Abstract
Haptokinetic cell migration across surfaces is mediated by adhesion receptors including β1 integrins and CD44 providing adhesion to extracellular matrix (ECM) ligands such as collagen and hyaluronan (HA), respectively. Little is known, however, about how such different receptor systems synergize for cell migration through three-dimensionally (3-D) interconnected ECM ligands. In highly motile human MV3 melanoma cells, both β1 integrins and CD44 are abundantly expressed, support migration across collagen and HA, respectively, and are deposited upon migration, whereas only β1 integrins but not CD44 redistribute to focal adhesions. In 3-D collagen lattices in the presence or absence of HA and cross-linking chondroitin sulfate, MV3 cell migration and associated functions such as polarization and matrix reorganization were blocked by anti-β1 and anti-α2 integrin mAbs, whereas mAbs blocking CD44, α3, α5, α6, or αv integrins showed no effect. With use of highly sensitive time-lapse videomicroscopy and computer-assisted cell tracking techniques, promigratory functions of CD44 were excluded. 1) Addition of HA did not increase the migratory cell population or its migration velocity, 2) blocking of the HA-binding Hermes-1 epitope did not affect migration, and 3) impaired migration after blocking or activation of β1 integrins was not restored via CD44. Because α2β1-mediated migration was neither synergized nor replaced by CD44–HA interactions, we conclude that the biophysical properties of 3-D multicomponent ECM impose more restricted molecular functions of adhesion receptors, thereby differing from haptokinetic migration across surfaces.
INTRODUCTION
Tumor cell invasion and migration are supported by different adhesion receptor systems, including integrins and CD44 (Stetler-Stevenson et al., 1993; Sherman et al., 1994; Friedl and Bröcker, 1999). Integrins and CD44 interact with an array of extracellular matrix (ECM) components, including collagen, fibronectin, laminin, vitronectin, and hyaluronan (HA), respectively (Etoh et al., 1992; Danen et al., 1993; Aznavoorian et al., 1996; Goebeler et al., 1996; Klominek et al., 1997), simultaneously acting as structural links from the cytoskeleton to the ECM as well as signaling molecules (Lokeshwar et al., 1994; Clark and Brugge, 1995; Entwistle et al., 1996; Cox and Huttenlocher, 1998).
In human melanoma, the expression of α2β1 integrin is correlated with metastatic behavior (Klein et al., 1991b) and is involved in melanoma cell migration on collagen surfaces (Etoh et al., 1992) and in the reorganization of collagen matrices (Klein et al., 1991b; Schiro et al., 1991; Riikonen et al., 1995). Likewise, α3β1 and αvβ3 integrins mediate chemotactic and haptotactic motility toward their respective ligands collagen (Lauer et al., 1998) and vitronectin (Aznavoorian et al., 1996), contributing to tumor progression and metastasis in melanoma (Mitjans et al., 1995; Friedl and Bröcker, 1999).
CD44 comprises a family of adhesion receptors binding to HA as principal ligand and, at lower affinity, to chondroitin sulfate (CS), heparan sulfate, fibronectin, and osteopontin (Rudzki and Jothy, 1997; Knudson, 1998). The CD44 cytoplasmic domain binds to the actin-based cytoskeleton via ankyrin and/or members of the ezrin–radixin–moesin family (Lokeshwar et al., 1994; Tsukira et al., 1994). Overexpression of CD44 or alternatively spliced CD44 variants appears to correlate with aggressiveness or metastasis of various human tumors, relating CD44 to tumor progression (Sherman et al., 1994; Knudson, 1996, 1998; Kincade et al., 1997). CD44 was shown to mediate neoplastic cell migration that predominantly depends on CD44–HA interactions (Rudzki and Jothy, 1997; Knudson, 1998), such as 1) migration across HA-coated surfaces in vitro (Thomas et al., 1992; Goebeler et al., 1996; Lamb et al., 1997; Ladeda et al., 1998) and 2) tumor growth and metastasis in vivo (Bartolazzi et al., 1994; Guo et al., 1994). These CD44 functions are dependent on the HA-binding Hermes-1 epitope and are efficiently inhibited by corresponding mAbs (Thomas et al., 1992; Guo et al., 1994; Goebeler et al., 1996) or soluble CD44 (Bartolazzi et al., 1994). Therefore, adhesive interaction of CD44 with HA-mediating cell motility has been implicated as a putative mechanism in tumor invasion and metastasis in some cells (Goebeler et al., 1996; Iida and Bourguignon, 1997; Lamb et al., 1997; Knudson, 1998; Ladeda et al., 1998), whereas for other cells negative results were reported (Driessens et al., 1995; Sleeman et al., 1996).
The cellular and molecular mechanisms by which cell adhesion receptors contribute to cell migration were extensively established for fibroblasts migrating across surfaces, termed haptokinetic migration (Lauffenburger and Horwitz, 1996; Cox and Huttenlocher, 1998). Haptokinetic migration results from a cyclic process of interdependent functions, including 1) cell polarization and pseudopod protrusion, 2) formation of graded cell-substratum attachments via adhesion receptor clustering at substrate-binding sites, 3) temporary assembly of localized molecular complexes composed of integrins and cytoskeletal and signaling proteins, termed focal contacts, 4) contraction of the cell body by myosin motors, and 5) release of focal contacts at the trailing edge and localized retraction of the posterior edge (Bretscher, 1996; Burridge and Chrzanowska-Wodicka, 1996; Lauffenburger and Horwitz, 1996; Palecek et al., 1997). In contrast to β1 integrins, it is not established whether CD44-mediated motility follows these principles of haptokinetic migration. Similarly, it is not known whether the haptokinetic migration model correctly predicts potentially more complex cell—matrix interactions and migration processes within three-dimensional (3-D) tissues consisting of multiple ligands (Friedl and Bröcker, 1999). Three-dimensionally interconnected ECM ligands provide an adhesive substrate, as do two-dimensional (2-D) surfaces, but they also impose a significant biomechanical barrier toward the advancing cell body, supporting both adhesion-dependent and adhesion-independent cell migration strategies (Friedl et al., 1998b,c). Furthermore, 3-D multicomponent ECM is likely to simultaneously display diverse ECM ligands to different sets of adhesion receptors, supporting cell motility by collaboration of adhesion receptors (Bauer et al., 1992). To this end, it is unclear how simultaneously expressed sets of adhesion receptors both synergize and counteract each other, depending on ligand availability and density.
