Abstract
The “cut” mutants of Schizosaccharomyces pombe are defective in spindle formation and/or chromosome segregation, but they proceed through the cell cycle, resulting in lethality. Analysis of temperature-sensitive alleles of cut11+ suggests that this gene is required for the formation of a functional bipolar spindle. Defective spindle structure was revealed with fluorescent probes for tubulin and DNA. Three-dimensional reconstruction of mutant spindles by serial sectioning and electron microscopy showed that the spindle pole bodies (SPBs) either failed to complete normal duplication or were free floating in the nucleoplasm. Localization of Cut11p tagged with the green fluorescent protein showed punctate nuclear envelope staining throughout the cell cycle and SPBs staining from early prophase to mid anaphase. This SPB localization correlates with the time in the cell cycle when SPBs are inserted into the nuclear envelope. Immunoelectron microscopy confirmed the localization of Cut11p to mitotic SPBs and nuclear pore complexes. Cloning and sequencing showed that cut11+ encodes a novel protein with seven putative membrane-spanning domains and homology to the Saccharomyces cerevisiae gene NDC1. These data suggest that Cut11p associates with nuclear pore complexes and mitotic SPBs as an anchor in the nuclear envelope; this role is essential for mitosis.
INTRODUCTION
Accurate chromosome segregation requires proper assembly and function of a mitotic spindle. The spindle is constructed from microtubules (MT)1 whose polymerization is nucleated by the centrosome (reviewed in Kellogg et al., 1994), which is known in fungi as the spindle pole body (SPB) (reviewed in Snyder, 1994). Although these two organelles are structurally distinct, genetic and biochemical approaches have identified several common components of centrosomes and SPBs, including γ-tubulin (reviewed in Kellogg et al., 1994), CDC31/centrin (reviewed in Schiebel and Bornes, 1995), and p34cdc2 (Bailly et al., 1989; Raibowol et al., 1989). Thus, analyses of SPBs have been informative about centrosomes in general.
Recent work has demonstrated that the SPB of Schizosaccharomyces pombe is a dynamic organelle, undergoing significant changes in morphology and cellular localization as cells progress through their growth and division cycle (Ding et al., 1997). The nature of these changes distinguishes the fission yeast centrosome from that of other organisms. For example, the SPBs of the budding yeast Saccharomyces cerevisiae duplicate in G1 and remain in the nuclear envelope through the entire cell cycle (Byers, 1981; Winey and Byers, 1993). The fission yeast SPB, on the other hand, resides in the cytoplasm through most of interphase, where it duplicates during late G2. As the cell enters mitosis, the nuclear envelope invaginates beneath the SPB and forms an opening, or fenestra, into which the duplicated SPB settles. Each part of the double SPB initiates intranuclear MTs; then the two parts separate to lie in distinct fenestrae, bound to the polar ends of the spindle MTs. As anaphase proceeds, the nuclear fenestrae close, and the SPBs are extruded back into the cytoplasm. The movement of the SPB in and out of the nuclear envelope during the cell cycle indicates the presence of a membrane-anchoring system, but the mechanism for attaching the SPB to the nuclear envelope has yet to be described.
Here, we provide evidence that the product of the cut11+ gene is an essential component of the anchoring system, at least during mitosis. Loss-of-function alleles show that this gene is essential for bipolar spindle formation, proper chromosome segregation, and cell viability. Temperature-sensitive alleles of this gene were isolated from a screen for mutants that overreplicated their DNA (Broek et al., 1991) but were named cut11ts because they shared the defining phenotype of “cut” cells, or cells untimely torn (Horio et al., 1988). At restrictive temperatures, cut mutants fail in chromosome segregation but proceed through the cell cycle into cytokinesis and septation. This leads to an asymmetric separation of chromosomes, resulting in either aneuploidy or aploidy and cell death (Hirano et al., 1986; Samejima et al., 1993). Several cut mutants have been characterized; their gene products cover a broad range of functions, including components of the spindle pole body (Bridge et al., 1998) and the anaphase-promoting complex (Samejima and Yanagida, 1994a,b), enzymes involved in DNA topology (Hirano et al., 1986; Saka et al., 1994) and replication (Saka et al., 1994), and microtubule-dependent motors (Hagan and Yanagida, 1990). cut11+, on the other hand, appears to be an essential component of the mitotic spindle pole body as well as a component of the nuclear pore complex.
METHODS AND MATERIALS
Strains and Cell Culture
All of the strains used are listed in Table 1. The haploid strains 972,h− and 975,h+ were used as wild type. Cell culture and genetic manipulation were performed using standard techniques (Moreno et al., 1991). Permissive temperature is defined as 25°C for temperature-sensitive (ts) mutants and 32°C for cold-sensitive mutants. Restrictive temperature was generally 36°C for ts mutants and 20°C for cold-sensitive strains. Cell transformations were performed using lithium acetate/sorbitol (Moreno et al., 1991) or lithium acetate/polyethylene glycol (Elble, 1992).
Table 1.
Strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| 37 | leu1-32, ura4-D18, h− | Nurse 513 |
| 38 | leu1-32, ura4-D18, h+ | This study |
| 76 | cut11-1, leu1-32, ura4-D18, h− | This study |
| 80 | cut11-2, leu1-32, ura4-D18, h− | This study |
| 83 | cut11-3, leu1-32, ura4-D18, h− | This study |
| 86 | cut11-4, leu1-32, ura4-D18, h+ | This study |
| 89 | cut11-5, leu1-32, ura4-D18, h− | This study |
| 91 | cut11-6, leu1-32, ura4-D18, h− | This study |
| 160 | cut11∷ura4+, ade6-M210, leu1-32, ura4-D18, pcut11+, LEU2, h− | This study |
| 161 | cut11+/cut1∷ura4+, ade6-M210/M216, leu1−/leu1−, ura4−/ura4−, h+/− | This study |
| 147 | cdc10-V50, ura4-D18, h+ | Nurse 1211 |
| 58 | cdc25-22, h+ | Nurse 143 |
| 110 | nda3-311, leu1-32, h+ | Nurse 780 |
| 128 | nuc2-663, leu1-32, ura4-D18, his2−, pnuc2+, LEU2, h+ | Yanagida NC101 |
| 69 | cut12-1, leu1-32, ura4-D18, h+ | Nurse v6 |
| 317 | cut11:GFP:ura4+, leu1-32, ura4-D18, h− | This study |
| 318 | cut11:GFP:ura4+, leu1-32, ura4-D18, h+ | This study |
| 319 | cut11:GFP:ura4+, cdc25-22, leu1-32, ura4-D18, h− | This study |
| 320 | cut11:GFP:ura4+, cdc10-V50, leu1-32, ura4-D18, h− | This study |
| 321 | cut11:GFP:ura4+, nda3-311, leu1-32, ura4-D18, h− | This study |
| 322 | cut11:GFP:ura4+, nuc2-663, leu1-32, ura4-D18, h− | This study |
The cut11ts strains were first isolated by Broek et al. (1991) with temperature sensitivity defined as a lack of colony formation at 36°C. These strains have been designated cut11–1, -2, -3, -4, -5, and -6 in order of the apparent severity of the phenotypes, based on cell morphology and growth at temperatures from 29 to 36°C. The phenotypes of the cut11ts strains were assessed by growing cells at the permissive temperature (25°C) to early log phase (OD595 < 0.5) and then shifting the culture to the restrictive temperature (36°C).
Microscopy
For immunofluorescence, cells in early-to-mid log phase (OD595 ∼0.3–0.7) were fixed by the double aldehyde method (Hagan and Hyams, 1988). The antibody to tubulin was a mouse monoclonal antibody raised against Drosophila α-tubulin (M. T. Fuller, Stanford University), and the antibody to Sad1p from Hagan and Yanagida (1995). Secondary antibodies were either rhodamine- or fluorescein-labeled goat anti-mouse or goat anti-rabbit immunoglobulin (Jackson Laboratories, Bar Harbor, ME) and were used as suggested by the manufacturer. DNA was visualized with 4,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Sigma, St. Louis, MO) (Moreno et al., 1991).
For live-cell light microscopy, cells were grown to early-to-mid log phase, concentrated by centrifugation in an Eppendorf microfuge for 3 sec, and resuspended in YES medium (Moreno et al., 1991). A 1-μl sample of these cells was placed on a 1.5% agar/YES pad on a microscope slide and covered with a glass coverslip, and images were collected on a Zeiss fluorescence microscope with an Empix charge-coupled device camera and MetaMorph imaging software (Universal Imaging, West Chester, PA).
For electron microscopy, cells were grown in liquid culture to early-to-mid log phase, shifted to the restrictive temperature for 4 h, and processed for electron microscopy and three-dimensional reconstruction as described in Ding et al. (1993). Immunoelectron microscopy was done as described in Ding et al. (1997) with antibodies against the green fluorescent protein (GFP) (a generous gift of J. Kahana, Harvard University).
Cloning and Sequencing
The cut11+ cDNA was cloned from an S. pombe cDNA library (a generous gift of Drs. C. Norbury and B. Edgar, I.C.R.F., London, England) in the pREP3 vector (Maundrell, 1993) by complementation of the cut11–1 temperature-sensitive allele. Rescue was assessed by growth at 36°C in the presence of 5 μg/ml thiamine, which reduces the level of expression from this vector (Forsburg, 1993). One transforming plasmid, pREP49, was determined to contain the complete cut11+ gene by several criteria. First, it gave complete rescue of all six cut11ts alleles and the cut11::ura4+ stain (see Construction of the cut11-null Allele). Second, genomic clones were also isolated from libraries (generously provided by Dr. A. Carr [Barbet et al., 1992]) probed with the pREP49 insert, and these plasmids also rescued the cut11ts and cut11::ura4+ alleles. Third, this plasmid spontaneously integrated as a single copy, and backcrosses of this integrant to cut11ts strains mapped the integrated plasmid to the cut11+ locus, with a resolution of ∼0.2 cM, (∼1 kb in S. pombe [Hayles and Nurse, 1989]). Southern blot analysis with probes from the pREP49 insert confirmed that the plasmid had integrated at its homologous locus.
DNA sequencing and cloning were performed using standard protocols (Sambrook et al., 1989). Comparisons of the sequences from pREP49 and genomic clones indicated that there is a single open reading frame (ORF) with no introns and a polyadenylation site 33 bp downstream from the termination codon in the cut11+ gene (GenBank accession number AF079307). Part of the cut11+ ORF (bases 912-1803) has been sequenced by the S. pombe genome project (see Gene Mapping).
