Abstract
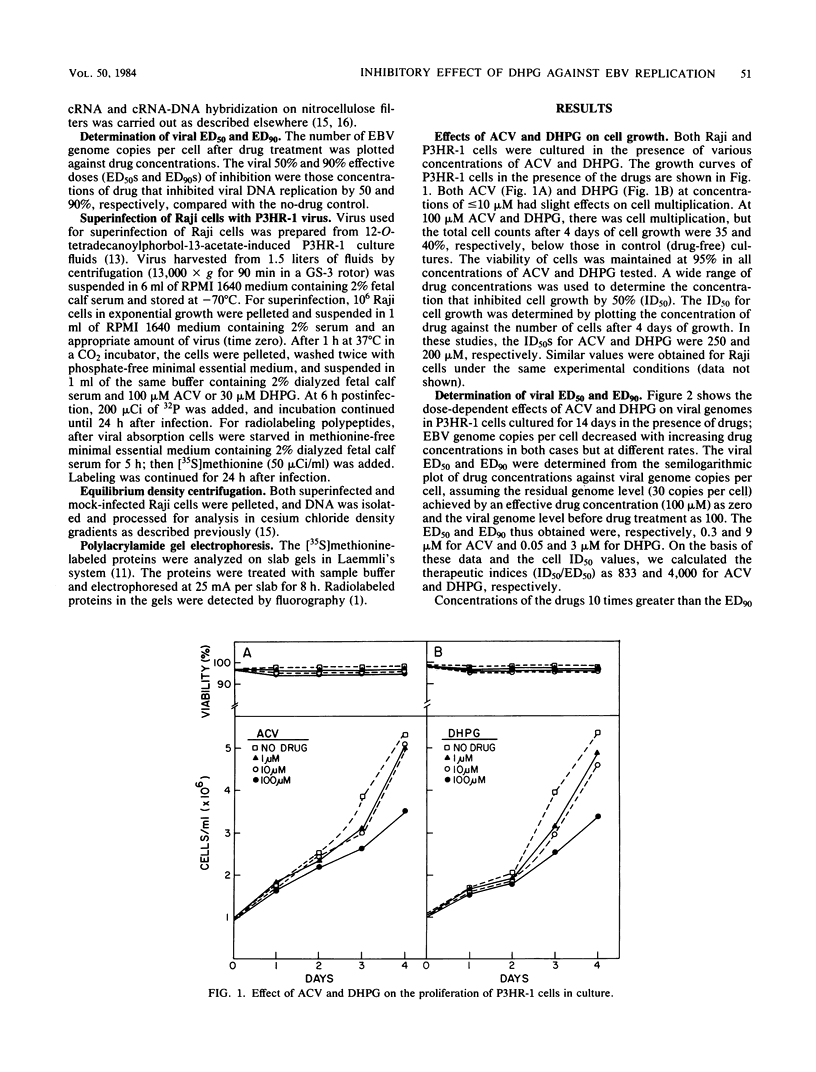
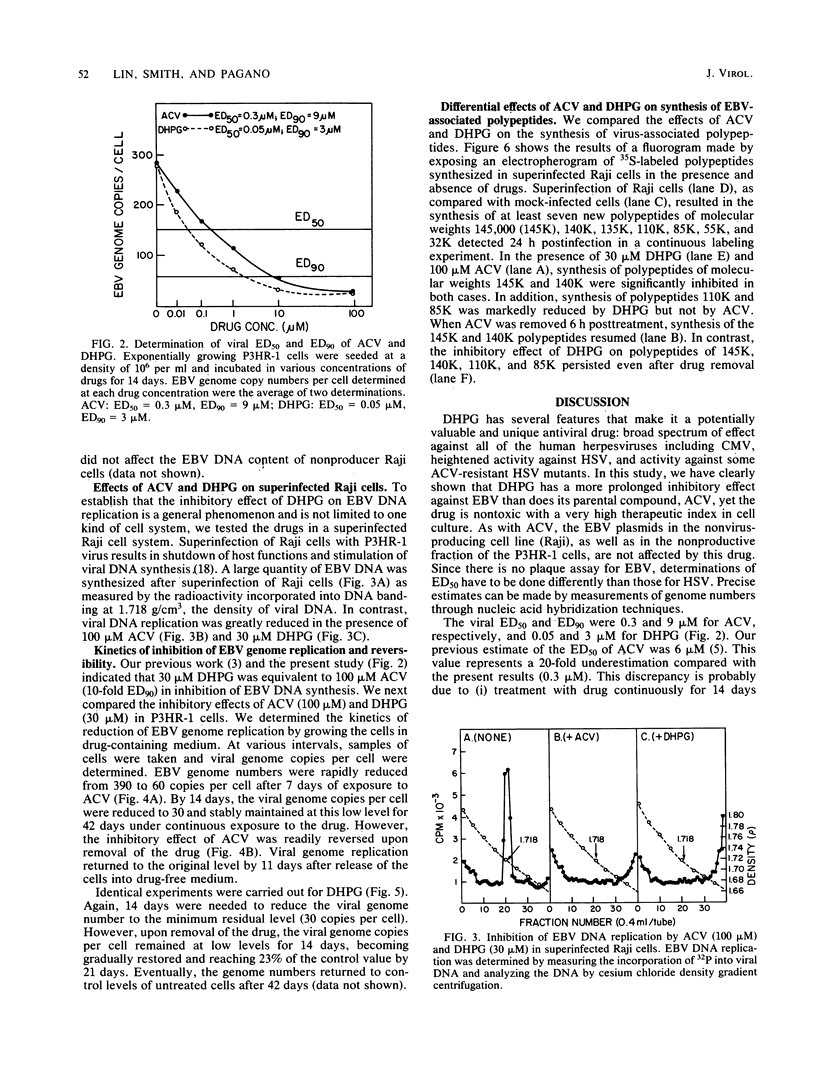
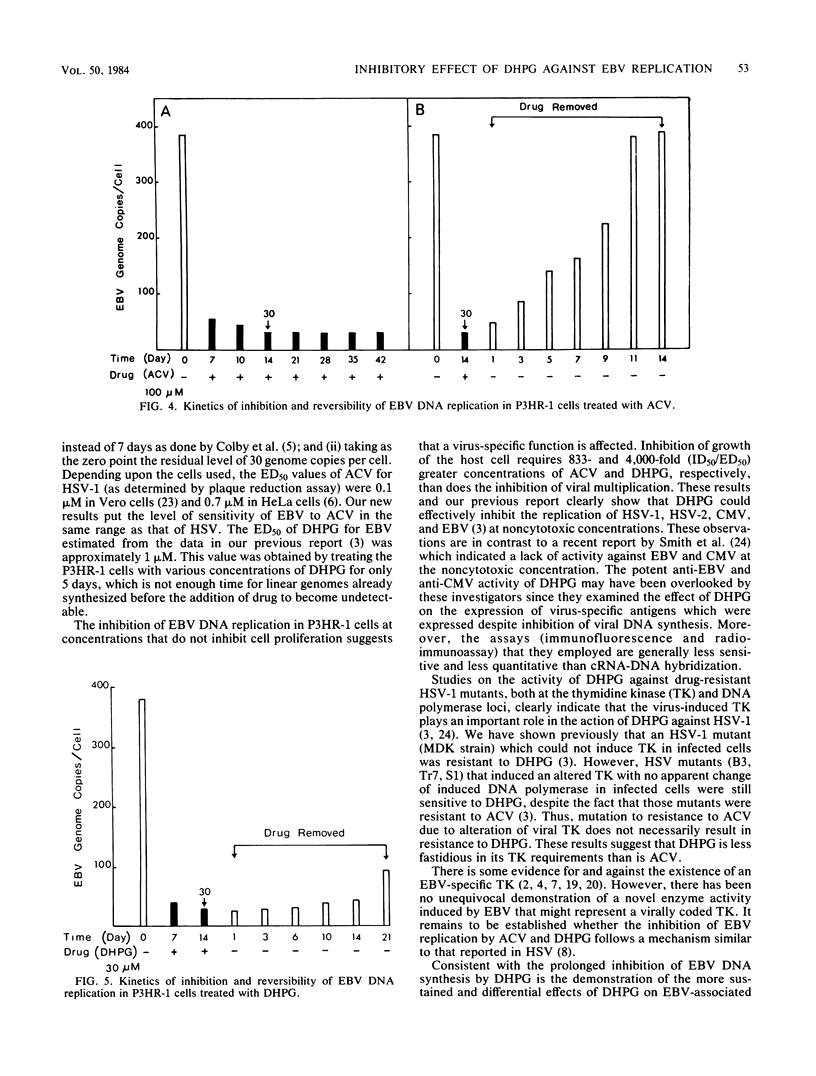
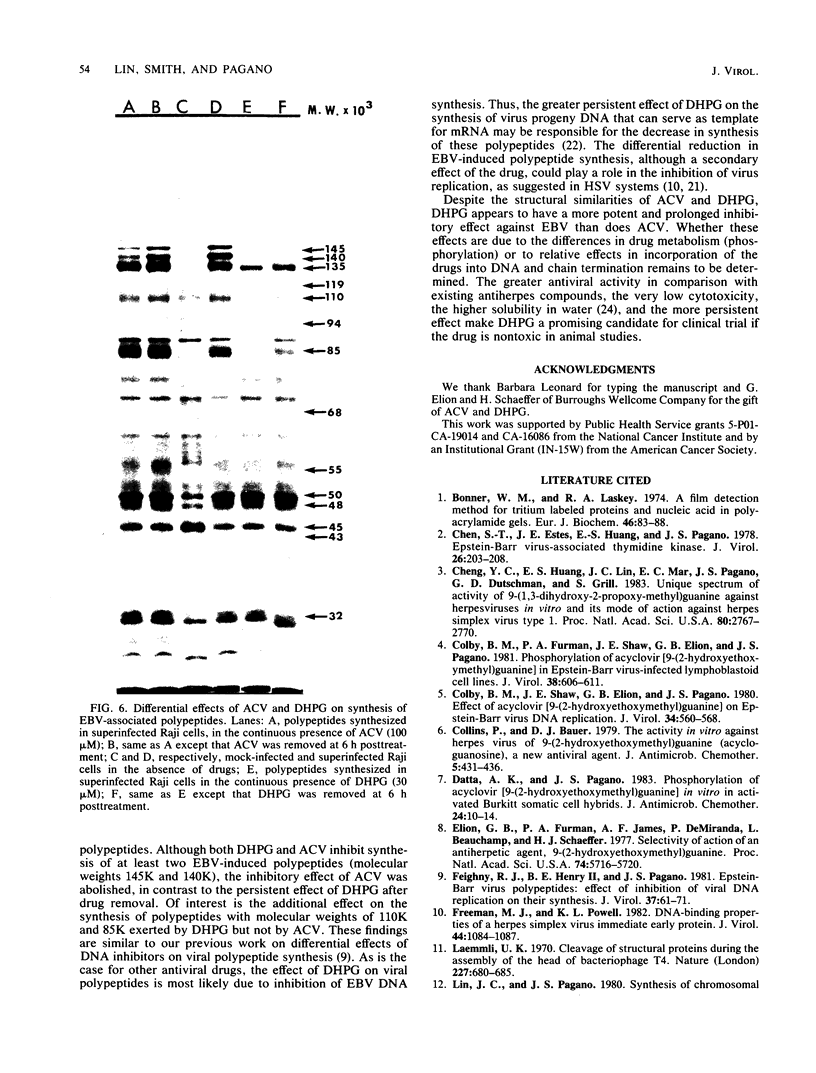
The effects of 9-(1,3-dihydroxy-2-propoxymethyl)guanine (DHPG), a new antiviral drug, and acyclovir (ACV) [9-(2-hydroxyethoxymethyl)guanine] on the replication of Epstein-Barr virus (EBV) were compared. Both drugs inhibited EBV DNA replication in P3HR-1 cells and superinfected Raji cells, but neither inhibited replication of the plasmid form of the EBV genome in latently infected Raji cells. However, DHPG had a more prolonged inhibitory effect than ACV. Although the effect of the drugs is prompt, the kinetics of inhibition of EBV replication indicated that a drug exposure of 14 days was needed to reduce the EBV genome copy number to the residual plasmid level (30 copies per cell). The inhibitory effect of ACV was readily reversed within 11 days after removal of the drug, in contrast to the more prolonged effect exerted by DHPG, which persisted for more than 21 days. The 50% inhibitory doses for cell growth of ACV and DHPG were estimated to be 250 and 200 microM, respectively. The viral 50% and 90% effective doses of inhibition were, respectively, 0.3 and 9 microM for ACV and 0.05 and 3 microM for DHPG. The therapeutic indices (50% inhibitory dose/50% effective dose) for ACV and DHPG were 833 and 4,000, respectively. Synthesis of EBV-associated polypeptides was also affected. In superinfected