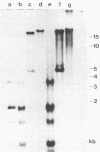
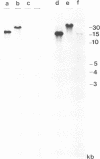
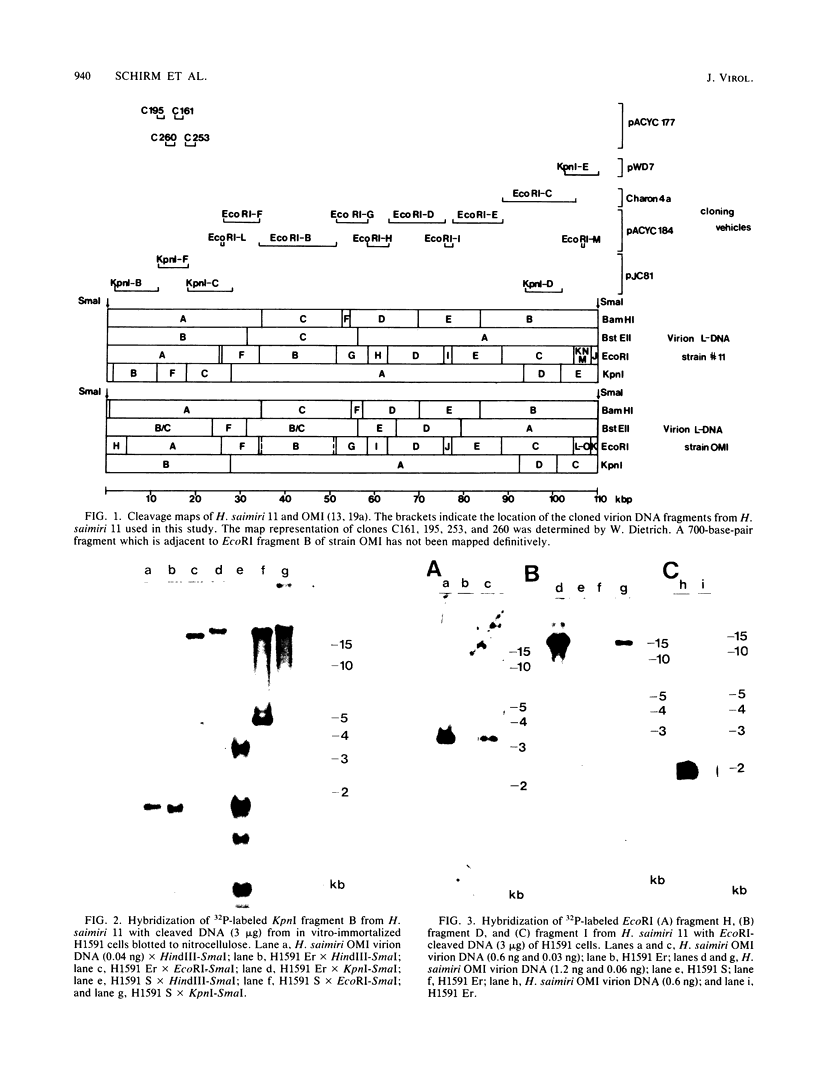
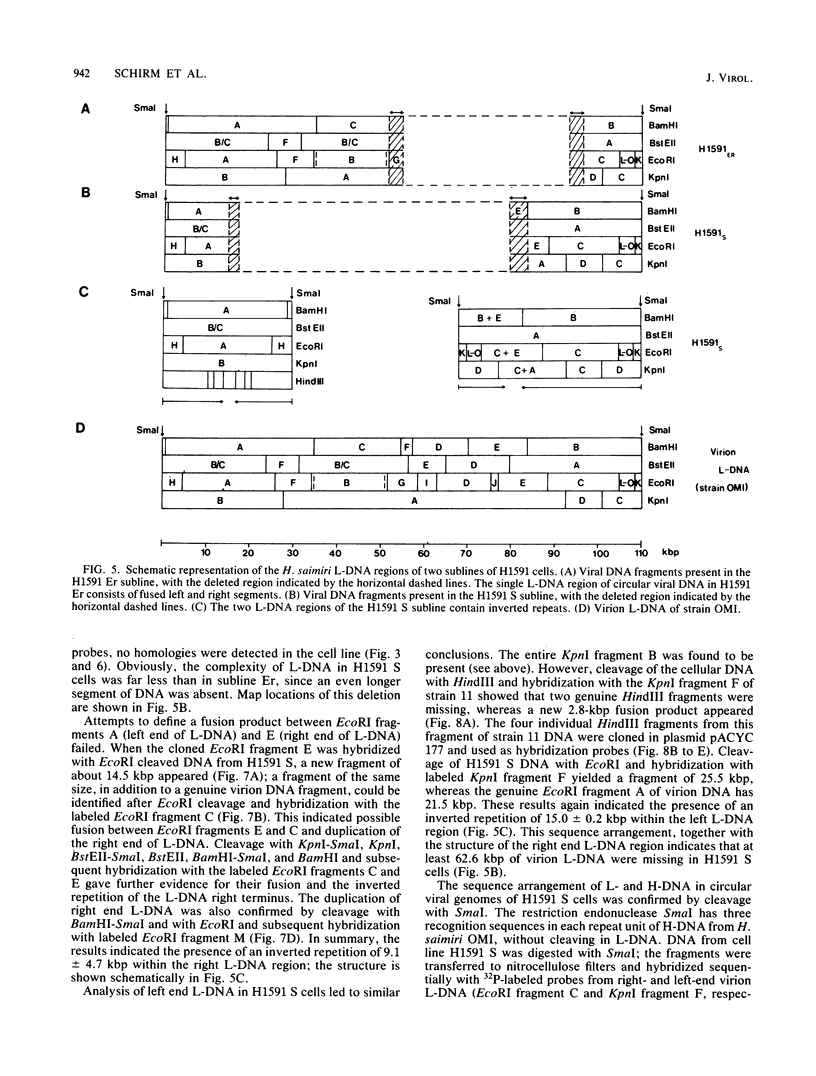
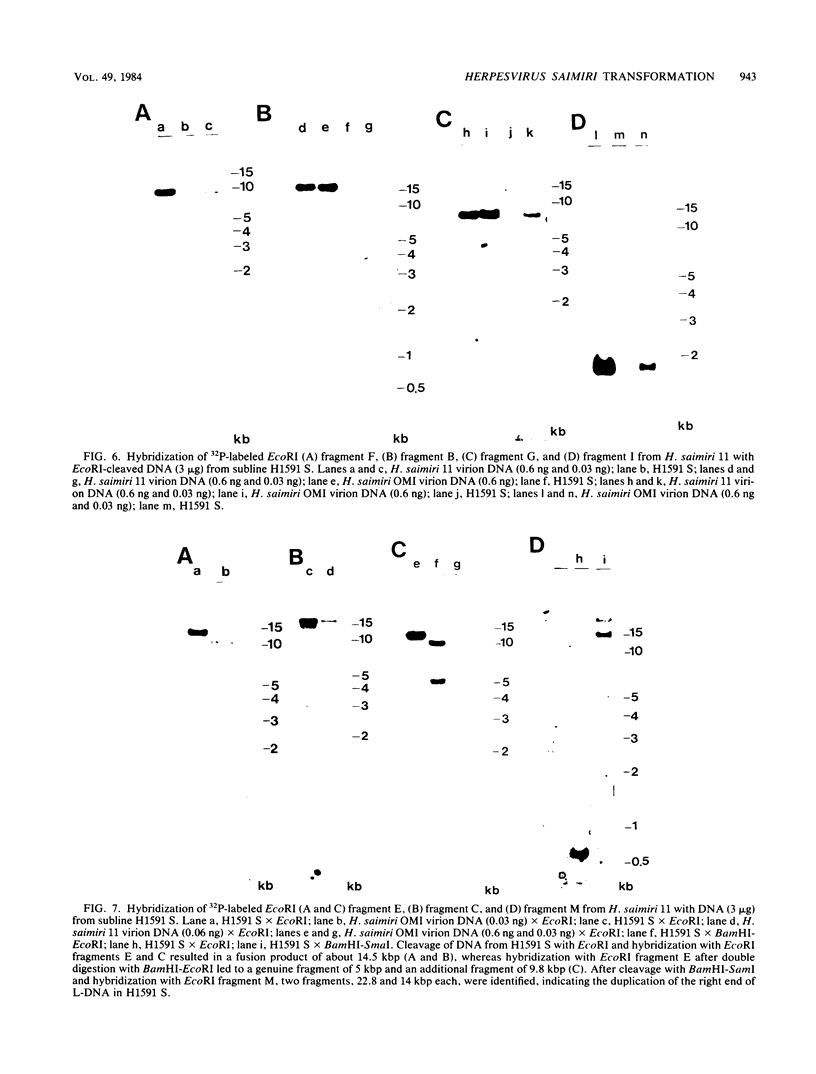
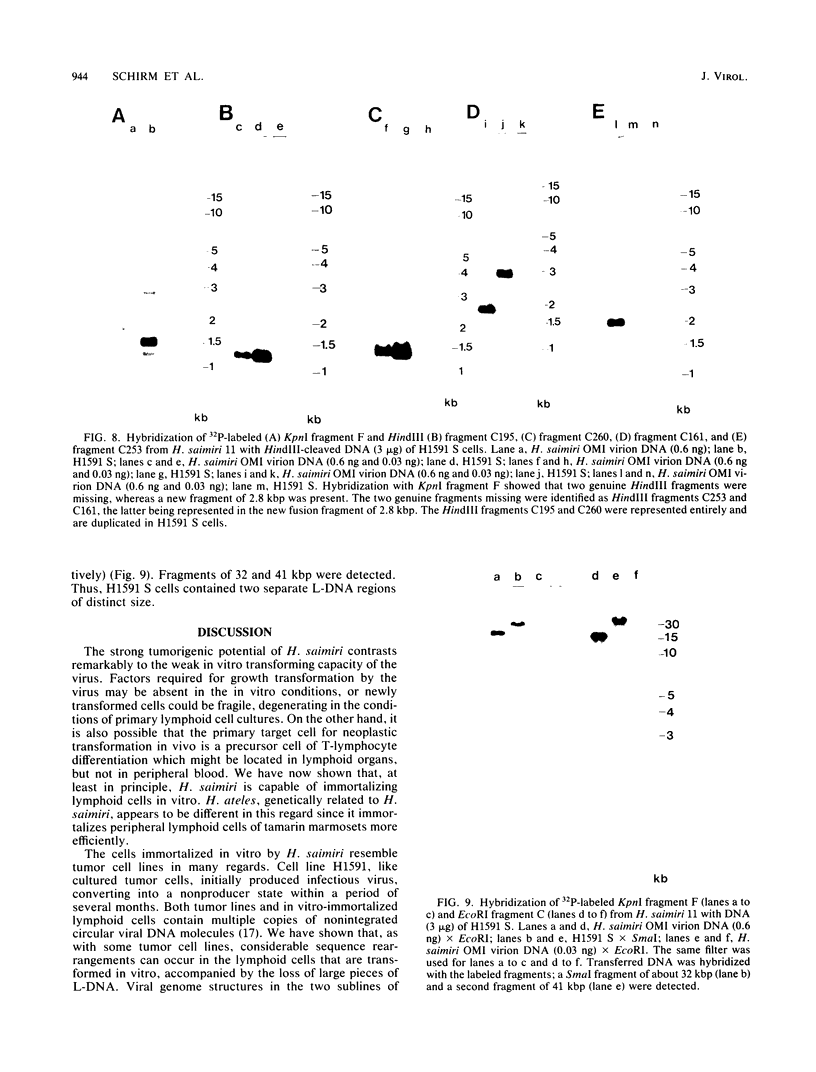
A lymphoid T-cell line (H1591) was established by infecting peripheral blood mononuclear cells from a cotton top marmoset with Herpesvirus saimiri OMI. Analysis of these in vitro-immortalized cells revealed nonintegrated, covalently closed circular viral DNA molecules in high multiplicities with substantial rearrangements and large deletions in their L-DNA (unique) regions. One subline, designated H1591 Er, contained circular viral DNA with one stretch of H-DNA (repetitive) and one of L-DNA; the L-DNA segment consisted of a linear fusion of a 53.2-kilobase-pair piece of L-DNA (left half of L-DNA) with a 15.2-kilobase-pair L-DNA fragment from the right end of the L-DNA region. The other subline, H1591 S, contained two short regions of L-DNA, each derived from the extreme ends of virion L-DNA. Both L-DNA regions of H1591 S cells contained inverted repetitions (15.0 +/- 0.2 and 9.1 +/- 4.7 kilobase pairs). The extensive deletions of L-DNA sequences in cell line H1591 indicate that at least 73% of the genetic information in H. saimiri is not required to maintain the persistence of viral DNA and the state of transformation in lymphoid T-cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Da Silva M. P. Cuidados de enfermagem nos pacientes com "shunt" e fistula artério-venosa. Rev Enferm Nov Dimens. 1976 Nov;2(5):290–294. [PubMed] [Google Scholar]