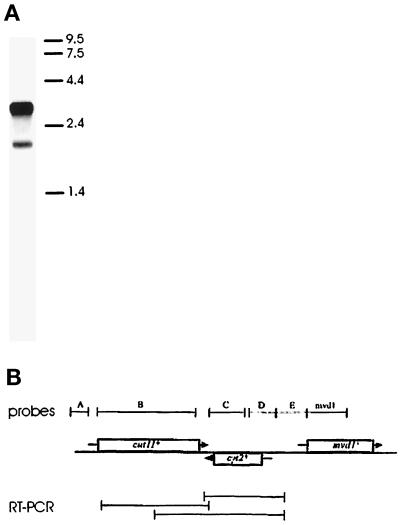
Northern blots and reverse transcription followed by PCR (RT-PCR) were performed with poly(A+) fractions of RNA isolated from wild-type cells (Sambrook et al., 1989). The Northern blots were done using formaldehyde-agarose gels with 1 μg per lane of RNA and were probed with 32P-labeled DNA under high-stringency conditions. The probes were generated by PCR from wild-type genomic DNA (A, ctcctttaatatctattaagtcgtgcg/caacaattccaaaggtagtaacag; B, atggtcatgttaaggactagttttcc/ggg ggagttgcttttgtattctcg; C, gatttgttcacttgctattttgtg/caacaattccaaaggtagtaacag; D, gttcagtaggagcattgtgg/gaaagctaaaaaggaacgaagg; E, ttagctttcttcttttgtaaagttg/aacagttaagtatggtcgaatcc; mvd1, atggacaaaaaggtttatcaatg/ggtagaagatgcaactgtagc) (see Figure 7B).
Figure 7.
A single cut11+ gene is expressed as two mRNAs. (A) Northern blot prepared with poly(A+) RNA from wild-type cells and probed with a DNA fragment corresponding to the cut11+ open reading frame (probe B). A major band of 3.7 kb and a minor band of 2.2 kb are detected. The mobilities of RNA markers are indicated on the right, in kb. (B) Diagram of the gene organization around the cut11+ locus. The cut11+ ORF and its orientation on the chromosome are indicated with the corresponding box and arrow. Two neighboring genes, cyt2+ and mvd1+, have been identified on the basis of their similarity to sequences found in S. cerevisiae. The relative positions of probes used for Northern blots (top) and the products from RT-PCR (bottom) are also shown.
RT-PCR was performed with Thermus aquaticus DNA polymerase (Taq; Promega, Madison, WI) following the manufacturer’s specifications. The substrate consisted of RNA samples that had been exhaustively treated with RNase-free DNase I (Promega) to insure that amplification was from the RNA and not from contaminating genomic DNA. Negative control reactions were done with single primers or with no added nucleic acids. The primer combinations used were gatttgttcacttgctattttgtg/caacaattccaaaggtagtaacag (A), atggtcatgttaaggactagttttcc/gaaagctaaaaaggaacgaagg (B), and cggttaatgaaggtggaatttctg/cacatacagctcaccaactttcg (C). A second nested PCR was done with Taq polymerase on 1 μl of a 1:1000 dilution of the A reaction with primers cggttaatgaaggtggaatttctg/gcgtgtaactcctaaactccg. The products were size fractionated by electrophoresis on agarose gels.
Construction of the cut11-null Allele
A cut11+-null strain was constructed using a single-step gene replacement protocol with flanking regions of the cut11+ gene and ura4+ as the selectable marker. First, the 1.8 kb HindIII fragment containing the ura4+ gene (Grimm et al., 1988) was subcloned into the SmaI site of the bacterial cloning vector pSPORT1 (Life Technologies–Bethesda Research Laboratories, Gaithersburg, MD), creating pSPORT1-URA4. An 800 bp piece of DNA corresponding to the sequence from nucleotide −770 to the first 30 bp of the cut11+ open reading frame was amplified from a genomic clone by PCR with a cut11+ primer (gtaggaagttattcaacata) and the vector primer M13-reverse (agcggataacaatttcacacagg) and then directionally cloned into the SphI and BamHI sites, 5′ of ura4+ in pSPORT1-URA4. A 1.7 kb piece of DNA corresponding to the 3′-flanking sequence of the cut11+ ORF was amplified by PCR with a cut11+-specific primer (cctaagtcatcctataaggt) and the vector primer M13-forward (cccagtcacgacgttgtaaaacg); the resulting product was directionally cloned into a blunted AgeI site and the KpnI site, 3′ of the ura4+ in pSPORT1-URA4. The resulting plasmid was then digested with SphI and HindIII to excise the cut11-ura4+ cassette, which was used to transform a Ura− diploid strain (ade6-M210/ade6-M216, leu1–32/leu1–32, ura4-D18/ura4-D18, h+/h−). Transformants with a Ura+ phenotype were identified and sporulated by nitrogen starvation, and 35 tetrads were analyzed. Only Ura− colonies were produced, indicating the cut11::ura4+ allele was lethal. Homologous integration was confirmed by PCR and Southern blot analyses of the transformed diploid strain. This strain was transformed with the pREP49 plasmid, and Leu+, Ura+ colonies subsequently were identified. Stability tests (Moreno et al., 1991) indicated that growth of the cut11::ura4+ strain depended on the presence of pREP49.
Construction of the Integrated cut11-GFP Strain
A plasmid vector containing the green fluorescent protein (S65T allele [Heim et al., 1995]), followed in frame by three tandem copies of the Pk1 epitope tag (Southern et al., 1991), and the ura4+ gene was generously provided by B. Carson and C. Troxell (University of Colorado). PCR was used to amplify a continuous ∼3.5 kb fragment containing the Pk1 and GFP tags together with the ura4+ gene. The amplification reaction was performed with the Pfu DNA polymerase (Stratagene, La Jolla, CA) under conditions that would minimize errors introduced by the reaction, using the manufacturer’s specifications. The 5′ oligonucleotide primer consisted of 71 bp corresponding to the sense strand 3′-end of the cut11+ ORF and 22 bp in-frame corresponding to the Pk1 sequence (underlined) (ctcaacttaacttatctccaaggatagagcgtcgctgctgggtattgtttcgagaatacaaaagcaactccgagctcatgggtattcctaacc). The 3′ oligonucleotide primer consisted of 70 bp corresponding to the antisense strand of cut11+ and 26 bp corresponding to the 3′-end of the ura4+ fragment (underlined) (ggatgcgtgtatatcgttggactaacgaacatttttcacaaaatagcaagtgaacaaatcccctctcttcctgttccaacaccaatgtttataacc). The GFP-Pk1-ura4+ PCR product was used directly to transform cells (leu1–32, ura4-D18, h−), and Ura+ colonies were selected for further analysis. A single integration of the GFP-Pk1-ura4+ fragment was confirmed by observing 2:2 segregation of the Ura+ phenotype in tetrads when the transformants were backcrossed with a ura4-D18–marked wild-type strain. Colony PCR was performed on these transformants using combinations of two cut11+ primers 5′ of the integration site (taacttatctccaaggatagagcg; gctgctgggtattgtttcgag) and either a cut11+ primer 3′ of the integration site (caacaattccaaaggtagtaacag) or a GFP primer (tcagggatcgtctttaaggctttg). Three independent cut11:GFP:ura4+ strains were isolated, and each gave identical results in backcrosses, PCR, and fluorescence microscopy.
Gene Mapping
The cut11+ gene was mapped to the left arm of chromosome I by probing filters containing ordered arrays of cosmids with the cut11+ sequence from pREP49. A cosmid library was probed (Hoheisel et al., 1993) using standard high-stringency hybridization techniques (Sambrook et al., 1989), and the cut11+ gene was mapped to NotI fragment F. Subsequently, the 3′ 912 bp of the cut11+ ORF through the 3′-end of the genomic clones used here were sequenced by the S. pombe genome project (cosmid c24C9; accession number Z98601: http://www.sanger.ac.uk/Projects/S_pombe/).
RESULTS
Temperature-sensitive alleles of cut11+ were isolated as mutants that would diploidize or die when grown at the restrictive temperature of 36°C (Broek et al., 1991). Genetic analysis showed that six such mutants were linked to within ∼0.1 cM (<500 bp in fission yeast) and are therefore likely to represent alleles of the same gene. No interallelic complementation was observed among these alleles, and all of these strains were rescued by the same plasmid, pREP49 (see below). These strains will be referred to collectively as cut11ts, although subsequent analyses demonstrated that each of them is distinct, on the basis of their growth rates at intermediate temperatures, morphologies at restrictive temperatures, and genetic interactions with other mutant loci. Analysis of heterozygous cut11ts/wt strains demonstrated that all six cut11ts alleles are recessive.
cut11ts Cells Form Aberrant Mitotic Spindles and Fail to Segregate DNA at the Restrictive Temperature
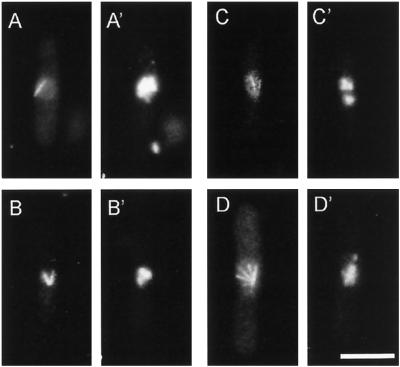
After 3 or more hours at the restrictive temperature, cut11ts strains become lumpy and branched and have asymmetric morphologies, like those previously described for other cut mutants (Hirano et al., 1986; Samejima et al., 1993). To determine whether the cut11ts strains developed defective spindles similar to those described for other cut mutants, we grew the six cut11ts alleles at 36°C for 4 h and then fixed and prepared the cells for fluorescence microscopy with antibodies to tubulin and DNA-specific staining with DAPI (Figure 1). Microtubules of mitotic cells were organized in diverse, abnormal bundles, including tapered shafts (Figure 1A), V shapes (Figure 1B), and fans (Figure 1, C and D). DAPI staining revealed that the DNA failed to segregate in >90% of the cells (n = 425) but remained clustered near one end of a single shaft or at the focal point of multiple microtubule bundles (Figure 1, A′–D′). Wild-type cells show a straight bar of microtubules, with symmetric division of DNA (for examples, see Hagan and Hyams [1988], their Figures 1 and 2). Consistent with the defining phenotype of cut mutants, the cut11ts cells underwent cytokinesis and septation regardless of failed chromosome segregation, leading to aneuploidy and cell death. Interphase microtubule arrays, on the other hand, looked normal (our unpublished results). Images for cut11–2 are shown in Figure 1, but all of the alleles showed similar phenotypes.