Raji cells, ACV (100 microM) and DHPG (30 microM) inhibited synthesis of polypeptides with molecular weights of 145,000 and 140,000; in addition, synthesis of polypeptides with molecular weights of 110,000 and 85,000 was markedly reduced by DHPG but not by ACV. However, after drug removal, the inhibitory effect of ACV on polypeptide synthesis was abolished in contrast to the more persistent effect of DHPG.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chen S. T., Estes J. E., Huang E. S., Pagano J. S. Epstein-Barr virus-associated thymidine kinase. J Virol. 1978 Apr;26(1):203–208. doi: 10.1128/jvi.26.1.203-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Huang E. S., Lin J. C., Mar E. C., Pagano J. S., Dutschman G. E., Grill S. P. Unique spectrum of activity of 9-[(1,3-dihydroxy-2-propoxy)methyl]-guanine against herpesviruses in vitro and its mode of action against herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1983 May;80(9):2767–2770. doi: 10.1073/pnas.80.9.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby B. M., Furman P. A., Shaw J. E., Elion G. B., Pagano J. S. Phosphorylation of acyclovir [9-(2-hydroxyethoxymethyl)guanine] in Epstein-Barr virus-infected lymphoblastoid cell lines. J Virol. 1981 May;38(2):606–611. doi: 10.1128/jvi.38.2.606-611.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby B. M., Shaw J. E., Elion G. B., Pagano J. S. Effect of acyclovir [9-(2-hydroxyethoxymethyl)guanine] on Epstein-Barr virus DNA replication. J Virol. 