- Deinhardt F. W., Falk L. A., Wolfe L. G. Simian herpesviruses and neoplasia. Adv Cancer Res. 1974;19(0):167–205. doi: 10.1016/s0065-230x(08)60054-8. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C. Herpesvirus saimiri DNA in tumor cells--deleted sequences and sequence rearrangements. J Virol. 1981 Aug;39(2):497–509. doi: 10.1128/jvi.39.2.497-509.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C. Specifically unmethylated cytidylic-guanylate sites in Herpesvirus saimiri DNA in tumor cells. J Virol. 1982 Aug;43(2):427–435. doi: 10.1128/jvi.43.2.427-435.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk L. A., Nigida S. M., Deinhardt F., Wolfe L. G., Cooper R. W., Hernandez-Camacho J. I. Herpesvirus ateles: properties of an oncogenic herpesvirus isolated from circulating lymphocytes of spider monkeys (Ateles sp.). Int J Cancer. 1974 Oct 15;14(4):473–482. doi: 10.1002/ijc.2910140407. [DOI] [PubMed] [Google Scholar]
- Falk L. A., Wolfe L. G., Deinhardt F. Isolation of Herpesvirus saimiri from blood of squirrel monkeys (Saimiri sciureus). J Natl Cancer Inst. 1972 May;48(5):1499–1505. [PubMed] [Google Scholar]
- Falk L., Johnson D., Deinhardt F. Transformation of marmoset lymphocytes in vitro with Herpesvirus ateles. Int J Cancer. 1978 May 15;21(5):652–657. doi: 10.1002/ijc.2910210517. [DOI] [PubMed] [Google Scholar]