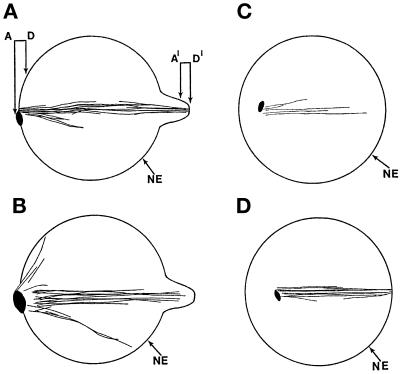
Figure 1.
cut11ts cells have aberrant spindles and fail to segregate their chromosomes when grown at the restrictive temperature. cut11–2 cells were grown at 36°C for 4 h and then fixed and stained for tubulin (A–D) and DNA (A′–D′). (A and A′) A mitotic spindle shaped like a taper shaft with one area of unsegregated DNA. (B and B′) A V-shaped spindle with the DNA near the focal point of the microtubules. (C and D) Cells with multiple microtubule bundles radiating from a single focal point, giving a fan-shaped spindle. (C′ and D′) The DNA segregated into two unequal masses. Bar, 5 μm.
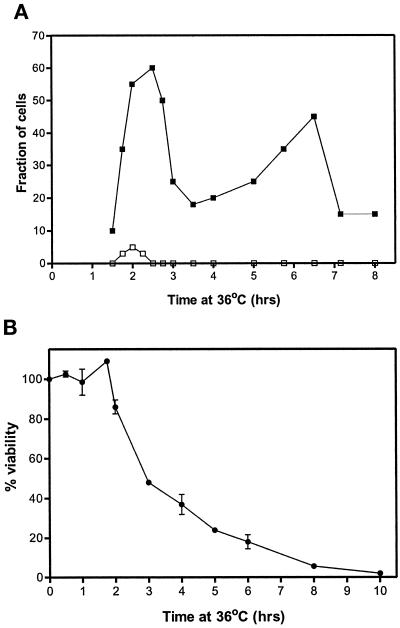
To determine whether the defective mitosis apparent in cut11ts mutants was the cause of the lethality observed at the restrictive temperature, we grew cells at the permissive temperature to early log phase, synchronized the cells in early G2 by centrifugal elutriation, shifted them to the restrictive temperature, and analyzed for spindle morphology (Figure 2). Before their first mitosis (t < 1.5 h), the microtubule arrays and chromatin distributions were normal. Mutant phenotypes, like those shown in Figure 1, became apparent after 1.5 h, when the cells attempted mitosis. Greater than 95% of the cells had an abortive mitosis and then septated and re-entered interphase. The timing of these events suggests that progression through the cell cycle is not seriously affected despite failed spindle function. An abrupt loss of viability after 2 h of growth at the restrictive temperature was observed, coinciding with the appearance of abnormal spindle morphology (Figure 2B). Together, these data suggest that lethality occurs as a result of an aberrant mitosis.
Figure 2.
The appearance of aberrant spindles and of lethality coincide in cut11–1 cells grown at the restrictive temperature. Aliquots of synchronized cells were removed at the times indicated, fixed, and stained for tubulin and DNA (A) or assayed for viability by determining the percent that retained the ability to form colonies on plates incubated at the permissive temperature (B). The fraction of cells with abnormal mitosis is indicated with ▪, and normal mitosis is shown with □ (A). Abnormal mitoses were cells with a spindle morphology similar to that shown in Figure 1. Approximately 300 cells were assayed for each data point. Viability was assessed by the ability to form colonies at the permissive temperature; the means and SD from these trials are indicated (B). The viability of the cells taken immediately from the gradient was defined as 100%. Similar results were obtained for cut11–2.
cut11ts Cells Fail to Anchor the Spindle Pole Body in the Nuclear Envelope and to Form a Bipolar Spindle
Electron microscopy and computer-assisted three-dimensional reconstruction were used to describe the phenotype of the cut11ts spindles and SPBs further. cut11–2 cells were grown at the restrictive temperature for 4 h and prepared for electron microscopy and three-dimensional reconstruction as described in MATERIALS AND METHODS. Nine mutant spindles were reconstructed, four in their entirety. Their fine structures show some heterogeneity, but a phenotypic pattern emerges. Samples of the electron micrographs taken from serial sections of one such cell are shown in Figure 3. These micrographs are from the two ends of the spindle diagrammed in Figure 4A; they are labeled A–D for one pole and A′–D′ for the opposing pole. An SPB is apparent at one pole, although its orientation relative to the spindle MTs is oblique, compared with wt (see Ding et al., 1993) (Figures 3 and 4). The microtubules at the opposite “pole” appear to end in a herniation of the nuclear envelope, and no identifiable SPB is present (Figures 3, A′–D′, and 4).
Figure 3.
cut11–2 cells fail to form a symmetric bipolar spindle or attach SPB(s) to the nuclear envelope. Cells were grown at the restrictive temperature for 4 h and prepared for electron microscopy. Serial sections were imaged, allowing the visualization of the nucleoplasm (N) and both ends of the spindle (A–D and A′–D′). Microtubules (MT) first appear in B and disappear after C′. The nuclear envelope (NE) and the SPB are also indicated. The three-dimensional reconstruction of this spindle is shown as a planar axial projection (see Figure 4A). Bar, 0.1 μm. Arrow, cluster of microtubules.
Figure 4.
Three-dimensional models of cut11–2 spindles reveal a monopolar spindle after growth at the restrictive temperature for 4 h. In each reconstruction the nuclear envelope (NE) is indicated, and the spindle pole bodies are shown as black oval spots. (A) Computer-generated reconstruction of the spindle shown in part in Figure 3. The relative positions of the panels in Figure 3 are indicated. (B–D) Three additional reconstructions.
Computer-generated models based on the complete reconstruction of this spindle and of three others are shown in Figure 4. Figure 4A shows two MT bundles emanating from one pole; this configuration probably corresponds to the V-shaped spindles observed by immunofluorescence (Figure 1B). There is a dimple in the nuclear envelope where the other SBP would be expected. MTs project from this point, but there is no visible SPB structure associated with these MTs. Figure 4B shows three bundles of MTs projecting from a single SPB, probably corresponding to a star-shaped spindle similar to that shown in Figure 1D. Unlike the model in Figure 4A, no MTs emanate from a second pole, although a dimple in the nuclear envelope is present. The third example (Figure 4C) shows a spindle that contains only five MTs, all emanating from one SPB within the nucleus. Complete serial sections demonstrated that this SPB was not attached to the nuclear envelope; it is, therefore, interpreted as free floating in the nucleoplasm. Despite some variation among the cut11ts spindle reconstructions, they all appear to have a single active SPB, which may or may not be free floating in the nucleoplasm. These observations suggest that the SPBs in cut11ts cells fail to anchor properly in the nuclear envelope and at least one SPB fails to mature properly. The data presented in Figures 3 and 4 suggest, however, that nuclear MT growth can be initiated in the absence of normal SPBs in cut11ts cells.
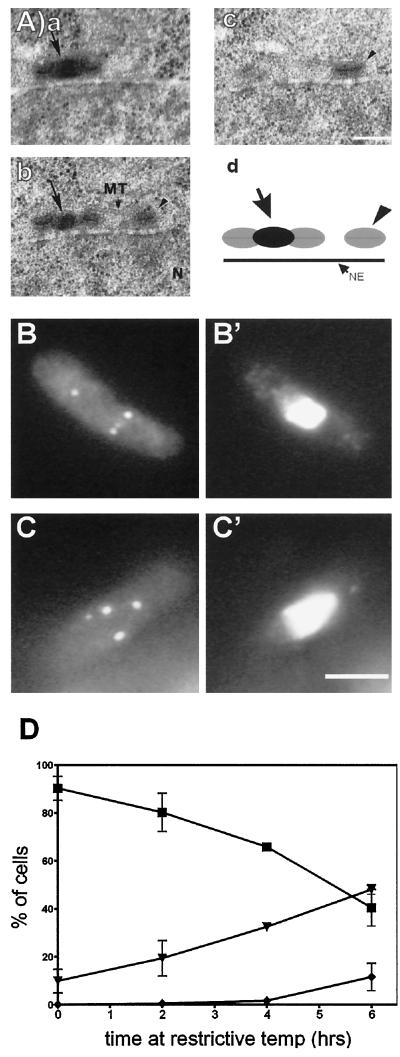
One cut11ts spindle reconstruction showed evidence of overreplication of the SPBs at the restrictive temperature (Figure 5A). Serial sections of this sample revealed one SPB complex that was duplicated but unseparated (Figure 5A, a and b) and an additional SPB lying beside it (Figure 5A, c). The duplicated SPBs look indistinguishable from wild-type SPBs (Ding et al., 1997), but the unduplicated SPB shows no evidence of a bridge structure on any portion of its surface. The position of these SPBs relative to each other and to the nuclear envelope is diagrammed in Figure 5A, d.
Figure 5.
cut11ts cells continue to replicate their SPBs when grown at the restrictive temperature. Cultures of cut11–1 were grown at the permissive temperature through early log phase and then shifted to the restrictive temperature and fixed for immunofluorescence with Sad1p antibodies or prepared for electron microscopy. (A, a–c) Electron micrographs of serial sections of a cut11–1 cell in interphase that had been grown at the restrictive temperature for 4 h. The nucleoplasm (N) and a cytoplasmic MT are marked. An arrow indicates the bridge between the two parts of the replicating SPB, and the arrowhead indicates a second SPB. Bar, 0.1 μm. (d) A diagrammed reconstruction. The duplicated SPBs appear to be in a late G2 configuration (Ding et. al., 1997). (B and C) Representative cells from samples stained for Sad1p at 4 and 6 h after the shift to the restrictive temperature, respectively. (B’ and C’) The same cells stained for DNA with DAPI. (D) Graph of the number of SPBs per cell, as revealed by Sad1p staining, after various times at the restrictive temperature. The fraction of cells with a single SPB (▪), two SPBs (▾), or three or more SPBs (♦) is given as a function of time. Three independent cultures of cut11–1 were analyzed, and at least 100 cells per trial were scored for each time point. Bar, 5 μm.