In the highly metastatic human MV3 melanoma cell line, β1 integrins as well as standard CD44 are highly expressed (Danen et al., 1993; Van Muijen et al., 1995), and both receptor systems were implicated in migration-associated cell-substrate adhesion, cell migration, and matrix reorganization in these cells (Klein et al., 1991a; Danen et al., 1993; Goebeler et al., 1996; Friedl et al., 1997). In the present study, using 3-D multicomponent matrices consisting of collagen, HA, and CS, we investigated 1) whether both β1 integrins and CD44 are equally involved in MV3 cell migration and concomitant matrix remodeling and 2) whether, after the loss of integrin function, CD44-mediated compensatory mechanisms exist for maintenance of migration.
MATERIALS AND METHODS
Cells and Cell Culture
The highly metastatic melanoma cell line MV3 (Van Muijen et al., 1991) was kindly provided by G. Van Muijen (University of Nijmegen, Nijmegen, The Netherlands). MV3 cells were shown to express high levels of standard CD44 (Goebeler et al., 1996), α2β1 and α3β1 integrins, and intermediate levels of α5β1, α6β1, and αvβ3 integrins (Danen et al., 1993), whereas α1β1 integrin was not detected (Klein et al., 1991a; K. Maaser, unpublished observations). Cells were cultured in RPMI 1640 supplemented with penicillin (50 U/ml), streptomycin (50 μg/ml), 2 mM glutamine, and 10% heat-inactivated FCS (Boehringer Mannheim, Mannheim, Germany) and maintained at 37°C in humidified 5% CO2 atmosphere. To exclude adhesive interference provided by serum proteins, migration studies on isolated integrin function were performed in FCS-free medium. In contrast, CD44 function was assessed in the presence of 5% FCS with the exception of some experiments. In comparison, the absence of FCS did not alter blocking effects of mAbs on β1 integrins and CD44, whereas the time-dependent percentage of spontaneously migrating cells was slightly reduced from ∼60–80 to 45–60%.
Antibodies
The following affinity-purified, adhesion-perturbing mAbs were used: anti-β1 mAb 4B4 (Morimoto et al., 1985) (Coulter, Hamburg, Germany), anti-α2 mAb 6F1 (Coller et al., 1989) (kindly provided by B. S. Coller, Mount Sinai Medical Center, New York, NY), and P1E6 (Carter et al., 1990) (Becton Dickinson, San Jose, CA); anti-α3 mAb P1B5 (Carter et al., 1990) (Becton Dickinson), anti-α5 mAb Sam-1 (Arroyo et al., 1992) (Life Technologies, Eggenstein, Germany), anti-α6 GoH3 (Sonnenberg et al., 1986) (Immunotech, Hamburg, Germany), anti-αv mAb 17E6 (Mitjans et al., 1995) (Merck, Darmstadt, Germany), and anti-CD44 mAb Hermes-1 (Jalkanen et al., 1987) (Endogen, Cambridge, MA). Activating anti-β1 mAb 8A2 (Kovach et al., 1992) was kindly provided by N. L. Kovach (University of Washington, Seattle, WA). Nonblocking anti-β1 integrin mAb K20 (Arroyo et al., 1992) was obtained from Immunotech, and nonblocking mAb Hermes-3 was kindly provided by S. Jalkanen (University of Turku, Turku, Finland). Polyclonal FITC- or lissamine–rhodamine sulfonyl chloride (LRSC)-conjugated goat anti-mouse F(ab)′ fragments and R-phycoerythrin-conjugated goat anti-mouse F(ab′)2 fragments (Jackson Laboratories, West Grove, PA) were used as secondary antibodies.
Preparation of 3-D ECM Lattices
Bovine dermal collagen (Vitrogen 100) consisting of 99.9% fibrillar collagen (97.1% type I and 2.9% type III collagen) was obtained from Celtrix Pharmaceuticals (Santa Clara, CA). MV3 cells were incorporated within 3-D collagen lattices as previously described (Friedl et al., 1997). In brief, cells from subconfluent cultures were detached using EDTA (2 mM), washed in PBS, and suspended in 100 μl buffered collagen solution, pH 7.4, containing 1.67 μg/ml collagen in minimal essential Eagle’s medium (Flow Laboratories, McLean, VA; final concentration 8 × 105 cells/ml) supplemented with 5% FCS. The suspension was allowed to polymerize for 20–30 min at 37°C in a 5% CO2 atmosphere in a self-constructed chamber (Friedl et al., 1993).
For the construction of multicomponent in vitro lattices in approximation of ECM characteristics present in interstitial tissues, HA concentrations ranging from 0.001 to 5 mg/ml and CS of 20 mg/ml were used. In tissues, HA contents vary greatly, ranging from 0.2 mg/g (human adult dermis) and 1 mg/g (mouse dermis) to 4 mg/g (umbilical cord) or 3.5 mg/g in solid tumors (Comper and Laurent, 1978; Knudson and Knudson, 1995; Knudson, 1996; Fraser et al., 1997), whereas synovial fluid contains 1–3 mg/ml (Comper and Laurent, 1978; Fraser et al., 1997). The concentration of CS in polysaccharide-rich tissues varies from 10 mg/g (dermal interstitial tissue) to 100 mg/g wet weight (cartilage) (Comper and Laurent, 1978). To construct multicomponent lattices, highly purified human umbilical cord HA (Sigma, Deisenhofen, Germany) lacking major protein contaminants (<2% protein contaminants) was copolymerized with chondroitin-4-sulfate (20 mg/ml; Sigma) and collagen (1.67 mg/ml), in modification of the protocol previously described for migration studies on hematopoietic cells (Friedl et al., 1995). In some experiments, human umbilical cord HA of determined high molecular weight (3.5–5 × 106 Da, as detected by low-angle laser light scattering, according to a protocol provided by the manufacturer) was used.
Haptokinetic Migration Assay
Glass coverslips were incubated with medium (control) or highly purified human umbilical cord HA at a concentration of 1 or 10 μg/ml (20°C, 18 h), washed twice, and overlaid with MV3 cells in suspension. In a series of initial experiments, a varying time interval required for initial seeding and cell spreading resulted in inconsistent migration baselines from experiment to experiment. Therefore, a period of preincubation was standardized to 7 h followed by time-lapse videomicroscopy over an observation period of an additional 12–18 h.
