Abstract
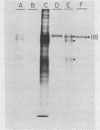
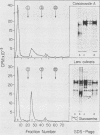
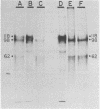
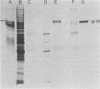
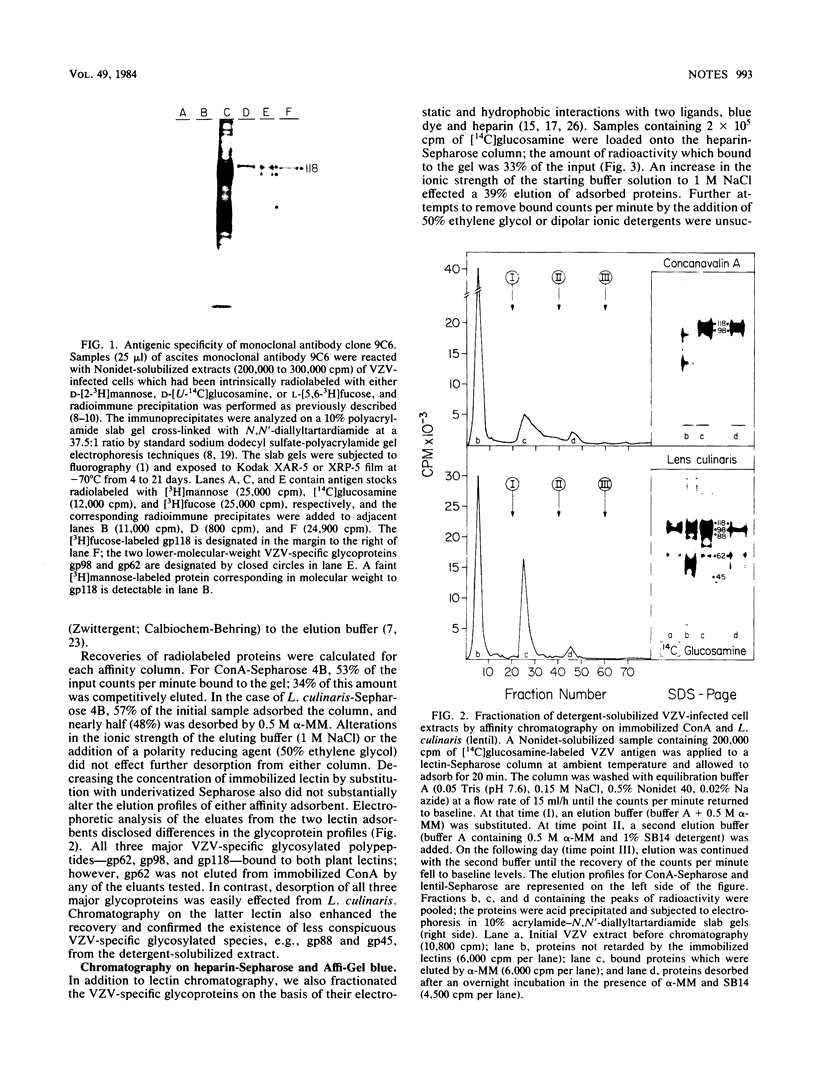
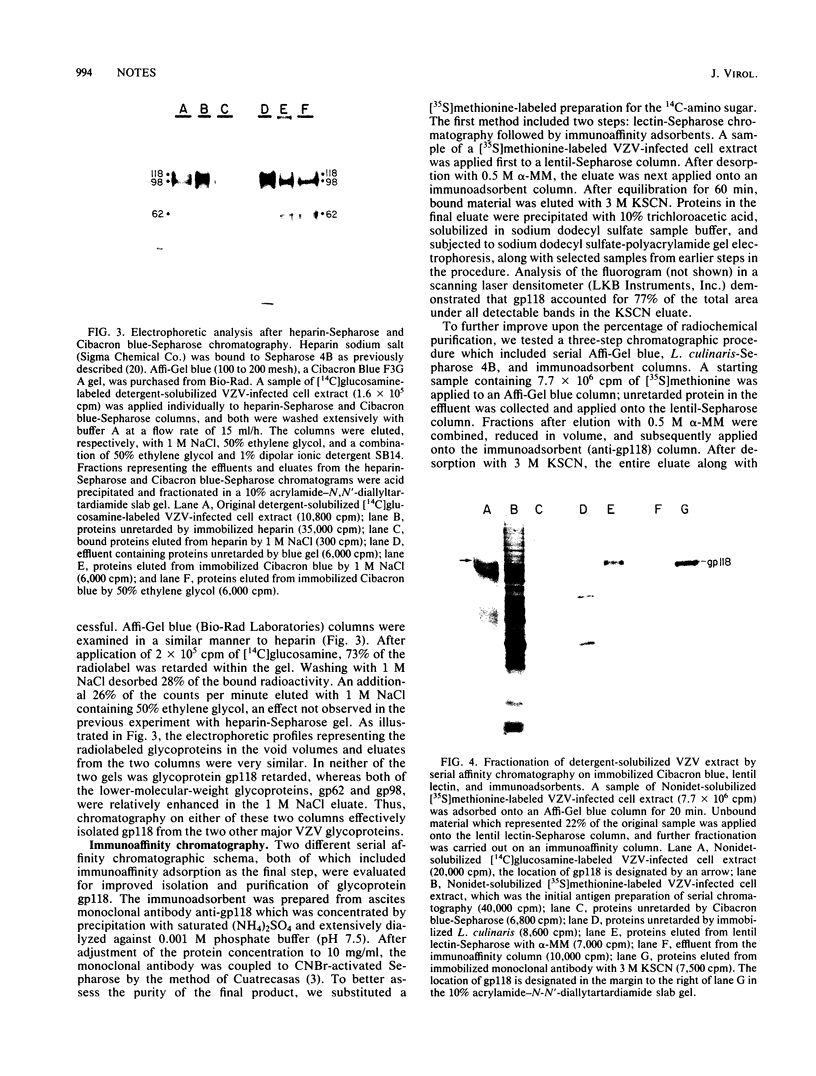
Glycoprotein gp118, one of the major glycosylated proteins specified by varicella-zoster virus, is biologically of great importance since it possesses an epitope which elicits a complement-independent neutralizing antibody response. To purify this glycoprotein from a Nonidet-solubilized extract of varicella-zoster virus-infected cells, we examined its affinity to a variety of ligands, including two lectins--concanavalin A and Lens culinaris, Cibacron blue and heparin, and finally an immunoadsorbent anti-gp118 monoclonal antibody. By serial affinity chromatography on three different columns consisting of, respectively (i) Cibacron blue dye-Sepharose, (ii) L. culinaris-Sepharose, and (iii) anti-gp118 murine monoclonal antibody bound to CNBr-activated Sepharose, we isolated varicella-zoster virus-specific gp118 essentially free of contamination by any other radiolabeled viral or cellular polypeptide. The fold purification was estimated at 1,025 and the percent recovery at 13.6. On the basis of its chromatographic properties, gp118 appeared to contain mainly asparagine-linked, biantennary, complex-type, and hybrid-type oligosaccharides.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S. Fractionation of asparagine-linked oligosaccharides by serial lectin-Agarose affinity chromatography. A rapid, sensitive, and specific technique. J Biol Chem. 1982 Oct 10;257(19):11235–11240. [PubMed] [Google Scholar]
- Eisenberg R. J., Ponce de Leon M., Pereira L., Long D., Cohen G. H. Purification of glycoprotein gD of herpes simplex virus types 1 and 2 by use of monoclonal antibody. J Virol. 1982 Mar;41(3):1099–1104. doi: 10.1128/jvi.41.3.1099-1104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Gonenne A., Ernst R. Solubilization of membrane proteins by sulfobetaines, novel zwitterionic surfactants. Anal Biochem. 1978 Jun 15;87(1):28–38. doi: 10.1016/0003-2697(78)90565-1. [DOI] [PubMed] [Google Scholar]