The data presented in Figures 4 and 5A show three variations of the SPB defect in cut11ts cells: unreplicated, overreplicated, and unanchored SPBs. To determine the extent of each of these phenotypes, we grew cells at the restrictive temperature for various times and prepared the cells for staining with antibodies to Sad1p, a component of the SPB (Hagan and Yanagida, 1995). These data suggest that SPB duplication continues normally in cut11ts cells at the restrictive temperature, because more than one Sad1p-positive spot was apparent in many cells (Figure 5, B and C). Moreover, the number of SPBs per cell increased with time, indicating that the SPBs were probably duplicating, even though all the cells were losing viability (Figure 5D). In some cases, multiple SPBs appeared to be associated with the nuclear envelope (Figure 5B), but in others the situation was more ambiguous (Figure 5A). These data indicate that SPB duplication, as measured by Sad1p staining, continues in cut11ts cells at the restrictive temperature.
cut11ts Alleles Are Synthetically Lethal with cut12ts
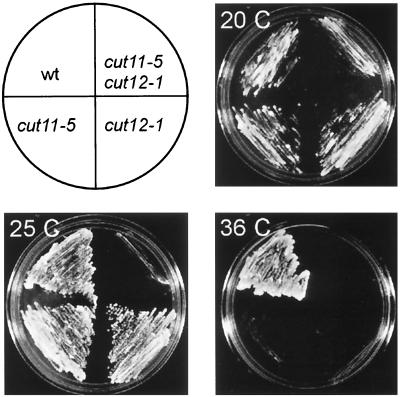
The cytological description of the cut11ts phenotype indicates a defect in SPB function at the restrictive temperature. Additional evidence of a role for cut11+ in SPB function was obtained via an examination of the genetic interactions between cut11ts and other mutants that affect the SPB, particularly cut12–1ts. The phenotype of cut12–1ts at the restrictive temperature is similar to that of cut11ts, and Cut12p has been localized to the nuclear proximal surface of the SPB by immunoelectron microscopy in wild-type cells (Bridge et al., 1998). The six cut11ts alleles were crossed to cut12–1, and the resultant double mutants showed allele-specific, synthetic lethal interactions. The cut11–4,cut12–1 strain failed to germinate at 25°C from the 20 tetrads examined, whereas strains cut11–2,cut12–1 and cut11–3,cut12–1 germinated at 25°C but produced a cut cell in the first or second division (our unpublished results). The cut11–5,cut12–1 and cut11–7,cut12–1 strains showed growth at a reduced temperature (20°C) (Figure 6) but not at 25°C, a temperature that is permissive for either single mutant (Figure 6, bottom left). These results suggest that cut11+ and cut12+ cooperate in the establishment of a functional mitotic SPB.
Figure 6.
cut11ts alleles are synthetically lethal with the SPB mutant cut12–1ts. One example of limited growth is observed between cut11–5 and cut12–1, as shown. Top Left, the position of the parental strains, double mutant, and wild type on each of the plates. Top Right and Bottom, plates with strains grown on rich medium at 20, 25, and 36°C. The results with other allelic combinations are described in the text.
A loss of viability was also observed in double mutants containing cut11ts and the cold-sensitive β-tubulin allele nda3–311 (Toda et al., 1983) (our unpublished results). These double mutants germinated but produced small colonies at 25°C, when compared with the parental strains. Consistent with an hypothesis of an interaction with tubulin, cut11ts strains also showed an increased sensitivity to the microtubule drug thiabendazole relative to that in wild-type cells (our unpublished results).
cut11+ Is Encoded by a Single Gene Expressed as Two mRNAs
The cut11+ gene was cloned by plasmid complementation by screening a cDNA library for plasmids that rescued the growth of cut11–1 at the restrictive temperature. Several criteria confirmed that one plasmid, pREP49, contained a single ORF encoding the cut11+ gene. First, the plasmid was integrated into the genome of strain cut11–1 by homologous integration, as confirmed by Southern blot and PCR analyses (our unpublished results). The LEU2 marker from the integrated pREP49 failed to segregate from the cut11ts phenotype when the strain was crossed to wild-type or other cut11ts strains (>4000 random spores scored). The pREP49 plasmid rescued both a cut11-null allele (described below) and all of the cut11ts strains. A clone containing an identical open reading frame was isolated from a genomic library using the pREP49 insert as a probe for hybridization, and this clone also rescued the cut11ts phenotype. The cut11+ gene was mapped to the left arm of chromosome I by probing ordered cosmid libraries (Hoheisel et al., 1993).
The cut11+ cDNA was used to probe genomic Southern blots to search for related genes in S. pombe and in other organisms. Low-stringency hybridization gave no indication of related genes, either in fission yeast or in other organisms (our unpublished results). Northern blot analysis, however, revealed that the cut11+ gene is expressed as two mRNAs (Figure 7A). The minor message is ∼2 kb, the size expected from the open reading frame found in the cDNA and genomic clones, whereas the more abundant mRNA is ∼3.7 kb. The significance of the larger mRNA is not known, but further analyses demonstrated that this mRNA includes additional sequences 3′ of the cut11+ ORF (Figure 7B). When Northern blots were probed with several DNA fragments that flank the cut11+ ORF, only those probes 3′ of the cut11+ coding sequence hybridized to the larger mRNA (Figure 7B, probes C–E). A probe for the 3′-neighboring gene mvd1+ did not hybridize to the cut11+ messages. These results were confirmed by isolating cDNA clones with RT-PCR with oligonucleotide primers derived from sequences throughout the 3′ region (Figure 7B, RT-PCR). Given the strand specificity conferred by RT-PCR, these cDNA products could only be generated from an RNA molecule consisting of the cut11+ ORF and regions immediately downstream. Furthermore, Northern blots of RNA isolated from the cut11-null expressing the pREP49 plasmid did not detect either of the mRNAs found in wild type but only a transcript corresponding to pREP49 (our unpublished results). Neither the hybridization pattern nor the RT-PCR products gave any indication of alternative splice sites or additional open reading frames outside that found on pREP49. These data suggest that alternative mRNA termination sites account for cut11+ message heterogeneity.
This analysis identified two neighboring genes: cyt2+, which overlaps with cut11+ on the opposite DNA strand, and mvd1+, which lies downstream on the same strand. These genes were named on the basis of significant sequence similarity to the budding yeast genes CYT2 (cytochrome-c1 heme lyase) (Zollner et al., 1992) and MVD1 (mevalonate diphosphate decarboxylase) (Toth and Huwyler, 1996).
cut11ts Encodes a Novel Gene Product with Seven Potential Membrane-Spanning Domains
The pREP49 plasmid contains an insert sequence that encodes a predicted translation product of 601 amino acids with a molecular weight of ∼72 kDa. Within this sequence, there are seven putative membrane-spanning domains in the amino-terminal half and a relatively charged carboxy-terminal end, as predicted by the methods of Rost et al. (1995) (Figure 8). A graph depicting the organization of the membrane-spanning domains reveals a striking symmetry around the second outer loop, a feature that is not common among seven pass-membrane proteins (Figure 8B). No other recognized structural motifs or modification sites are apparent in the Cut11p sequence. Database searches with the BLAST algorithm (Altschul et al., 1990) gave the best match as the S. cerevisiae gene NDC1, with 49% similarity and 22% identity throughout the protein (Winey et al., 1993). There are no regions of particularly high or low similarity between these two proteins, but their overall charge profiles are very similar. Evidence of a functional homology between cut11+ and NDC1 is described below. No other significant similarities were found, but all of the matches found are membrane-spanning proteins of varying types.
Figure 8.
The cut11+ gene encodes a single open reading frame of 601 amino acids. (A) The primary sequence for Cut11p, with the seven predicted membrane-spanning domains highlighted with the gray boxes. This sequence has GenBank accession number AF079307. (B) The topology of Cut11p indicated as either an inner loop, transmembrane helix (labeled 1–7), or outer loop, as predicted by Rost et al. (1995) (http://www.embl-heidelberg.de/predictprotein/predictprotein.html). The probability of assigning each helical transmembrane is as follows: 1, 0.8737; 2, 0.8040; 3, 0.8280; 4, 0.8881; 5, 0.8820; 6, 0.8515; and 7, 0.7780.
cut11ts Mutants Are Partially Rescued by Expression of S. cerevisiae NDC1
Although the similarity and identity between cut11+ and NDC1 are relatively low, there is also considerable phenotypic similarity between cut11ts and ndc1–1cs mutants. In particular, ndc1–1cs cells duplicate the SPB at the restrictive temperature, but the new SPB fails to insert into the nuclear membrane, resulting in a monopolar spindle phenotype (Winey et al., 1993). To examine the relationship between these two genes directly, we cloned NDC1 into the fission yeast vector pREP3X (Maundrell, 1993) and expressed the gene in cut11ts cells. Ndc1p rescued the cut11ts lethality at intermediate temperatures (29 and 32°C) that are lethal to cut11ts but not at the highest restrictive temperature (36°C). This rescue was not observed when the expression from the vector was repressed with thiamine. However, GAL1-CEN1 driven expression of cut11+ in ndc1–1cs cells did not give rescue. Together, these results suggest that cut11+ and NDC1 have some functional homology, both serving to anchor the SPBs to the nuclear envelope.
cut11+ Is an Essential Gene
To determine whether the cut11+ gene is essential for growth, we deleted the entire coding sequence of one copy in a diploid cell with the nutritional marker ura4+. Heterozygous cut11::ura4+/cut11+(ura4−) cells were selected on medium lacking uracil, and transformation was confirmed by Southern blot and PCR analyses (described in MATERIALS AND METHODS). Twenty tetrads from sporulated diploid strains were dissected, and no Ura+ colonies were identified, indicating that the cut11+ gene is essential. To determine the phenotype of the cut11::ura4+ cells, spores were germinated in liquid medium lacking uracil, grown for several hours, and prepared for tubulin immunofluorescence and DNA staining. One-half of the spores did not germinate and are presumed to be cut11+,ura4-, whereas cut11::ura4+ cells had short spindles with unsegregated DNA in the first division, reminiscent of the cut11ts phenotype (see Figure 1A). These data suggest that cut11+ is an essential gene and that the cut11ts mutants represent loss-of-function alleles.