Blocking Experiments and Immunofluorescence Staining
Cells were preincubated with mAb (10 μg/ml, 30 min, 4°C) and washed before incorporation within the lattice. In most experiments mAb was additionally added to collagen solution and supernatant, or, alternatively, added to the supernatant only after the onset of cell migration. To study the distribution and trafficking of β1 integrins during migration, cells were preincubated with primary blocking mAb 4B4 or nonblocking mAb K20 (10 μg/ml, 30 min, 4°C), washed, and stained with secondary LRSC-conjugated goat anti-mouse F(ab)′ fragments (1.3 μg/ml, 30 min, 4°C). Subsequently cells were incorporated within the collagen lattice, fixed after varying time intervals (4% paraformaldehyde, 10 min, 20°C), washed, and stained with FITC-conjugated mAb 4B4 within the lattice.
Flow Cytometry
Cells were cultured for 14 h in liquid culture or within a 3-D collagen matrix. For collagen matrix digestion, cells within matrices were incubated with highly purified collagenase type VII (Sigma). Cell surface receptors were stained with primary mAb and secondary with R-phycoerythrin–conjugated goat anti-mouse F(ab′)2 fragments. Surface expression was measured using FACS Calibur (Becton Dickinson). In control experiments using collagenase-treated cells from liquid culture, neither integrin nor CD44 expression were affected by the digestion procedure (Friedl et al., 1995; Maaser, unpublished observations).
Confocal Laser Microscopy
Three-channel confocal laser microscopy (Leica TCS 4D, Bensheim, Germany) was performed as previously described (Friedl et al., 1997). For imaging of collagen fibers, laser light (488 and 568 nm) at a low intensity was introduced into the sample, and the light reflected by the sample was detected, allowing a sensitive detection of fibers up to a scanning depth of 50 μm and at a minimal pixel resolution of 70 nm from fixed as well as viable samples. 3-D reconstructions of sequential x- and y-sections were displayed as topographical images using the Leica TCS-4D software. In some experiments, time-series of migrating cells after prestaining with primary mAb and secondary Fab fragments were obtained as simultaneous two-channel scans, as described (Friedl et al., 1998b).
Time-Lapse Videomicroscopy and Computer-assisted Cell Tracking
Migration of MV3 cells within 3-D collagen lattices or on 2-D substrate was recorded by time-lapse videomicroscopy using up to five independent units and analyzed by computer-assisted cell tracking as previously described (Friedl et al., 1993) and modified (Friedl et al., 1997). In brief, 40 cells were randomly selected from the video screen, and 2-D projections of the paths were digitized as x/y coordinates in 17-min–step intervals. Four principally different migration parameters were obtained: the percentage of cells migrating, and the time migrating, velocity, and speed.
Time-dependent percentages of “cells locomoting” were calculated from step to step. A cell was considered locomoting if the centroid of the cell was translocating at least one pixel per step, corresponding to a step length of 1.5 μm/17 min or 0.09 μm/min, as described (Friedl et al., 1993 and 1995). The cumulative percentage of cells locomoting resulted from the fraction of cells that migrated at least one pixel corresponding to the half cell diameter of spherical morphology during a given time interval. The “time locomoting” as percentage of the observation time was obtained for each individual cell and averaged for cell populations.
“Velocity” describes the actual translocation efficiency of cells in the process of migration, delineating the “true” speed without interference of stopping frequencies. Velocity was calculated as the sum of all step lengths per minute divided by the number of steps migrated for each locomoting cell, and pooled or averaged for cell populations.
“Speed” represents a more general summation parameter describing overall motility as a function of the fraction of locomoting cells and their locomotor duration, individual step lengths, and step number, which was used in some experiments if all of these independent parameters showed the same tendency. The speed of a cell population was calculated from step to step as the sum of all step lengths per minute divided by the number of cells investigated (time-dependent speed containing both migrating and stopping cells). The speed of individual cells was calculated from the sum of step lengths per minute divided by the total number of steps (including both locomoting and nonlocomoting cells) and was averaged for cell populations (“mean speed”).
Viability of cells within collagen lattices subsequent to videomicroscopy was measured using calcein and ethidium homodimer (LIFE/DEAD Viability/Cytotoxicity Kit, Molecular Probes, Eugene, OR). Cell viability of simultaneous control versus antibody-treated samples after 20 h culture in collagen differed on average by 3.5% (maximum 6%); the total range varied from 60 to 99% for different sets of experiments after 24–48 h in the lattice.
Statistical Analysis
Statistics of the cumulative percentage of cells locomoting was performed as follows. Because the variables were discrete (cells were either moving or not), the nonparametric analysis of odds ratios was performed (Nikolai et al., 1998). Zelen’s exact test was used to determine the homogeneity of a series of experiments, and subsequently common odds ratio for a series of experiments was estimated by the conditional maximum likelihood estimator for P < 0.05 (including Bonferroni adjustment). Time locomoting and velocity were analyzed by two-way analysis of variance (P < 0.05, Bonferroni adjustment), taking into account the variance of these parameters between single experiments. For analysis of velocities, the two-way analysis of variance was performed using the natural logarithm of velocities (according to their asymmetric distribution). For two-way analysis of variance, gaussian distribution of residuals was confirmed (Q-Q-Plot of residues).
RESULTS
Expression of β1 Integrins and CD44 on MV3 Cells
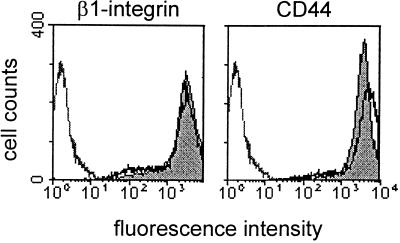
In highly motile MV3 melanoma cells, β1 integrins and CD44 were abundantly expressed at the cell surface in liquid culture as well as in collagen lattices (Figure 1). Previously, both receptors were shown to mediate melanoma cell migration across ligand-coated surfaces (Etoh et al., 1992; Goebeler et al., 1996; Ladeda et al., 1998), although integrin clustering at substrate interactions was shown for β1 integrins but not for CD44 (Friedl and Bröcker, 1997; Friedl et al., 1997; Ladeda et al., 1998). Upon migration, considerable amounts of both β1 integrins and CD44 were deposited into the lattice (Friedl, 1997); however, no down-modulation in cell surface staining was obtained for cells after 14 h culture in collagen (Figure 1, solid black line), as compared with liquid culture (Figure 1, gray area). Hence, high levels of both receptors are available for prolonged time periods on the surface of these cells.