- Falk L., Wright J., Wolfe L., Deinhardt F. Herpesvirus ateles: transformation in vitro of marmoset splenic lymphocytes. Int J Cancer. 1974 Aug 15;14(2):244–251. doi: 10.1002/ijc.2910140213. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B., Bornkamm G. W., Ludwig H. Repetitive sequences in complete and defective genomes of Herpesvirus saimiri. J Virol. 1975 Feb;15(2):398–406. doi: 10.1128/jvi.15.2.398-406.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein B., Müller I., Werner J. The presence of Herpesvirus Saimiri genomes in virus-transformed cells. Int J Cancer. 1977 Apr 15;19(4):546–554. doi: 10.1002/ijc.2910190416. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B. Oncogenic herpesviruses of non-human primates. Biochim Biophys Acta. 1979 Nov 30;560(3):301–342. doi: 10.1016/0304-419x(79)90007-6. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hunt R. D., Garcia F. G., Barahona H. H., King N. W., Fraser C. E., Meléndez L. V. Spontaneous Herpesvirus saimiri lymphoma in an owl monkey. J Infect Dis. 1973 Jun;127(6):723–725. doi: 10.1093/infdis/127.6.723. [DOI] [PubMed] [Google Scholar]
- Kaschka-Dierich C., Werner F. J., Bauer I., Fleckenstein B. Structure of nonintegrated, circular Herpesvirus saimiri and Herpesvirus ateles genomes in tumor cell lines and in vitro-transformed cells. J Virol. 1982 Oct;44(1):295–310. doi: 10.1128/jvi.44.1.295-310.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E., Dietrich W., Fleckenstein B., Bodemer W. Virus-specific transcription in a Herpesvirus saimiri-transformed lymphoid tumor cell line. J Virol. 1983 Nov;48(2):377–383. doi: 10.1128/jvi.48.2.377-383.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E., Schirm S., Dietrich W., Bodemer W., Kolb E., Fleckenstein B. Cloning of Herpesvirus saimiri DNA fragments representing the entire L-region of the genome. Gene. 1983 Nov;25(2-3):281–289. doi: 10.1016/0378-1119(83)90232-9. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Sherr C. J., Sen A., King N., Daniel M. D., Fleckenstein B. Endogenous New World primate type C viruses isolated from owl monkey (Aotus trivirgatus) kidney cell line. Proc Natl Acad Sci U S A. 1978 Feb;75(2):1004–1008. doi: 10.1073/pnas.75.2.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen W. C., Neubauer R. H., Rabin H., Cicmanec J. L. Nonimmune rosette formation by lymphoma and leukemia cells from Herpesvirus saimiri-infected owl monkeys. J Natl Cancer Inst. 1973 Sep;51(3):967–975. doi: 10.1093/jnci/51.3.967. [DOI] [PubMed] [Google Scholar]
- Werner F. J., Bornkamm G. W., Fleckenstein B. Episomal viral DNA in a Herpesvirus saimiri-transformed lymphoid cell line. J Virol. 1977 Jun;22(3):794–803. doi: 10.1128/jvi.22.3.794-803.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]