Cut11p Localizes to Nuclear Pore Complexes and Mitotic SPBs
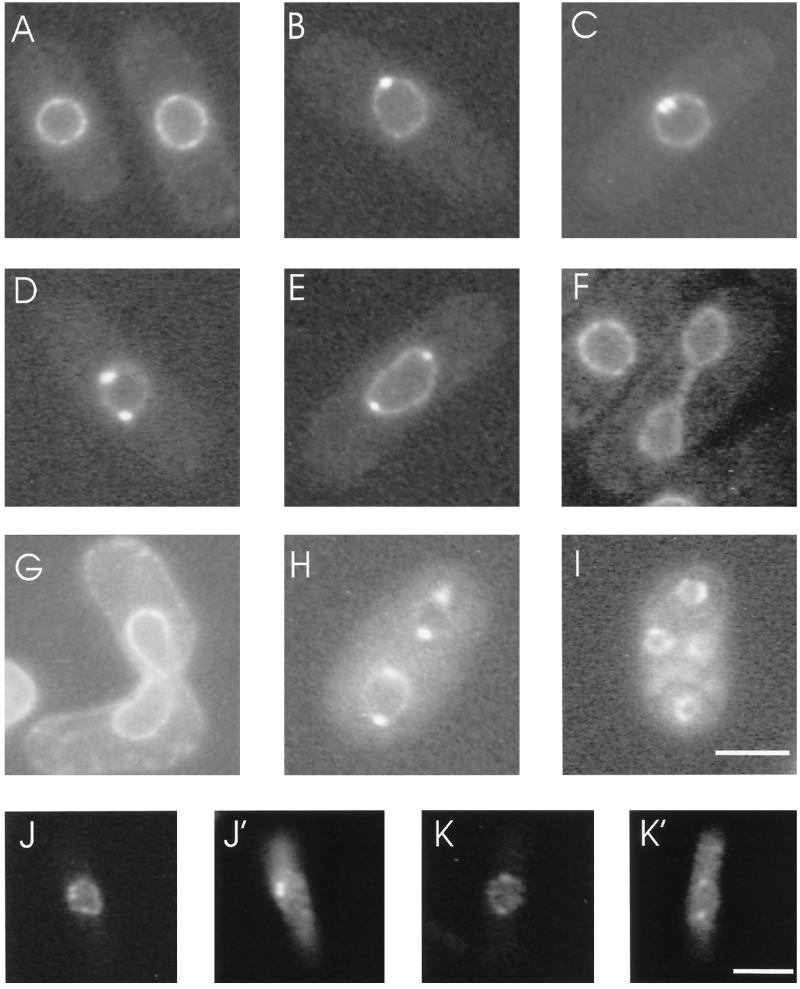
A fusion protein of cut11+ and the GFP was made to examine the localization of Cut11p in living cells as they progressed through the cell cycle. GFP and three tandem copies of the Pk1 epitope tag were integrated into chromosome I at the 3′-end of the cut11+ ORF (see MATERIALS AND METHODS), giving a single copy-tagged allele of cut11+ under the transcriptional control of the endogenous promoter. Homologous integration was confirmed by PCR and tetrad analysis of crosses between the cut11:GFP strain and wild-type cells. Live cells from early log phase cultures were observed by light microscopy, and virtually all cells showed staining of the nuclear envelope (Figure 9). This punctate nuclear envelope pattern is similar to the nuclear pore complex (NPC) straining previously reported for fission yeast (Demeter et al., 1995) and was confirmed as NPC staining by immunoelectron microscopy (see below).
Figure 9.
Cut11p localizes to the nuclear envelope and spindle pole body in live cells. (A) A representative field from an early log phase culture shows nuclear envelope staining in interphase cells. (B–E) SPB staining first appears in prophase (B) and is present in early prometaphase (C), late prometaphase (D), and early anaphase (E). (F) By late anaphase/telophase, the staining is again restricted to the nuclear envelope. (G–I) Cut11-GFPp shows nuclear envelope staining in conjugating cells (G) and mature asci (I) and additional SPB staining during meiotic division (H). (J, J’, K, and K’) Costaining of the Cut11-GFP with Sad1p antibodies is shown: GFP (J and K) and the Sad1p staining (J’ and K’). Bars: A–I and J–K’, 5 μm.
Additionally, a subset of cells in these preparations showed one or two prominent spots at the periphery of the nuclear envelope (Figure 9, B–E and H). When cells from five different cultures (a total of ∼1400 cells) were scored for the presence of bright spots, a single bright spot was relatively rare (0.5 ± 0.3%) compared with the frequency of cells with two spots (8 ± 2%). The ratio of cells with and without spots was similar to the ratio of interphase and mitotic cells in a log phase culture of fission yeast (Mitchison and Nurse, 1985). The predominance of two spots versus one spot is consistent with the association of unseparated and separated mitotic SPBs within the nuclear envelope (Ding et al., 1997). Therefore, the bright spot(s) were interpreted as mitotic spindle pole bodies. A pattern similar to the GFP fluorescence in live cells was observed when Pk1-tagged Cut11p was localized by immunofluorescence using antibodies against Pk1 (our unpublished results).
SPB localization was first confirmed by staining cells for the SPB marker Sad1p (Hagan and Yanagida, 1995). This staining revealed that the spots seen with Cut11:GFP colocalized with Sad1p (Figure 9, J and K vs. J’ and K’).
The localization of Cut11p to the SPB appears from prophase (Figure 9B) through early anaphase (Figure 9E) but not in late anaphase (Figure 9F). The mitotic stages described for images in Figure 9 are inferred from the configuration of the SPBs and the nuclear envelope at specific times as previously reported (Ding et al., 1993, 1997). These stages of mitosis correspond to the time when the SPB is anchored in the nuclear envelope (Ding et al., 1997).
Cut11p has the same localization pattern in meiotic cells as that described for vegetative growth. cut11:GFP cells of the opposite mating type were induced to mate by starving for nitrogen on malt agar plates (Moreno et al., 1991) and were observed at various stages of the meiotic life cycle. All of these cells showed NPC staining. Conjugating cells (Figure 9G) and mature asci (Figure 9I) lacked Cut11p staining at the SPBs, but cells in meiotic divisions showed SPB staining (Figure 9H).
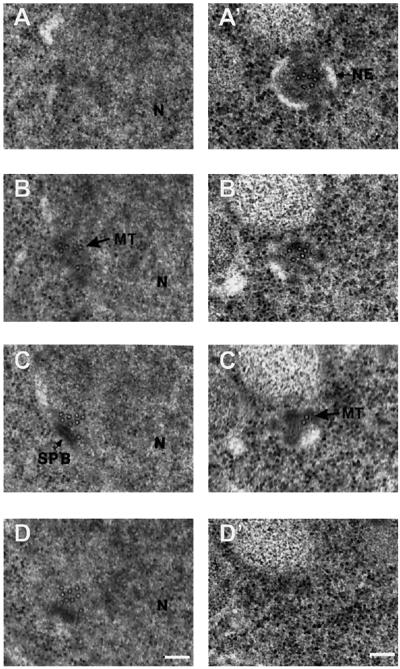
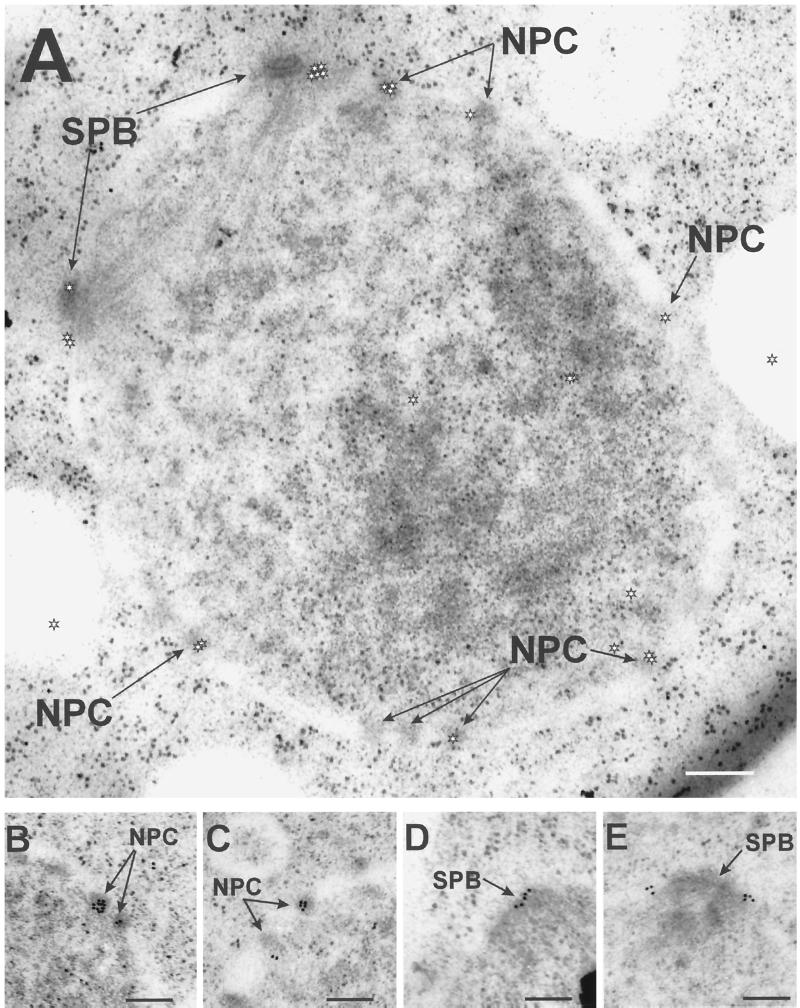
The Cut11p-GFP localization pattern described from light microscopy was confirmed by immunoelectron microscopy with antibodies against GFP (Figure 10). An example of staining around the entire nucleus is shown in Figure 10A, whereas views of individual NPCs (Figure 10, B and C) and SPBs (Figure 10, D and E) are shown at a higher magnification. The gold-labeled secondary antibodies are marked with a star symbol in Figure 10A, because they are difficult to distinguish from ribosomes, given the dynamic range of journal prints. The specificity of the signal was calculated by determining the density of gold-labeled secondary antibody per unit of surface area over NPCs and SPBs and through the entire cell area within the section. Eighty NPCs were scored, of which 38 showed staining, with an average of 2.0 ± 0.4 gold particles per NPC (area = ∼6500 nm2/NPC). NPCs lacking signal are presumed to be below the surface of the section and therefore inaccessible to antibody staining. Six mitotic SPBs were scored, with 5.0 ± 0.8 particles per SPB (area = ∼24,000 nm2/SPB). The signal-to-noise ratio for both SPBs and NPCs was ∼200:1. An interphase SPB, located in the cytoplasm, did not show staining (our unpublished results).