Figure 1.
β1 integrin and CD44 surface expression on MV3 melanoma cells incorporated into 3-D collagen lattices. Cells were cultivated for 14 h in liquid culture (gray area) or within collagen lattice (solid black line), followed by incubation in collagenase and staining with mAbs 4B4 (β1 integrin) or Hermes-1 (CD44) (gray line: isotypic control). Compared with cells cultivated in liquid culture CD44 but not β1 integrin, expression was up-regulated after 14 h of culture in collagen lattices. Additionally, high levels of α3 integrins (fluorescence intensity: 2–6 × 102) and moderate staining of α5, α6, and αv integrins were detected (0.5–1 × 102), which is in accordance with previously published data (Klein et al., 1991b; Danen et al., 1993; Goebeler et al., 1996).
β1 Integrin-dependent Migration of MV3 Cells within 3-D Collagen Matrices
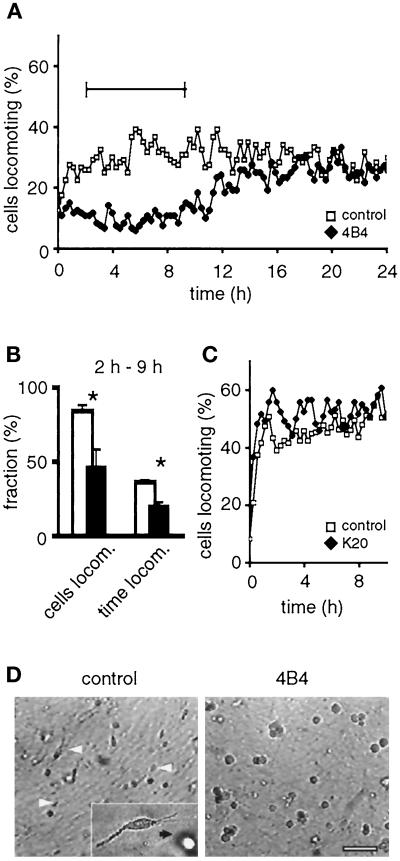
To investigate the relative contribution of β1 integrins and CD44 to melanoma cell migration in direct comparison, MV3 cells were incubated with blocking mAbs 4B4 (anti-β1 integrin) or Hermes-1 (anti-CD44) before incorporation into collagen matrices. Blocking of β1 integrins by mAb 4B4 immediately inhibited MV3 cell migration (Figure 2A). At the single-cell level, both the number of migrating cells as well as duration and frequency of locomotor periods were significantly reduced by mAb 4B4 (Figure 2B). This inhibition was associated with impaired cell polarization (Figure 2D) and, as detected by confocal microscopy, the loss of integrin clustering at contacts to collagen fibers and lacking fiber alignment (Figure 3B). In contrast, adhesion nonperturbing anti-β1 integrin mAb K20 had no effect on migration (Figure 2C) or on initial microspike formation (Figure 3D), cell polarization (Figure 3E), substrate binding, and collagen fiber alignment toward the cell body (Figure 3E, white arrowheads).
Figure 2.
Inhibition of MV3 cell migration in 3-D collagen lattices by adhesion-perturbing anti-β1 integrin mAb 4B4 (A, B, and D), but not by nonblocking mAb K20 (C). Paths of individual cells were digitized, and the percentage of cells locomoting from step to step was obtained. Cumulative percentage of cells locomoting and fraction of time locomoting (B, white bars: control; black bars: mAb 4B4) and representative cell morphologies (D) were obtained for the time period of steady-state locomotion indicated in A (‖—‖). Cells were preincubated with mAb (10 μg/ml) or PBS, washed, and embedded within the collagen matrix. No additional mAb was added to the lattices (A–C), except in D, which contained additional mAb 4B4 in the supernatant. Data represent the mean values (+SD) of three independent experiments (120 cells in total). Asterisks indicate significant differences for P < 0.05. (D) Polarized cell morphology (white arrowheads) was abolished in the presence of mAb 4B4, as detected from video recordings. Black and white cells correspond to cells at different level in depth within the collagen lattice. Bar, 100 μm.
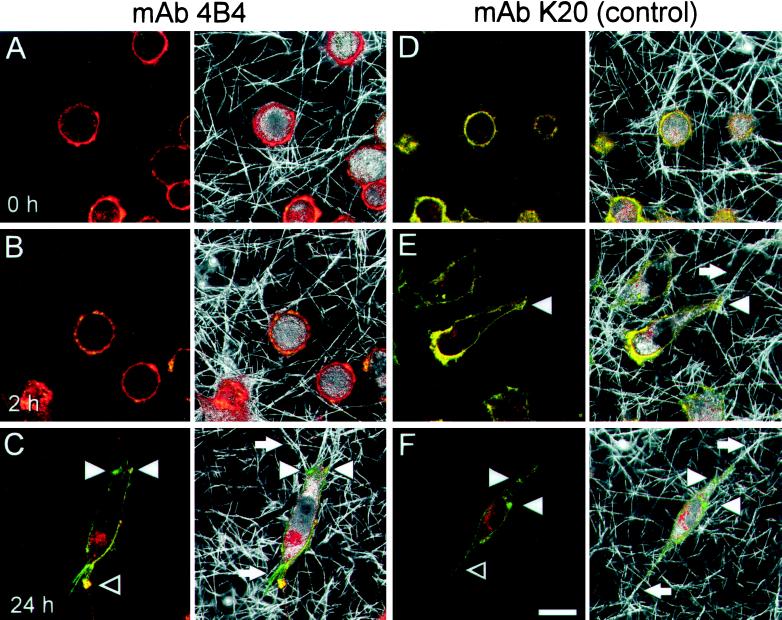
Figure 3.
