Abstract
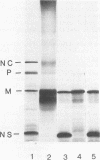
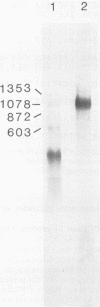
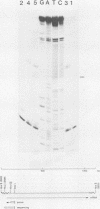
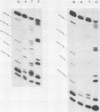
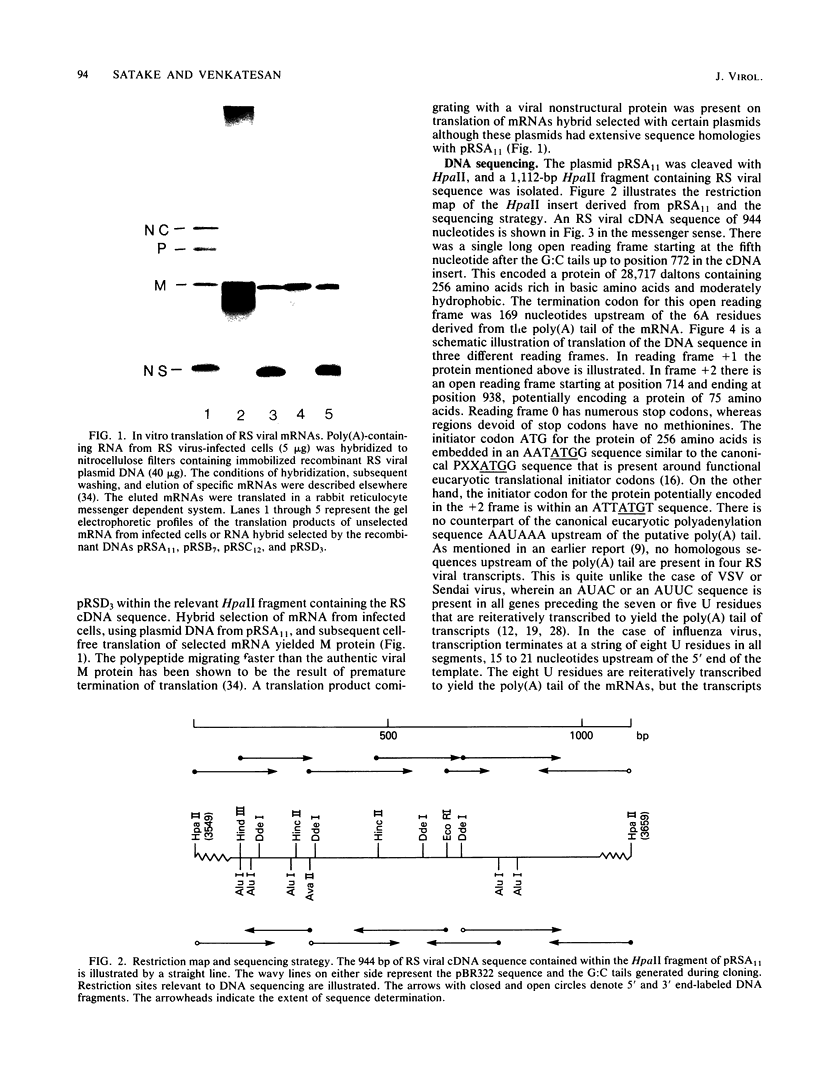
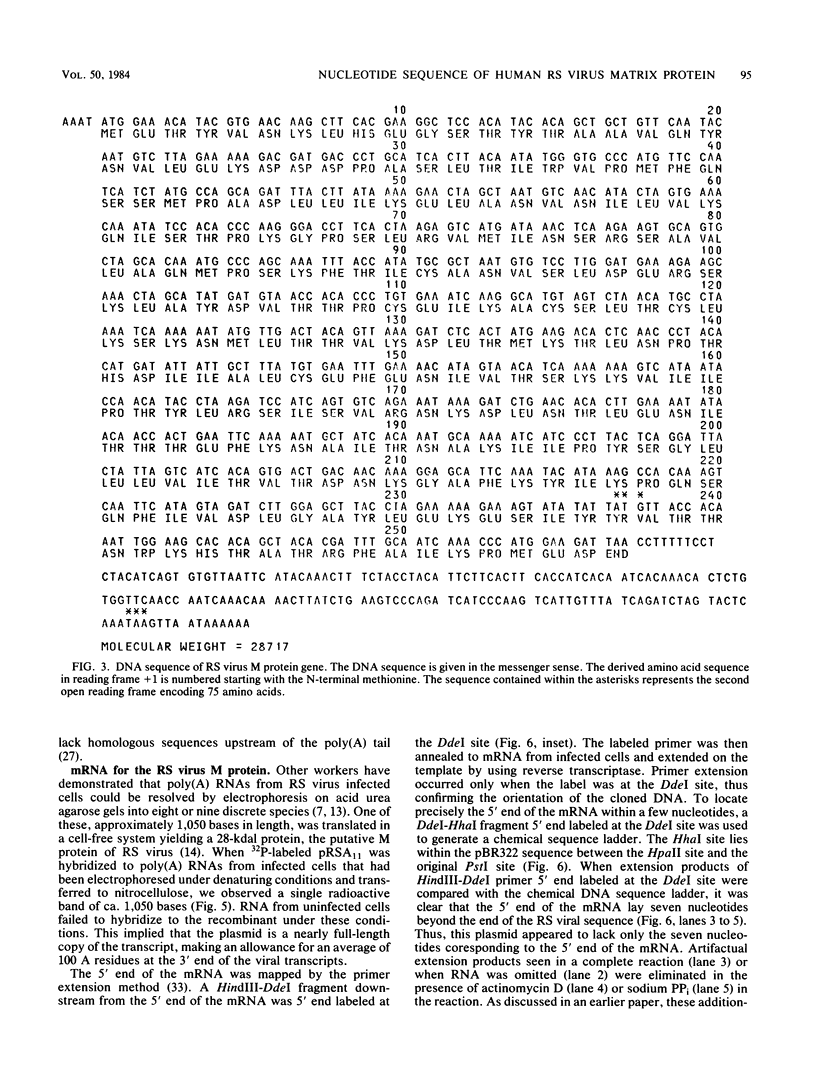
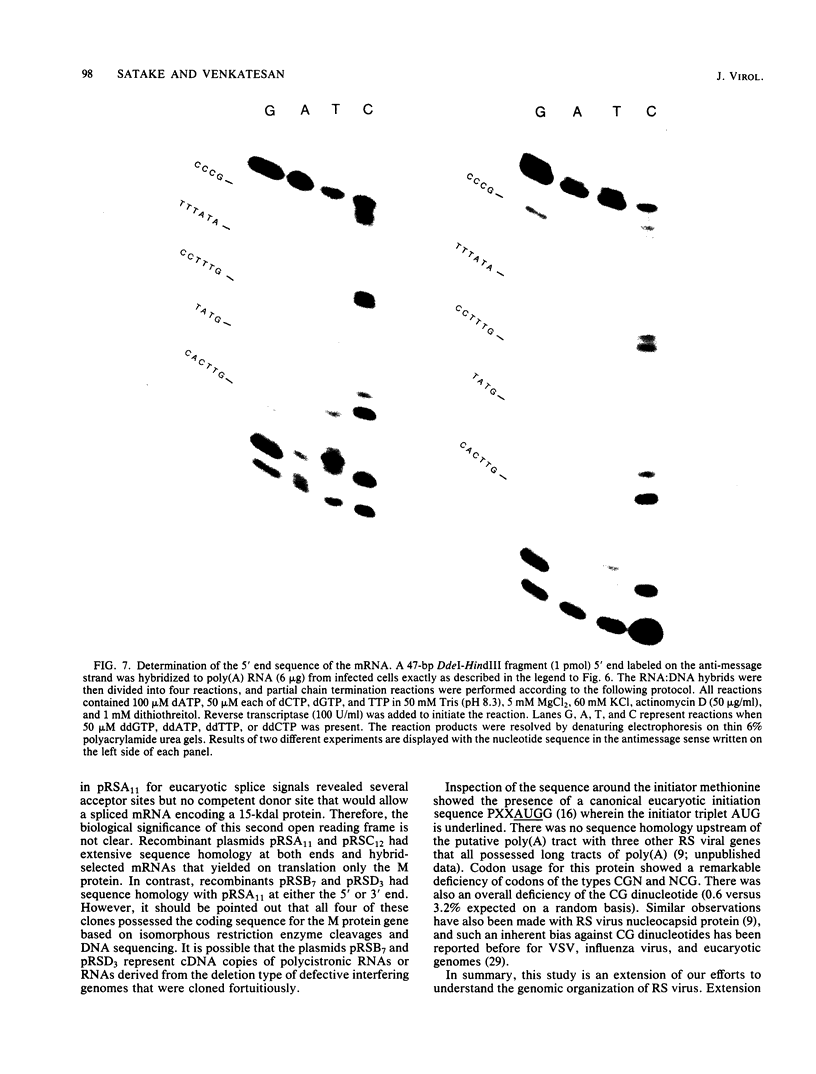
The amino acid sequence of the matrix protein of the human respiratory syncytial virus (RS virus) was deduced from the sequence of a cDNA insert in a recombinant plasmid harboring an almost full-length copy of this gene. It specifically hybridized to a single 1,050-base mRNA from infected cells. The recombinant containing 944 base pairs of RS viral matrix protein gene sequence lacked five nucleotides corresponding to the 5' end of the mRNA. The nucleotide sequence of the 5' end of the mRNA was determined by the dideoxy sequencing method and found to be 5' NGGGC, wherein the C residue is one nucleotide upstream of the cloned viral sequence. The initiator ATG codon for the matrix protein is embedded in an AATATGG sequence similar to the canonical PXXATGG sequence present around functional eucaryotic translation initiation codons. There is no conserved sequence upstream of the polyadenylate tail, unlike vesicular stomatitis virus and Sendai virus, in which four nucleotides upstream of the polyadenylate tail are conserved in all genes. There is no equivalent of the eucaryotic polyadenylation signal AAUAAA upstream of the polyadenylate tail. The matrix protein of 28,717 daltons has 256 amino acids. It is relatively basic and moderately hydrophobic. There are two clusters of hydrophobic amino acid residues in the C-terminal third of the protein that could potentially interact with the membrane components of the infected cell. The matrix protein has no homology with the matrix proteins of other negative-strand RNA viruses, implying that RS virus has undergone extensive evolutionary divergence. A second open reading frame potentially encoding a protein of 75 amino acids and partially overlapping the C terminus of the matrix protein was also identified.