Figure 10.
Cut11:GFPp localizes to the mitotic SPBs and the NPCs by immunoelectron microscopy. The cut11:GFP strain was grown to mid log phase at 32°C and prepared for immunoelectron microscopy with antibodies against GFP and with gold-labeled secondary antibodies. (A) Cross-section through the nucleus showing an early mitotic spindle and several nuclear pore complexes with the gold labeling on several NPCs and the SPBs. The position of the gold particles is marked with a white star, to distinguish them from ribosomes. (B and C) Higher magnification showing staining of two NPCs. (D and E) Higher magnification showing staining around the SPB. Bars, 0.2 μm.
Cut11p Localization to the SPB Is Cell Cycle Dependent
The apparent redistribution of the Cut11p to mitotic SPBs suggested a cell cycle-dependent change in its binding. This possibility was further investigated by constructing double mutants of cut11-GFP with four different mutant strains that arrest at a specific time in the cell cycle when incubated at restrictive temperatures.
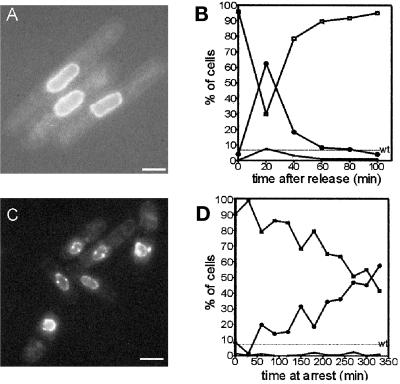
Cells were arrested in late G2 with cdc25–22 grown at 36°C (Nasmyth and Nurse, 1981). After 4 h in arrest, <4% of the cells showed SPB staining, whereas all of the cells had NPC staining. Release to the permissive temperature allows these cells to progress into mitosis, and the frequency of SPB staining peaked at ∼65% 20 min after this temperature shift (Figure 11). One hour after release, most of the cells once again showed only nuclear envelope staining (Figure 11). A second, less prominent peak in SPB staining occurred ∼3.5 h after release from the cell cycle arrest. Thus, the dynamics of the appearance of Cut11p at the SPB in a cdc25–22–mediated cell cycle arrest and release is consistent with its localization to mitotic SPBs. Similar results were obtained when cut11:GFP was expressed in cdc10-V50 cells. This mutant blocks in G1 at the restrictive temperature (Marks et al., 1992). In this case, virtually none of the cells showed SPB localization at the arrest point, but a peak in SPB localization appeared ∼2.5 h after release (our unpublished results).
Figure 11.
Cut11-GFPp localizes to the SPB in a cell cycle-dependent manner. (A and B) Cultures of cut11:GFP,cdc25–22 were grown to mid log phase at the permissive temperature, arrested at the restrictive temperature for 4 h, and then released back to the permissive temperature. (A) cut11:GFP,cdc25–22 cells arrested for 4 h, showing no SPB staining. (B) Graph showing the fraction of cells in the culture with zero (□), one (▴), or two (○) SPBs as a function of the time after release from arrest. (C and D) Cultures of cut11:GFP,nuc2–663 were grown to mid log phase at the permissive temperature and then arrested at the restrictive temperature. (C) cut11:GFP,nuc2-663 cells 4 h after arrest with SPB staining. (D) Graph of the fraction of cells in the culture with zero (□), one (▴), or two (○) SPBs as a function of time at the restrictive temperature. The frequency of two SPBs in wild-type cells is indicated with the dashed line marked wt. Approximately 300 cells were scored for each time point. Bars, 5 μm.
Two additional cell cycle arrest mutants have been used to refine the time at which Cut11p localizes to the SPB within the specific stages of mitosis. Cells arrested at prophase were obtained using the cold-sensitive β-tubulin mutant nda3–311 (Hiraoka et al., 1984). These cells lack microtubules when arrested at 20°C but form a bipolar mitotic spindle within a few minutes of being returned to the permissive temperature. There was a threefold increase in the frequency of SBP staining over wild type when cells were held at the restrictive temperature for 6 h. The SPB staining was lost within 20 min of return to the permissive temperature because the cells passed through mid-to-late anaphase, as assessed by the structure and orientation of the nuclear envelope (our unpublished results).
The temperature-sensitive strain nuc2–663 carries a mutation in the gene for a component of the anaphase-promoting complex; it blocks at the restrictive temperature with an apparent metaphase arrest. These cells contain a short spindle with DNA at an equatorial position (Horio et al., 1988; Yamada et al., 1997). At metaphase, the SPBs are separated by ∼2 μm and embedded in the nuclear envelope (Ding et al., 1997). The Cut11p staining pattern observed in nuc2–663 cells at the restrictive temperature (Figure 11C) showed two spots at opposite sides of the nuclear envelope, as expected for the poles of a metaphase spindle. The frequency of cells with two SPBs increased for several hours in cultures held at the restrictive temperature, peaking at ∼65% of the population (Figure 11).
Cultures of cdc25–22, cdc10-V50, nda3–311, and nuc2–663 grown continuously at the permissive temperature showed Cut11p patterns that were indistinguishable from those described for wild-type cells (our unpublished results). Thus, the distribution of Cut11:GFP in wild-type and cell cycle arrest mutant cells indicates that Cut11p is localized to the nuclear envelope throughout the cell cycle and at the SPBs in mitotic cells. The appearance and subsequent disappearance of Cut11p at the SPBs correspond tightly with the times in mitosis when the SPBs are anchored in the nuclear envelope (Ding et al., 1997).
DISCUSSION
Our work suggests that cut11+ is essential for the formation of a bipolar mitotic spindle because it helps to anchor the spindle poles to the nuclear envelope during cell division. Tubulin immunofluorescence and electron microscopy of cut11ts or null deletion mutant cells grown at the restrictive temperature showed a monopolar spindle with unsegregated chromosomes, demonstrating the necessity of Cut11p for normal spindle formation. The localization of Cut11p tagged with GFP suggests that it associates with nuclear pore complexes and mitotic spindle pole bodies. The redistribution of Cut11p to include SPBs was cell cycle dependent, occurring only between prophase and mid anaphase. The timing of the appearance of Cut11p to and its disappearance from the SPBs corresponds to the time of its entry and exit from the nuclear envelope (Ding et al., 1997). Furthermore, expression of Cut11:GFPp in cell cycle arrest mutant backgrounds revealed that the changes in SPB localization required passage through the cell cycle. The localization of Cut11p to the nuclear envelope may be achieved through the seven putative membrane-spanning domains in the amino-terminal half of the protein, consistent with this protein playing an essential role in the attachment of NPCs and SPBs to fenestrae in the nuclear envelope.
Cell Cycle-specific SPB Anchoring Requires cut11+
Fission yeast, like other fungi, have a “closed” mitosis, a term referring to the lack of nuclear envelope breakdown during cell division. Thus, spindle formation and subsequent chromosome segregation occur within the nucleus (McCully and Rabinow, 1971; Tanaka and Kanbe, 1986; Ding et al., 1993; reviewed in Kubai, 1975). Before formation of the mitotic spindle in fission yeast, mitotic spindle poles move from the cytoplasm into fenestrae in the nuclear envelope, where they remain from prophase through mid anaphase (Ding et al., 1997). Thus, S. pombe requires a cell cycle-specific tethering system that can grab and hold the SPBs in the nuclear envelope as the cells enter mitosis but can release them as mitosis is nearing completion. Although Cut11p staining and the mitotic anchoring of SPBs in the nuclear envelope correlate well, it is curious that late anaphase and meiotic cells engaged in karyogamy do not have Cut11p SPB staining, given that migrating nuclei appear to be led by the SPBs (Chikashige et al., 1994; Hagan and Yanagida, 1997). These observations suggest that the SPB is tethered to the nuclear envelope by two mechanisms, one that functions during mitosis, when the SPBs are positioned in the fenestrae, and another that binds it to the surface of the nuclear envelope in interphase, when the SPB is in the cytoplasm.
The three-dimensional models generated from electron microscopy of serial thin sections provide the most direct evidence that cut11ts cells fail to anchor the SPB into the nuclear envelope at mitosis. In particular, two out of four complete reconstructions showed a single SPB with a monopolar spindle attached free floating in the nucleoplasm. Given that all of the models showed a SPB either in the nuclear envelope or within the nucleus itself, the defect in cut11ts cells is not likely to be the formation of the fenestrae observed in prophase.
Images from Sad1p staining suggest that SPB duplication continues in cut11ts cells at the restrictive temperature. Our understanding of the relationship between the cut11ts phenotype and Sad1p staining, however, remains incomplete. For example, the distribution of cut11ts spindle MTs, seen both by light and electron microscopy, shows that a second SPB fails to nucleate MTs or is completely absent. Therefore, Sad1p staining may mark an early event in SPB duplication, before the establishment of MT-organizing activity, an event that is independent of cut11+ function. Sad1p staining may also be present in the absence of an SPB structure that is recognizable by electron microscopy. Our electron micrographs indicate that some of the nuclear MT-organizing activity in cut11ts cells is no longer associated with an identifiable SPB (Figures 3, A′–D′, and 4). In this context, it is interesting that the single SPB in Figure 5A lacks a bridge structure with which it might attach to a nascent SPB. Perhaps the bridge structure fails to form during SPB duplication in cut11ts cells at the restrictive temperature. We infer that in the absence of a normal association with the nuclear envelope, essential components of a mitotic SPB, such as γ-tubulin, lose their customary binding to the SPB. Perhaps a spindle like that in Figure 4 is the result of one normal SPB (presumably the mother) and of active γ-tubulin that is still associated with the nuclear envelope but not with an SPB. Certainly, interphase γ-tubulin in wild-type S. pombe shows only indirect association with the SPB; although the two are close, they are separated by the nuclear envelope (Ding et al., 1997). A better understanding of the relationship between cut11+, γ-tubulin, and other components of the MT-initiating complex will be essential to understanding SPB function in fission yeast.
cut11+ and NDC1
The protein most similar to Cut11p, on the basis of BLAST sequence alignment (Altschul et al., 1990), is Ndc1p of S. cerevisiae (Winey et al., 1993). There is limited sequence similarity throughout the entire sequence but a striking similarity in the charge profiles, including predicted membrane-spanning domains in the amino-terminal half and a relatively charged carboxy-terminal end of these two proteins.