Distribution of β1 integrins (left) and corresponding interactions with collagen fibers (right) after incubation with blocking mAb 4B4 and nonblocking mAb K20. MV3 cells were incubated with mAb 4B4 (A–C) or nonblocking mAb K20 (D–F), additionally stained with LRSC-conjugated F(ab)′ fragments (red fluorescence), washed, and subsequently embedded into the collagen lattice. Samples were incubated for 0 h (A and D), 2 h (B and E), or 24 h (C and F) at 37°C, fixed, and stained again with FITC-conjugated anti-β1 integrin mAb 4B4 (green fluorescence). Red color (A and B) indicates low secondary staining intensity by green mAb 4B4 caused by previous occupation of existing epitopes by 4B4–LRSC conjugates. Yellow color (D and E) indicates the superimposition of K20 (red) and 4B4 staining (green) detecting different epitopes on the β1 chain. Bar, 20 μm.
If the collagen lattice was free of additional mAb, 4B4-induced effects after cell pretreatment were immediate but transient. Ten hours after incubation with mAb 4B4, migration was progressively recovered and reached control levels after 10–16 h (Figure 2A). The late onset of migration and concomitant cell polarization were accompanied by the appearance of free 4B4 epitopes on the cell surface (Figure 3C, green fluorescence), whereas 4B4-coated β1 integrins remained internalized (Figure 3C, red fluorescence). The resulting migratory phenotype was again characterized by clustering of β1 integrins at attachment sites to collagen fibers (Figure 3C, white arrowheads), shedding of β1 integrins from the trailing edge (Figure 3C, black arrowheads), and fiber traction at the leading (Figure 3C right, top white arrow) and trailing edge (Figure 3C, bottom), as seen in spontaneously migrating MV3 cells. The internalization of receptor–mAb complexes in the process of migration was not a consequence of blocking, because integrins coated with nonblocking mAb K20 were equally internalized (Figure 3F, red fluorescence), which was reminiscent of migration-associated recycling of receptor–membrane complexes in other cells (Bretscher, 1996). Because continuous internalization of mAb-coated cell surface receptors and the reappearance of mAb-free β1 integrins greatly interfered with mAb blocking efficiency in migrating MV3 cells, all subsequent experiments were performed after prelabeling of the cells followed by addition of mAb to the collagen lattice and supernatant likewise. If blocking mAb 4B4 (10 μg/ml) was permanently present in the lattice, a more pronounced and prolonged inhibition of migration was obtained (Table 1) that persisted for >48 h.
Table 1.
Function of different integrin α-chains and β1 integrins in MV3 cell migration
| Cells locomoting (%)
|
Time locomoting (%)
|
|||
|---|---|---|---|---|
| Control | mAb | Control | mAb | |
| α2 (1) | 83 ± 4 | 28 ± 7*** | 38 ± 3 | 20 ± 2*** |
| α2 (2) | 88 ± 14 | 58 ± 13*** | 46 ± 7 | 36 ± 7** |
| α2 (1, 2) | 88 ± 14 | 27 ± 4*** | 46 ± 7 | 30 ± 4*** |
| α2 (1, 2) + α3 | 93 ± 5 | 30 ± 8*** | 48 ± 8 | 24 ± 3*** |
| α2 (1, 2) + αv | 97 ± 1 | 40 ± 9*** | 50 ± 4 | 34 ± 7*** |
| α2 (1, 2) + α3 + αv | 97 ± 1 | 38 ± 1*** | 50 ± 4 | 28 ± 5*** |
| α2 (1, 2) + α3 + α5 + α6 + αv | 93 ± 6 | 46 ± 18*** | 45 ± 8 | 26 ± 4*** |
| α3 | 88 ± 14 | 86 ± 13 | 46 ± 7 | 48 ± 7 |
| αv | 88 ± 14 | 95 ± 5 | 46 ± 7 | 45 ± 5 |
| β1 | 88 ± 3 | 23 ± 5*** | 39 ± 3 | 17 ± 4*** |
Cells were preincubated with mAbs (10 μg/ml), and mAbs were additionally added to the medium during and after polymerization of the collagen matrix. Steady-state locomotion was observed for at least 15 h and calculated from single-cell analysis as cumulative percentage of cells locomoting and time locomoting (means of three independent experiments ± SD). Significant differences of mAb treatment versus control cells are indicated by asterisks (** P < 0.001, *** P < 0.0001). mAbs: α2 (1), 6F1; α2 (2), P1E6; α3, P1B5; α5, SAM-1; α6, GoH3; αv, 17E6; β1, 4B4.
MV3 Cell Migration Is Dependent on α2β1 but Not on Other Integrins
To identify integrin α chains involved in MV3 migration and associated matrix remodeling, blocking mAb against α2, α3, α5, α6, and αv integrins were used alone and in combination. Blocking anti-α2 integrin mAbs 6F1 and P1E6 significantly reduced the percentage of cells locomoting as well as percentage of time locomoting (Table 1) but not, however, migration velocity of residually migrating cells. The inhibitory effect of mAb 6F1 was comparable with anti-β1 integrin mAb 4B4, whereas mAb P1E6 was less efficient. Blocking of other α-chains, including α3, α5, α6, and αv, did not inhibit migration or increase the effect of blocking anti-α2 mAb.