- Grose C., Edwards D. P., Friedrichs W. E., Weigle K. A., McGuire W. L. Monoclonal antibodies against three major glycoproteins of varicella-zoster virus. Infect Immun. 1983 Apr;40(1):381–388. doi: 10.1128/iai.40.1.381-388.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose C., Friedrichs W. E. Immunoprecipitable polypeptides specified by varicella-zoster virus. Virology. 1982 Apr 15;118(1):86–95. doi: 10.1016/0042-6822(82)90322-1. [DOI] [PubMed] [Google Scholar]
- Grose C., Friedrichs W. E., Smith G. C. Purification and molecular anatomy of the varicella-zoster virion. Biken J. 1983 Mar;26(1):1–15. [PubMed] [Google Scholar]
- Grose C., Perrotta D. M., Brunell P. A., Smith G. C. Cell-free varicella-zoster virus in cultured human melanoma cells. J Gen Virol. 1979 Apr;43(1):15–27. doi: 10.1099/0022-1317-43-1-15. [DOI] [PubMed] [Google Scholar]
- Grose C. The synthesis of glycoproteins in human melanoma cells infected with varicella-zoster virus. Virology. 1980 Feb;101(1):1–9. doi: 10.1016/0042-6822(80)90478-x. [DOI] [PubMed] [Google Scholar]
- HOPE-SIMPSON R. E. THE NATURE OF HERPES ZOSTER: A LONG-TERM STUDY AND A NEW HYPOTHESIS. Proc R Soc Med. 1965 Jan;58:9–20. doi: 10.1177/003591576505800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L. A. Lectin affinity chromatography of Sindbis and Rous sarcoma virus glycopeptides and oligosaccharides. J Virol Methods. 1982 May;4(4-5):283–295. doi: 10.1016/0166-0934(82)90075-1. [DOI] [PubMed] [Google Scholar]
- Kamata T., Takaki K., Hinuma Y., Watanabe Y. Protein kinase activity associated with Epstein-Barr virus-determined nuclear antigen. Virology. 1981 Sep;113(2):512–520. doi: 10.1016/0042-6822(81)90179-3. [DOI] [PubMed] [Google Scholar]
- Kornfeld K., Reitman M. L., Kornfeld R. The carbohydrate-binding specificity of pea and lentil lectins. Fucose is an important determinant. J Biol Chem. 1981 Jul 10;256(13):6633–6640. [PubMed] [Google Scholar]