The phenotypic similarities between cut11+ and NDC1 also suggest functional homology. Like cut11ts, the cold-sensitive mutant ndc1–1 shows a defect in chromosome segregation (Thomas and Botstein, 1986) and a failure in the SPB cycle (Winey et al., 1993); SPB duplication occurs, but the new SPB is not inserted into the nuclear envelope (Winey et al., 1993). Furthermore, like Cut11p, the NDC1 protein localizes to nuclear pore complexes (Winey et al., 1993) and SPBs (Chial and Winey, personal communication). A nonmitotic phenotype has not been observed for either mutant, suggesting either a nonessential function at the NPC or a bias toward isolating mitosis-specific alleles.
The most direct evidence of functional homology between these genes is the partial rescue of the cut11ts phenotype when NDC1 was expressed in these strains. NDC1 is, however, a rather poor substitute for cut11+; complementation was observed only when NDC1 was expressed at high levels and only at intermediate temperatures. High levels of expression of cut11+ in ndc1–1cs cells failed to rescue their cold sensitivity, but this may be caused by the particulars of the ndc1–1cs allele.
cut11+ and the Nuclear Pore Complex
The NPC and the mitotic SPB share a requirement for anchoring to the nuclear envelope (reviewed in Davis, 1995; Doyle and Hurt, 1995). The first indication that the NPC and SPB share components came from the analysis of NDC1 as described above (Winey et al., 1993). In addition, the budding yeast protein Nuf2p has been localized to the SPB but shows two hybrid interactions with the NPC protein Nup1p (Belanger et al., 1991; Osborne et al., 1994). The pattern of localization of Cut11p presented here suggests that it, too, is a component of both SPBs and NPCs.
The function, if any, of Cut11p at the NPCs remains unknown. The simplest model is that Cut11p anchors the NPC to the nuclear membrane in the same way we propose for the SPB. The data presented here, however, indicate that the lethality of cut11ts strains occurs specifically at mitosis, and not during interphase, as might be expected for a nuclear transport defect. The development of a NPC phenotype may, however, require more time at the restrictive temperature than a single passage through the cell cycle. Alternatively, cut11+ may play an essential role in SPB function but be redundant for NPC function.
Regulation of Cut11p Localization
The mechanism by which Cut11p changes its distribution remains unknown. The simplest model is that Cut11p redistribution at mitosis is a matter of accessibility to other components of the SPB. A strong candidate for this type of interaction is Cut12p because 1) it localizes to the SPB surface that is proximal to the nuclear envelope during interphase (Bridge et al., 1998), 2) cut12–1 has a phenotype that is similar to that of cut11ts, and 3) cut12–1 is synthetically lethal with cut11ts in an allele-specific manner.
The presence of two mRNAs for the cut11+ gene suggests an alternative mechanism for regulating the behavior of the protein. The two mRNAs may be produced differentially as a function of time in the cell cycle, although how this would affect Cut11p is not clear, because the larger message appears to arise from the inclusion of 3′-untranslated sequence.
It is also possible that Cut11p itself is modified to affect its localization in mitotic cells. The principal cell cycle regulatory enzyme in fission yeast is the cyclin-dependent kinase cdc2+, and its activity is required for entry into mitosis (Bailly et al., 1989; Alfa et al., 1990; reviewed in Forsburg and Nurse, 1991). There are, however, no putative modification sites for this enzyme in the Cut11p sequence. The polo kinase plo1+ (Ohkura et al., 1995; Lane and Nigg, 1996) and the cdc7+ kinase (Fankhauser and Simanis, 1994; Sohrmann et al., 1998) have also been localized to the SPB or affect SPB behavior. It will be interesting to explore any interactions between these enzymes and cut11+ in the future.
Finally, the fenestrae that form in the fission yeast nuclear envelope during prophase (Ding et al., 1997) may be analogous to nuclear envelope breakdown in metazoa. This process requires signals dependent on calcium ions and MPF activity (Steinhardt and Alderton, 1988; Kao et al., 1990; Sluder et al., 1995). A possible role for calcium is indicated by the importance of the calmodulin gene cam1+ in S. pombe; it is essential for proper spindle structure and chromosome segregation (Takeda and Yamamoto, 1987; Moser et al., 1997). Like Cut11p, Cam1p localizes to the SPBs, as well as to the points of cell growth (Moser et al., 1997).
Our characterization of the cut11+ gene has identified a novel protein product that localizes to both the spindle pole bodies and the nuclear pore complexes of fission yeast. It plays an essential role in the formation of a bipolar spindle and the proper segregation of chromosomes, possibly by anchoring the SPB in the nuclear envelope during mitosis. It remains to be determined whether Cut11p has a similar function for the nuclear pore complex.
ACKNOWLEDGMENTS
We thank Heidi Chial for NDC1 plasmids, ndc1–1cs strains, and helpful discussions and Dr. Sally Passion for the GFP plasmid SPG9. We thank Drs. Susan Forsburg and Shelly Sazer for many helpful discussions. We thank Dr. Cindy Troxell for assistance in mapping and isolating cut11+ genomic clones, Brenda Huneycutt for help in Sad1p staining, and Chad Baxter for help in analyzing the cut11:GFP, cell cycle mutants. We thank Mary Morphew for cut11:GFP immunoelectron microscopy localization. We thank Drs. Heidi Browning, Elisa Stone, and Mark Winey for critically reading the manuscript. This work was supported by National Institutes of Health grants GM-33787 and RR-0592 to J.R.M. and by American Cancer Society grant PF-4035 to R.R.W. J.R.M. is a Research Professor of the American Cancer Society.
Abbreviations used:
- DAPI
4,6-diamidino-2-phenylindole dihydrochloride
- GFP
green fluorescent protein
- MT
microtubule
- NE
nuclear envelope
- NPC
nuclear pore complex
- ORF
open reading frame
- PCR
polymerase chain reaction
- RT
reverse transcription
- SPB
spindle pole body
- ts
temperature sensitive
- wt
wild type
REFERENCES
- Alfa CE, Ducommun B, Beach D, Hyams JS. Distinct nuclear and spindle pole body populations of cyclin-cdc2 in fission yeast. Nature. 1990;347:680–682. doi: 10.1038/347680a0. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic logic alignment search tool. J Mol Biol. 1990;2:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bailly E, Doree M, Nurse P. p34cdc2 is located in both nucleus and cytoplasm; part is centrosomally associated at G2/M and enters vesicles at anaphase. EMBO J. 1989;8:3053–3058. doi: 10.1002/j.1460-2075.1989.tb08581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet N, Muriel WJ, Carr AM. Versatile shuttle vectors and genomic libraries for use with Schizosaccharomyces pombe. Gene. 1992;114:59–66. doi: 10.1016/0378-1119(92)90707-v. [DOI] [PubMed] [Google Scholar]
- Belanger KD, Kenna MA, Wei S, Davis L. Genetic and physical interactions between Srp1 and nuclear pore complex proteins Nup1p and Nup2p. J Cell Biol. 1994;126:619–630. doi: 10.1083/jcb.126.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge A, Morphew M, Bartlett R, Hagan IM. The fission yeast SPB component Cut12 links bipolar spindle formation to mitotic control. Genes Dev. 1998;9:927–942. doi: 10.1101/gad.12.7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broek D, Bartlett R, Crawford K, Nurse P. Involvement of p34cdc2 in establishing the dependency of S phase on mitosis. Nature. 1991;349:388–393. doi: 10.1038/349388a0. [DOI] [PubMed] [Google Scholar]
- Byers B. Cytology of the yeast life cycle. In: Strathern JN, Jones EW, Broach JR, editors. Molecular Biology of the Yeast Saccharomyces. I. Life Cycle Inheritance. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1981. pp. 59–96. [Google Scholar]
- Chikashige Y, Ding DQ, Imai Y, Yammamoto M, Haraguchi T, Hiraoka Y. Telomere-led premeiotic chromosome movement in fission yeast. Science. 1994;264:270–273. doi: 10.1126/science.8146661. [DOI] [PubMed] [Google Scholar]
- Davis LI. The nuclear pore complex. Annu Rev Biochem. 1995;64:865–896. doi: 10.1146/annurev.bi.64.070195.004245. [DOI] [PubMed] [Google Scholar]
- Demeter J, Morphew M, Sazer S. A mutation in the RCC1-related protein pim1 results in nuclear envelope fragmentation in fission yeast. Proc Natl Acad Sci USA. 1995;92:1436–1440. doi: 10.1073/pnas.92.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding R, McDonald KL, McIntosh JR. Three-dimensional reconstruction and analysis of mitotic spindles from the yeast, Schizosaccharomyces pombe. J Cell Biol. 1993;120:141–151. doi: 10.1083/jcb.120.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding R, West RR, Morphew M, Oakley BR, McIntosh JR. The spindle pole body of Schizosaccharomyces pombe enters and leaves the nuclear envelope as the cell cycle proceeds. Mol Biol Cell. 1997;8:1461–1479. doi: 10.1091/mbc.8.8.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle V, Hurt EC. Genetic approaches to nuclear pore structure and function. Trends Genet. 1995;6:235–241. doi: 10.1016/s0168-9525(00)89057-5. [DOI] [PubMed] [Google Scholar]
- Elble R. A simple and efficient procedure for transformation of yeasts. Biotechniques. 1992;13:18–20. [PubMed] [Google Scholar]
- Fankhauser C, Simanis V. The cdc7 protein kinase is a dosage dependent regulator of septum formation in fission yeast. EMBO J. 1994;13:3011–3019. doi: 10.1002/j.1460-2075.1994.tb06600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg SL. Comparison of different Schizosaccharomyces pombe expression systems. Nucleic Acids Res. 1993;21:2955–2956. doi: 10.1093/nar/21.12.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg SL, Nurse P. Cell cycle regulation in the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe. Annu Rev Cell Biol. 1991;7:227–256. doi: 10.1146/annurev.cb.07.110191.001303. [DOI] [PubMed] [Google Scholar]