HA Promotes MV3 Cell Migration on 2-D Surface but Not in 3-D Collagen Lattices
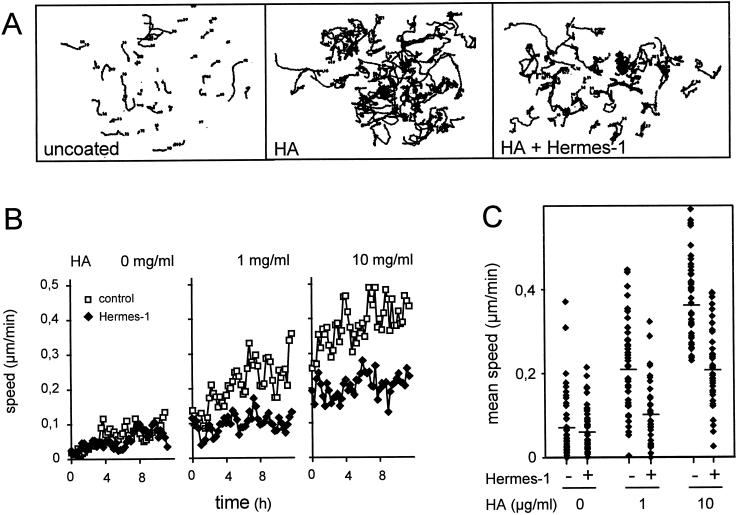
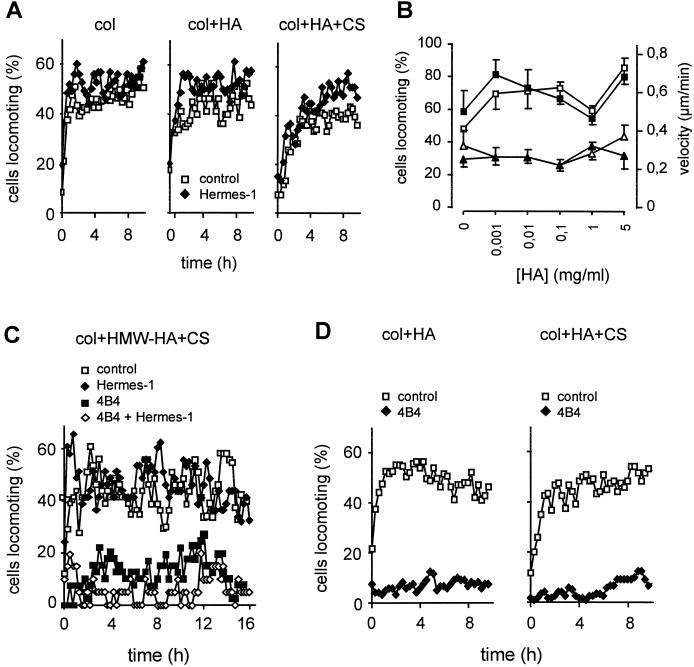
The CD44 ligand HA is ubiquitously distributed throughout interstitial tissues, forming soft interconnected lattices (Scott, 1995). With respect to HA binding, CD44 can exist in three functional states: nonactivable, activable (e.g., by substrate, mAb, or cytoplasmic serine phosphorylation), and constitutively active (Peck and Isacke, 1998). If seeded onto HA-coated coverglass, >95% of MV3 cells attached and spread on the substrate (Figure 4A) in a time-dependent manner, suggesting that CD44 was either constitutively active or inducible in MV3 cells. Subsequently, time- and dose-dependent induction of haptokinetic migration was seen on HA-coated substrate, as compared with cells on uncoated substrate (Figure 4, A and B). Both the number and time of cells locomoting and their velocities were increased, resulting in increased migration speed (Figure 4C). Haptokinetic migration on HA, but not on uncoated glass, was significantly reduced, although not completely blocked by anti-CD44 mAb Hermes-1, which is consistent with previously published data (Thomas et al., 1992; Goebeler et al., 1996).
Figure 4.
HA-induced migration on 2-D substrate is inhibited by mAb Hermes-1. Shown are individual cell paths at their actual position (A, n = 40 randomly selected cells), time-dependent migration speed (B), and migratory variation within the populations (C) in the presence or absence of mAb Hermes-1 (10 μg/ml). One representative out of at least two experiments is shown.
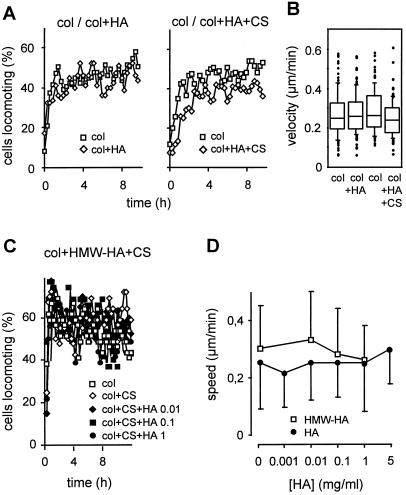
In vitro, HA spontaneously aggregates, forming multimeric branched strands (Scott et al., 1991), binds to fibrillar collagen (Turley et al., 1985), and is further multimerized and stabilized by cross-linking CS, resulting in increased biomechanical resistance of the lattice (Nishimura et al., 1998). For constitution of multicomponent lattices, HA and CS were used in concentrations found in interstitial tissues (see MATERIALS AND METHODS). In contrast to migration on 2-D HA-coated surfaces, no alteration in spontaneous MV3 cell locomotion was observed in 3-D collagen matrices supplemented with HA in the absence or presence of CS, as determined by the number of locomoting cells (Figure 5A) and their velocities (Figure 5B). The lack of HA-induced migration was present in highly purified HA as well as in high molecular weight HA (3.5–5 × 106 Da; Figure 5, C and D) for a wide dose range (Figure 5D).
Figure 5.
Effect of highly purified (A, B, and D) and high molecular weight HA (C and D) on MV3 cell migration within multicomponent matrices. Cells were embedded within matrices consisting of fibrillar collagen (col), or collagen supplemented with HA (1 mg/ml) (col+HA), or HA (1 mg/ml) and CS (20 mg/ml) (col+HA+CS). The spontaneous baseline migration in collagen as compared with multicomponent matrices was obtained from n = 3 independent experiments (A, 120 cells in total). C represents 40 cells (one representative experiment for n = 2). (B) Box plot analysis of cumulative velocities at the single-cell level, as derived from A. The lines within the boxes indicate the medians, and boxes represent range from 25th to 75th percentiles. Further scattering is indicated by whiskers (indicating values within 1.5 × box-length range), and extreme scattering values are indicated individually (●). (D) For dose–response to HA, the mean speed ± SD was assessed (40 cells, one representative experiment).
CD44 Neither Supports Migration within Three-Component Matrices Consisting of Collagen, HA, and CS, nor Restores Migration after β1 Integrin Blocking
Blocking of the HA-binding epitope of CD44 by mAb Hermes-1 did not affect migration, regardless of whether native 3-D collagen lattices or lattices supplemented with HA or HA and CS were used (Figure 6A). Neither increased mAb concentration (40 μg/ml) or incubation with Hermes-3 (detecting a different non-HA–binding epitope) nor variations in HA concentrations (Figure 6B) or the use of high molecular weight HA (Figure 6C) resulted in altered migration. These data indicate that the CD44 Hermes-1 epitope, despite its established function in HA-mediated motility on planar surfaces, may not be involved in the migration of MV3 cells, if a 3-D multicomponent ECM is used.
Figure 6.