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein J. M., Hruska J. F. Respiratory syncytial virus proteins: identification by immunoprecipitation. J Virol. 1981 Apr;38(1):278–285. doi: 10.1128/jvi.38.1.278-285.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Little S. P., Hagen F. S., Huang A. S. The matrix (M) protein of vesicular stomatitis virus regulates transcription. Cell. 1978 Dec;15(4):1455–1462. doi: 10.1016/0092-8674(78)90069-7. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Wertz G. W. cDNA cloning and transcriptional mapping of nine polyadenylylated RNAs encoded by the genome of human respiratory syncytial virus. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3208–3212. doi: 10.1073/pnas.80.11.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De B. P., Thornton G. B., Luk D., Banerjee A. K. Purified matrix protein of vesicular stomatitis virus blocks viral transcription in vitro. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7137–7141. doi: 10.1073/pnas.79.23.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elango N., Venkatesan S. Amino acid sequence of human respiratory syncytial virus nucleocapsid protein. Nucleic Acids Res. 1983 Sep 10;11(17):5941–5951. doi: 10.1093/nar/11.17.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez H. B., Pringle C. R. Seven complementation groups of respiratory syncytial virus temperature-sensitive mutants. J Virol. 1978 Sep;27(3):459–464. doi: 10.1128/jvi.27.3.459-464.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Huang Y. T., Wertz G. W. Respiratory syncytial virus mRNA coding assignments. J Virol. 1983 May;46(2):667–672. doi: 10.1128/jvi.46.2.667-672.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. T., Wertz G. W. The genome of respiratory syncytial virus is a negative-stranded RNA that codes for at least seven mRNA species. J Virol. 1982 Jul;43(1):150–157. doi: 10.1128/jvi.43.1.150-157.