- Grimm C, Kohli J, Murray J, Maundrell KG. Genetic engineering of Schizosaccharomyces pombe: a system for gene disruption and replacement using the ura4 gene as a selectable marker. Mol Gen Genet. 1988;215:81–86. doi: 10.1007/BF00331307. [DOI] [PubMed] [Google Scholar]
- Hagan I, Yanagida M. Novel potential mitotic motor protein encoded by the fission yeast cut7+ gene. Nature. 1990;347:563–566. doi: 10.1038/347563a0. [DOI] [PubMed] [Google Scholar]
- Hagan I, Yanagida M. The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J Cell Biol. 1995;129:1033–1047. doi: 10.1083/jcb.129.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan I, Yanagida M. Evidence for cell-cycle specific, spindle pole body-mediated, nuclear positioning in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1997;110:1851–1866. doi: 10.1242/jcs.110.16.1851. [DOI] [PubMed] [Google Scholar]
- Hagan IM, Hyams JS. The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1988;89:343–357. doi: 10.1242/jcs.89.3.343. [DOI] [PubMed] [Google Scholar]
- Hayles J, Nurse P. Genetics of the fission yeast Schizosaccharomyces pombe. Annu Rev Genet. 1989;26:323–402. doi: 10.1146/annurev.ge.26.120192.002105. [DOI] [PubMed] [Google Scholar]
- Heim R, Cubitt AB, Tsien RY. Improved green fluorescence. Nature. 1995;373:663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- Hirano T, Funahashi S, Uemura T, Yanagida M. Isolation and characterization of Schizosaccharomyces pombe cut mutants that block in nuclear division but not cytokinesis. EMBO J. 1986;5:2973–2979. doi: 10.1002/j.1460-2075.1986.tb04594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka Y, Toda T, Yanagida M. The NDA3 gene of fission yeast encodes β-tubulin: a cold-sensitive nda3 mutation reversibly blocks spindle formation and chromosome movement in mitosis. Cell. 1984;39:349–358. doi: 10.1016/0092-8674(84)90013-8. [DOI] [PubMed] [Google Scholar]
- Hoheisel JD, Maier E, Mott R, McCarthy L, Grigoriev AV, Schalkwyk LC, Nizetic D, Francis F, Lehrach H. High resolution cosmid and P1 maps spanning the 14 Mb genome of the fission yeast S. pombe. Cell. 1993;73:109–120. doi: 10.1016/0092-8674(93)90164-l. [DOI] [PubMed] [Google Scholar]
- Horio T, Hiraoka Y, Yanagida M. A temperature-sensitive mutation of the Schizosaccharomyces pombe gene nuc2+ that encodes a nuclear scaffold-like protein blocks spindle elongation in mitotic anaphase. J Cell Biol. 1988;106:1171–1183. doi: 10.1083/jcb.106.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao JPY, Alderton JM, Tsein RY, Steinhardt RA. Active involvement of Ca2+ in mitotic progression of Swiss 3T3 fibroblasts. J Cell Biol. 1990;111:183–196. doi: 10.1083/jcb.111.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg DR, Moritz M, Albert BM. The centrosome and cellular organization. Annu Rev Biochem. 1994;63:639–674. doi: 10.1146/annurev.bi.63.070194.003231. [DOI] [PubMed] [Google Scholar]
- Kubai D. The evolution of the mitotic spindle. Int Rev Cytol. 1975;43:167–227. doi: 10.1016/s0074-7696(08)60069-8. [DOI] [PubMed] [Google Scholar]
- Lane HA, Nigg EA. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J Cell Biol. 1996;135:1701–1713. doi: 10.1083/jcb.135.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J, Fankhauser C, Reymond A, Simanis V. Cytoskeletal and DNA structure abnormalities result from bypass of requirement of the cdc10 start gene in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1992;101:517–528. doi: 10.1242/jcs.101.3.517. [DOI] [PubMed] [Google Scholar]
- Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- McCully EK, Rabinow CF. Mitosis in the fission yeast Schizosaccharomyces pombe: a comparative study with light and electron microscopy. J Cell Sci. 1971;9:475–507. doi: 10.1242/jcs.9.2.475. [DOI] [PubMed] [Google Scholar]
- Mitchison JM, Nurse P. Growth in cell length in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1985;75:357–376. doi: 10.1242/jcs.75.1.357. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of the fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Moser MJ, Flory MR, Davis TN. Calmodulin localizes to the spindle pole body of Schizosaccharomyces pombe and performs an essential function in chromosome segregation. J Cell Sci. 1997;110:1805–1812. doi: 10.1242/jcs.110.15.1805. [DOI] [PubMed] [Google Scholar]
- Nasmyth K, Nurse P. Cell division cycle mutants altered DNA replication and mitosis in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1981;182:119–214. doi: 10.1007/BF00422777. [DOI] [PubMed] [Google Scholar]
- Ohkura H, Hagan IM, Glover DM. The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes Dev. 1995;9:1059–1073. doi: 10.1101/gad.9.9.1059. [DOI] [PubMed] [Google Scholar]
- Osborne MA, Schlenstedt G, Jinks T, Silver PA. Nuf2, a spindle pole body-associated protein required for nuclear division in yeast. J Cell Biol. 1994;125:853–866. doi: 10.1083/jcb.125.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibowol K, Draetta G, Brizulela L, Vandre D, Beach D. The cdc2 kinase is a nuclear protein that is essential for mitosis in mammalian cells. Cell. 1989;57:393–401. doi: 10.1016/0092-8674(89)90914-8. [DOI] [PubMed] [Google Scholar]
- Rost D, Casadio R, Fariselli P, Sanders C. Transmembrane helices predicted at 95% accuracy. Protein Sci. 1995;4:521–533. doi: 10.1002/pro.5560040318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saka Y, Fantes P, Sutani T, McInerry C, Creanor J, Yanagida M. Fission yeast cut 5 links nuclear chromatin and M phase regulator in the replication check point control. EMBO J. 1994;13:5319–5329. doi: 10.1002/j.1460-2075.1994.tb06866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Samejima I, Matsumoto T, Nakaseko Y, Beach D, Yanagida M. Identification of seven new cut genes involved in Schizosaccharomyces pombe mitosis. J Cell Sci. 1993;105:135–143. doi: 10.1242/jcs.105.1.135. [DOI] [PubMed] [Google Scholar]
- Samejima I, Yanagida M. Identification of cut8+ and cek1+, a novel protein kinase gene, which complement a fission yeast mutation that blocks anaphase. Mol Biol Cell. 1994a;14:6361–6371. doi: 10.1128/mcb.14.9.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima I, Yanagida M. Bypassing anaphase by fission yeast cut9 mutation: requirement of cut9+ initiates anaphase. J Cell Biol. 1994b;127:1655–1670. doi: 10.1083/jcb.127.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiebel E, Bornes M. In search of centrins. Trends Cell Biol. 1995;5:197–201. doi: 10.1016/s0962-8924(00)88999-0. [DOI] [PubMed] [Google Scholar]
- Sluder G, Thompson EA, Reider CL, Miller FJ. Nuclear envelope breakdown is under nuclear and not cytoplasmic control in sea urchin zygotes. J Cell Biol. 1995;129:1447–1458. doi: 10.1083/jcb.129.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. The spindle pole body of yeast. Chromosoma. 1994;103:369–380. doi: 10.1007/BF00362281. [DOI] [PubMed] [Google Scholar]
- Sohrmann M, Schmidt S, Hagan I, Simanis V. Asymmetric segregation on spindle poles of the Schizosaccharomyces pombe septum-inducing protein kinase cdc7p. Genes Dev. 1998;12:84–94. doi: 10.1101/gad.12.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern JA, Young DF, Heaney F, Baumgartner W, Randall RE. Identification of an epitope on the P & V proteins of simian virus 5 that distinguishes between two isolates with different biological characteristics. J Gen Virol. 1991;72:1551–1557. doi: 10.1099/0022-1317-72-7-1551. [DOI] [PubMed] [Google Scholar]
- Steinhardt RA, Alderton J. Intracellular free calcium rise triggers nuclear envelope breakdown. Nature. 1988;332:364–366. doi: 10.1038/332364a0. [DOI] [PubMed] [Google Scholar]
- Takeda K, Yamamoto M. Analysis and in vivo disruption of the gene coding for calmodulin in Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 1987;84:3580–3584. doi: 10.1073/pnas.84.11.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Kanbe T. Mitosis in the fission yeast Schizosaccharomyces pombe as revealed by freeze-substitution electron microscopy. J Cell Sci. 1986;80:253–268. doi: 10.1242/jcs.80.1.253. [DOI] [PubMed] [Google Scholar]
- Thomas JH, Botstein D. A gene required for the separation of chromosomes on the spindle apparatus in yeast. Cell. 1986;44:65–76. doi: 10.1016/0092-8674(86)90485-x. [DOI] [PubMed] [Google Scholar]
- Toda T, Umesono K, Hirata A, Yanagida M. Cold-sensitive nuclear division mutants of the fission yeast Schizosaccharomyces pombe. J Mol Biol. 1983;168:251–270. doi: 10.1016/s0022-2836(83)80017-5. [DOI] [PubMed] [Google Scholar]
- Toth MV, Huwyler L. Molecular cloning and expression of the cDNAs encoding human and yeast mevalonate pyrophosphate decarboxylase. J Biol Chem. 1996;271:7895–7898. doi: 10.1074/jbc.271.14.7895. [DOI] [PubMed] [Google Scholar]
- Winey M, Byers B. Assembly and functions of the spindle pole body in budding yeast. Trends Genet. 1993;9:300–334. doi: 10.1016/0168-9525(93)90247-f. [DOI] [PubMed] [Google Scholar]
- Winey M, Hoyt MA, Chan C, Goetsch L, Botstein D, Byers B. NDC1: a nuclear periphery component required for yeast spindle pole body duplication. J Cell Biol. 1993;122:743–751. doi: 10.1083/jcb.122.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Kumada K, Yanagida M. Distinct subunit functions and cell cycle regulated phosphorylation of 20S APC/cyclosome required for anaphase in fission yeast. J Cell Sci. 1997;110:1793–1804. doi: 10.1242/jcs.110.15.1793. [DOI] [PubMed] [Google Scholar]
- Zollner A, Rodel G, Haid A. Molecular cloning and characterization of the Saccharomyces cerevisiae CYT2 gene encoding cytochrome-c1-heme lyase. Eur J Biochem. 1992;207:1093–1100. doi: 10.1111/j.1432-1033.1992.tb17146.x. [DOI] [PubMed] [Google Scholar]