CD44-independent baseline migration (A and B) and lack of CD44-mediated migratory restoration after blocking of β1 integrin function (C and D). Experiments were performed as described in Figure 5. Cells were preincubated with blocking mAbs (anti-CD44 mAb Hermes-1 and anti-β1 integrin mAb 4B4; 10 μg/ml) or PBS, embedded within the different matrices, and mAb was additionally added to supernatant and matrix. (B) Mean steady-state values of baseline velocity (▵) and percentage of migrating cells (□) in the absence (open symbols) or presence (closed symbols) of mAb Hermes-1. Data represent 120 cells (n = 3 experiments) in A and D and one representative experiment for n = 2 (B and C).
To rule out that in contrast to migration across HA, CD44 function in 3-D collagen substrate may be masked by extensive β1 integrin-mediated motility, MV3 cell migration in HA-containing collagen lattices was investigated in the presence of mAb 4B4; however, similar to native collagen (Figure 2), the presence of HA or CS, or both, had no supportive effect on mAb 4B4-impaired migration in lattices containing highly purified (Figure 6D) or high molecular weight HA (Figure 6C). Hence, despite abolished integrin-mediated motility and the availability of HA substrate, CD44 did not compensate for the loss of β1 integrin function via HA-mediated interactions.
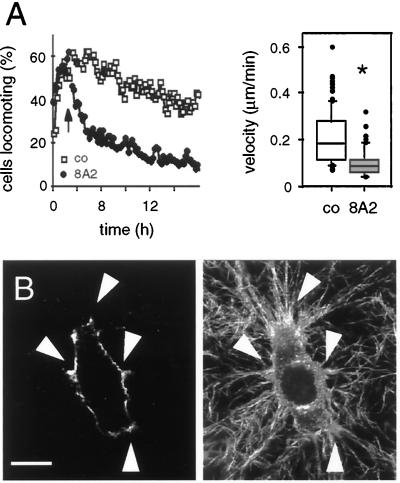
Stimulation of Adhesion to Collagen by Anti-β1 Integrin mAb 8A2 Results in Cell Immobilization
In non-neoplastic fibroblasts, optimal migration rates are reduced after 1) disruption of substrate adhesion leading to reduced maximal traction forces and, ultimately, detachment or rounding up of the cells as well as 2) increased attachment strength resulting in delayed detachment and reduced migration (Horwitz and Lauffenburger, 1996; Huttenlocher et al., 1996; Palecek et al., 1997). In MV3 cells similar to other tumor cells, blocked substrate attachment leads to detachment-related loss of traction forces, polarization, and migration (compare Figure 3B); however, it is not known whether neoplastic cells migrating in 3-D matrices cease migration if attachment is increased or whether more versatile compensatory de-adhesion strategies exist, such as CD44-mediated detachment (Knudson, 1998), to rescue migration. To monitor attachment-related changes in migration without changing collagen ligand density (which would increase fiber density and physical matrix resistance), MV3 cells were incubated with adhesion-inducing anti-β1 integrin mAb 8A2 (Kovach et al., 1992) 3 h after the onset of migration (Figure 7A, black arrow). mAb 8A2 induced a progressive loss of the number of locomoting cells. This inhibition was similarly effective as described for mAb 4B4; however, in contrast to reduced substrate binding and development of spherical morphology in the presence of mAb 4B4 (Figure 3B), mAb 8A2 favored a highly polarized morphology, pronounced traction, and bundling of collagen fibers and integrin clustering for >24 h (Figure 7C). This sustained fiber binding resulted in delayed cell detachment (as detected from time-lapse video recordings) and, consequently, decreased migration velocity (Figure 7B). Hence, reduction (4B4) and induction (8A2) of adhesion to collagen fibers impaired MV3 cell migration by different mechanisms, both of which were not compensated by CD44 function.
Figure 7.
Effect of adhesion-inducing anti-β1 integrin mAb 8A2 on MV3 cell migration (A and B), β1 integrin distribution (C, left), and interaction with collagen fibers (C, right). MV3 cells were embedded into collagen lattices. Three hours after the migratory onset mAb 8A2 was added to the supernatant (A, black arrow). After 24 h, samples were fixed, and 8A2-coated β1 integrins were stained with secondary LRSC-conjugated F(ab)′ fragments. Fluorescence (C, left) and reflection images (C, right) were detected by confocal microscopy. β1 integrin clustering and corresponding interactions with collagen fibers are indicated by white arrowheads. Bar, 20 μm.
DISCUSSION
β1 integrins and CD44 mediate adhesion to ubiquitously expressed ECM ligands collagen and HA, respectively, and hence are important candidate receptors for cell migration through the tissue. In many tumors, both collagen and HA are detected at the interface between invading tumor cells and the surrounding host tissue, as well as within the tumor (Yeo et al., 1996), potentially acting as a localized promigratory matrix (Knudson, 1996). The adhesive function of β1 integrins and CD44 in cell migration is well established for cellular locomotion on planar surfaces consisting of isolated ECM ligands (Thomas et al., 1992; Goebeler et al., 1996); however, circumstantial evidence suggests major differences in β1 integrin and CD44 usage in cell motility. β1 integrins cluster upon ligand binding to initiate the formation of focal contacts (Regen and Horwitz, 1992; Horwitz and Lauffenburger, 1996), whereas CD44 remains diffusely distributed and nonclustered and appears to be excluded from focal contacts in migrating cells (Friedl et al., 1997; Ladeda et al., 1998). In some cells, CD44-related motility is even seen independent of precoated HA substrate (Birch et al., 1991), suggesting substrate-independent mechanisms. Nevertheless, although not yet formally proven, CD44 function in cell migration—analogous to β1 integrin function—was proposed to result from direct attachment-driven interaction with ECM components, most notably HA (Bartolazzi et al., 1994; Knudson, 1996), followed by coupling of CD44 to the actin cytoskeleton (Peck and Isacke, 1998).