1980 May;34(2):560–568. doi: 10.1128/jvi.34.2.560-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P., Bauer D. J. The activity in vitro against herpes virus of 9-(2-hydroxyethoxymethyl)guanine (acycloguanosine), a new antiviral agent. J Antimicrob Chemother. 1979 Jul;5(4):431–436. doi: 10.1093/jac/5.4.431. [DOI] [PubMed] [Google Scholar]
- Datta A. K., Pagano J. S. Phosphorylation of acyclovir in vitro in activated Burkitt somatic cell hybrids. Antimicrob Agents Chemother. 1983 Jul;24(1):10–14. doi: 10.1128/aac.24.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feighny R. J., Henry B. E., 2nd, Pagano J. S. Epstein-Barr virus polypeptides: effect of inhibition of viral DNA replication on their synthesis. J Virol. 1981 Jan;37(1):61–71. doi: 10.1128/jvi.37.1.61-71.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M. J., Powell K. L. DNA-binding properties of a herpes simplex virus immediate early protein. J Virol. 1982 Dec;44(3):1084–1087. doi: 10.1128/jvi.44.3.1084-1087.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Pagano J. S. Synthesis of chromosomal proteins and Epstein-Barr virus DNA in activated Burkitt somatic cell hybrids. Virology. 1980 Oct 15;106(1):50–58. doi: 10.1016/0042-6822(80)90220-2. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Shaw J. E., Smith M. C., Pagano J. S. Effect of 12-O-tetradecanoyl-phorbol-13-acetate on the replication of Epstein-Barr virus. I. Characterization of viral DNA. Virology. 