- Leatherbarrow R. J., Dean P. D. Studies on the mechanism of binding of serum albumins to immobilized cibacron blue F3G A. Biochem J. 1980 Jul 1;189(1):27–34. doi: 10.1042/bj1890027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson S., Jeansson S., Lycke E. Unusual lectin-binding properties of a herpes simplex virus type 1-specific glycoprotein. J Virol. 1981 May;38(2):564–570. doi: 10.1128/jvi.38.2.564-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross L. J. Comparison of antigenic glycoproteins and glycoprotein receptors of concanavalin A isolated from duck embryo cells infected with Marek's disease virus and a herpes virus of turkeys (strain FC126). J Gen Virol. 1974 Sep;24(3):549–562. doi: 10.1099/0022-1317-24-3-549. [DOI] [PubMed] [Google Scholar]
- Scott J. V., Stowring L., Haase A. T., Narayan O., Vigne R. Antigenic variation in visna virus. Cell. 1979 Oct;18(2):321–327. doi: 10.1016/0092-8674(79)90051-5. [DOI] [PubMed] [Google Scholar]
- Shemer Y., Leventon-Kriss S., Sarov I. Isolation and polypeptide characterization of varicella-zoster virus. Virology. 1980 Oct 15;106(1):133–140. doi: 10.1016/0042-6822(80)90228-7. [DOI] [PubMed] [Google Scholar]
- Shiraki K., Okuno T., Yamanishi K., Takahashi M. Polypeptides of varicella-zoster virus (VZV) and immunological relationship of VZV and herpes simplex virus (HSV). J Gen Virol. 1982 Aug;61(Pt 2):255–269. doi: 10.1099/0022-1317-61-2-255. [DOI] [PubMed] [Google Scholar]
- Thompson S. T., Stellwagen E. Binding of Cibacron blue F3GA to proteins containing the dinucleotide fold. Proc Natl Acad Sci U S A. 1976 Feb;73(2):361–365. doi: 10.1073/pnas.73.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLER T. H., WITTON H. M., BELL E. J. The etiologic agents of varicella and herpes zoster; isolation, propagation, and cultural characteristics in vitro. J Exp Med. 1958 Dec 1;108(6):843–868. doi: 10.1084/jem.108.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyn-Jones A. P., Kaaden O. R. Induction of virus-neutralizing antibody by glycoproteins isolated from chicken cells infected with a herpesvirus of turkeys. Infect Immun. 1979 Jul;25(1):54–59. doi: 10.1128/iai.25.1.54-59.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]