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Separate pathways of maturation of the major structural proteins of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1128–1139. doi: 10.1128/jvi.21.3.1128-1139.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Grez M., Hauser H., Lindenmaier W., Schütz G. 5'-Terminal sequences of eucaryotic mRNA can be cloned with high efficiency. Nucleic Acids Res. 1981 May 25;9(10):2251–2266. doi: 10.1093/nar/9.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Keene J. D., Schubert M. The origins of defective interfering particles of the negative-strand RNA viruses. Cell. 1981 Oct;26(2 Pt 2):145–154. doi: 10.1016/0092-8674(81)90298-1. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lubeck M. D., Schulman J. L., Palese P. Susceptibility of influenza A viruses to amantadine is influenced by the gene coding for M protein. J Virol. 1978 Dec;28(3):710–716. doi: 10.1128/jvi.28.3.710-716.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Morrison T. G. Site of synthesis of membrane and nonmembrane proteins of vesicular stomatitis virus. J Biol Chem. 1975 Sep 10;250(17):6955–6962. [PubMed] [Google Scholar]
- Peeples M., Levine S. Respiratory syncytial virus polypeptides: their location in the virion. Virology. 1979 May;95(1):137–145. doi: 10.1016/0042-6822(79)90408-2. [DOI] [PubMed] [Google Scholar]
- Queen C. L., Korn L. J. Computer analysis of nucleic acids and proteins. Methods Enzymol. 1980;65(1):595–609. doi: 10.1016/s0076-6879(80)65062-9. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robertson J. S., Schubert M., Lazzarini R. A. Polyadenylation sites for influenza virus mRNA. J Virol. 1981 Apr;38(1):157–163. doi: 10.1128/jvi.38.1.157-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K. Complete intergenic and flanking gene sequences from the genome of vesicular stomatitis virus. Cell. 1980 Feb;19(2):415–421. doi: 10.1016/0092-8674(80)90515-2. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer R. E. An improved method for detecting foreign DNA in plasmids of Escherichia coli. Anal Biochem. 1979 Sep 15;98(1):60–63. doi: 10.1016/0003-2697(79)90705-x. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan S., Baroudy B. M., Moss B. Distinctive nucleotide sequences adjacent to multiple initiation and termination sites of an early vaccinia virus gene. Cell. 1981 Sep;25(3):805–813. doi: 10.1016/0092-8674(81)90188-4. [DOI] [PubMed] [Google Scholar]
- Venkatesan S., Elango N., Chanock R. M. Construction and characterization of cDNA clones for four respiratory syncytial viral genes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1280–1284. doi: 10.1073/pnas.80.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan S., Gershowitz A., Moss B. Complete nucleotide sequences of two adjacent early vaccinia virus genes located within the inverted terminal repetition. J Virol. 1982 Nov;44(2):637–646. doi: 10.1128/jvi.44.2.637-646.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg G., Hammarskjöld M. L. Isolation of DNA from agarose gels using DEAE-paper. Application to restriction site mapping of adenovirus type 16 DNA. Nucleic Acids Res. 1980 Jan 25;8(2):253–264. doi: 10.1093/nar/8.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter G., Fields S. Cloning of influenza cDNA ino M13: the sequence of the RNA segment encoding the A/PR/8/34 matrix protein. Nucleic Acids Res. 1980 May 10;8(9):1965–1974. doi: 10.1093/nar/8.9.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P. F., Gharpure M. A., Hodes D. S., Chanock R. M. Genetic studies of respiratory syncytial virus temperature-sensitive mutants. Arch Gesamte Virusforsch. 1973;41(3):238–247. doi: 10.1007/BF01252771. [DOI] [PubMed] [Google Scholar]
- Wunner W. H., Pringle C. R. Respiratory syncytial virus proteins. Virology. 1976 Aug;73(1):228–243. doi: 10.1016/0042-6822(76)90077-5. [DOI] [PubMed] [Google Scholar]
- Zvonarjev A. Y., Ghendon Y. Z. Influence of membrane (M) protein on influenza A virus virion transcriptase activity in vitro and its susceptibility to rimantadine. J Virol. 1980 Feb;33(2):583–586. doi: 10.1128/jvi.33.2.583-586.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]