1979 Nov;99(1):183–187. doi: 10.1016/0042-6822(79)90052-7. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Smith M. C., Cheng Y. C., Pagano J. S. Epstein-Barr virus: inhibition of replication by three new drugs. Science. 1983 Aug 5;221(4610):578–579. doi: 10.1126/science.6306771. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Smith M. C., Pagano J. S. Activation of latent Epstein-Barr virus genomes: selective stimulation of synthesis of chromosomal proteins by a tumor promoter. J Virol. 1983 Mar;45(3):985–991. doi: 10.1128/jvi.45.3.985-991.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. C., Smith M. C., Pagano J. S. Effects of 12-O-tetradecanoyl-phorbol-13-acetate on cell proliferation and Epstein-Barr virus DNA replication. Virology. 1982 Feb;117(1):186–194. doi: 10.1016/0042-6822(82)90518-9. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Smith M. C., Pagano J. S. Induction of replication of Epstein-Barr virus DNA by 12-O-tetradecanoyl-phorbol-13-acetate. II. Inhibition by retinoic acid and 9-(2-hydroxyethoxymethyl) guanine. Virology. 1981 May;111(1):294–298. doi: 10.1016/0042-6822(81)90675-9. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Replication of viral deoxyribonucleic acid and breakdown of cellular deoxyribonucleic acid in Epstein-Barr virus infection. J Virol. 1972 Apr;9(4):714–716. doi: 10.1128/jvi.9.4.714-716.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooka T., Calender A., de Turenne M., Daillie J. Effect of arabinofuranosylthymine on the replication of Epstein-Barr virus and relationship with a new induced thymidine kinase activity. J Virol. 1983 Apr;46(1):187–195. doi: 10.1128/jvi.46.1.187-195.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano J. S., Datta A. K. Perspectives on interactions of acyclovir with Epstein-Barr and other herpes viruses. Am J Med. 1982 Jul 20;73(1A):18–26. doi: 10.1016/0002-9343(82)90057-2. [DOI] [PubMed] [Google Scholar]
- Powell K. L., Littler E., Purifoy D. J. Nonstructural proteins of herpes simplex virus. II. Major virus-specific DNa-binding protein. J Virol. 1981 Sep;39(3):894–902. doi: 10.1128/jvi.39.3.894-902.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Purifoy D. J., Courtney R. J. The synthesis of herpes simplex virus proteins in the absence of virus DNA synthesis. Biochem Biophys Res Commun. 1975 Sep 2;66(1):262–271. doi: 10.1016/s0006-291x(75)80323-8. [DOI] [PubMed] [Google Scholar]
- Schaeffer H. J., Beauchamp L., de Miranda P., Elion G. B., Bauer D. J., Collins P. 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978 Apr 13;272(5654):583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- Smith K. O., Galloway K. S., Kennell W. L., Ogilvie K. K., Radatus B. K. A new nucleoside analog, 9-[[2-hydroxy-1-(hydroxymethyl)ethoxyl]methyl]guanine, highly active in vitro against herpes simplex virus types 1 and 2. Antimicrob Agents Chemother. 1982 Jul;22(1):55–61. doi: 10.1128/aac.22.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]