Previously, increased migration of melanoma cells on HA-covered surfaces was correlated with high CD44 expression induced by either CD44 overexpression after transfection (Thomas et al., 1992) or naturally occurring differences in endogenous CD44 expression (Goebeler et al., 1996). When HA served as the sole substrate mediating attachment to 2-D substrate such as HA-coated transwell chambers or coverglass, CD44-dependent migration was consistently decreased by mAbs directed to the Hermes-1 epitope, the putative HA binding site of human CD44 (Jalkanen et al., 1987; Thomas et al., 1992; Goebeler et al., 1996) as well as by other blocking anti-CD44 mAbs in murine cells (Ladeda et al., 1998). Additional non–Hermes-1 epitopes appear to facilitate migration if surfaces are coated with HA associated with reconstituted basement membrane (Koochekpour et al., 1995; Radotra and McCormick, 1997). Hence, an HA-dependent promigratory function of CD44 in haptokinetic migration, as confirmed in the present study using time-lapse videomicroscopy, as well as the interaction of CD44 with the actin cytoskeleton, is generally accepted (Lokeshwar et al., 1994; Tsukira et al., 1994); however, the precise promigratory mechanism remains unresolved. In most cases, in particular if HA is combined with chemoattractants and/or complex ECM (Radotra and McCormick, 1997), it is unclear whether the antibody simply blocked CD44-matrix interactions (Kincade et al., 1997) or whether HA-mediated CD44-ligation initiated a cytokine cascade or other responses rather than simply acting as an anchoring matrix. This latter notion is supported by our finding that contact-dependent HA-induced migration was a time-dependent delayed process developing independent of the onset of cell attachment and spreading. In the presence of anti-CD44 mAb Hermes-1, reduced migration was accompanied by persistent cell-substrate interaction, polarization, ruffling of filopodia, and spreading (Wolf and Friedl, unpublished observations, as detected from video recordings). Here, the mechanism of HA-mediated motility on 2-D surfaces greatly differs from integrin-mediated haptokinetic migration, the blocking of which results in reduced substrate adhesion, loss of polarization, and acquisition of a detached, spherical cell shape.
Two major differences exist between 2-D and 3-D tissue substrates, namely 1) the degree of polymerization and substrate rigidity to provide sufficient binding strength and 2) the biomechanical resistance imposed by 3-D tissues not present in 2-D models. These differences may force cells to develop different levels in attachment strength or deformation of the cell body. On 2-D surfaces, HA forming long and branched strands sticks to the surface, providing a highly stable and presumably nondeformable anchoring structure (Scott et al., 1991). In 3-D tissues, however, HA forms thick fibrillar aggregates 10–40 nm in diameter that multimerize to 3-D honeycomb-like networks starting at a low dilution of <1 μg/ml (Scott et al., 1992; Scott, 1995) and a low molecular weight of 300 kDa (Scott et al., 1992). HA self-assembly results in elastic gel-like meshworks that can occur independent of other ECM components (Scott et al., 1991). HA also interacts with and branches other ECM components such as collagen fibers (Turley et al., 1985; Scott et al., 1992), thereby potentially serving as a proadhesive substrate (Scott, 1995). Efficient interpenetration and immobilization of HA in collagen lattices are supported by ultracentrifugation data showing a considerably greater mechanical stability and resistance to compression in the presence of HA (Fessler, 1960). The polysaccharide CS additionally cross-links HA monomers to form stable heteroduplexes (Scott et al., 1992), thereby increasing HA polymer rigidity (Nishimura et al., 1998). CS at physiological concentrations (5–40 mg/ml), as detected in dermis or cartilage, does not self-assemble (Scott et al., 1991 and 1992), but increases the viscosity of HA gels by two- to threefold (Nishimura et al., 1998), avidly interacts with collagen substrate, and stabilizes the collagen triple-helical structure (Comper and Laurent, 1978). In the present study, addition of CS to collagen markedly increased collagen fiber bundling in the presence or absence of HA, as detected by phase-contrast and confocal reflection microscopy (Wolf and Friedl, unpublished observations). This suggests an impact of CS on collagen fiber assembly, however, that did not interfere with or support MV3 cell migration. Last, spontaneous HA binding to fibrillar collagen (Turley et al., 1985) is thought to be strengthened by CS (Rudzki and Jothy, 1997) without interfering with CD44–HA interaction (Samuelsson and Gustafson, 1998). To summarize current knowledge on spontaneous matrix assembly, multi-component lattices consisting of a collagen backbone supplemented with highly purified or high molecular weight HA or CS, or both, should provide a 3-D substrate suitable for simultaneous availability of interconnected ECM ligands.
Within such matrices, however, MV3 migration was dominated by integrin α2β1, whereas no synergistic or residual promigratory function was obtained by CD44, HA, or CS. Concomitant with migration, other important migration-associated cell functions were blocked by anti-α2 or -β1 integrin mAbs but not by mAb Hermes-1, including cell polarization, fiber traction and reorganization (Friedl et al., 1997), collagen gel contraction (Klein et al., 1991a; Maaser and Friedl, unpublished observations), and the shedding of β1 integrins and CD44 into the lattice (Regen and Horwitz, 1992; Friedl et al., 1997). These findings suggest that all of these events are functionally interconnected, dependent on α2β1 integrins, and not compensated by CD44–HA interactions. In addition to HA, CD44 has been observed to display affinity for CS (Aruffo et al., 1990); however, a promigratory function of CS was absent in both spontaneous locomotion as well as in cells after mAb 4B4 treatment. Hence, despite its abundant expression on MV3 cells and its promigratory action in MV3 cell migration across HA-coated surfaces, we found no indication for force-generating effects provided by CD44 interacting with HA or CS if a 3-D network of interconnected ECM ligands was used.
The lack of promigratory CD44 function in MV3 cells in vitro is in line with data from gene disruption experiments in vivo. MDAY-D2 lymphosarcoma cells lacking CD44 expression fully retain their invasive capacity into subcutaneous tissue and metastasis as compared with parental CD44-positive MDAY-D2 wild-type cells (Driessens et al., 1995). Similarly, in highly metastatic pancreatic carcinoma cells, CD44 variant binding to HA does not contribute to in vivo invasion and metastasis, as shown by hyaluronidase transfection resulting in undiminished metastasis despite a loss of the cellular capacity to bind HA (Sleeman et al., 1996). Although CD44 mediates cancer cell migration across surfaces via recognition of HA, the present negative findings raise the question of how CD44 function actually contributes to tumor biology. Several observations argue against a direct CD44-related function in the migratory, hence force-generating, action per se: 1) CD44 is excluded from focal contacts (Friedl et al., 1997), and 2) continuous cell spreading on HA is present even after Hermes-1 binding; 3) HA forms lattices of high elasticity and water content that may not provide the sufficient substrate rigidity that is required for anchoring CD44 in cooperation with integrin function. Other established CD44 functions may contribute to tumor progression both in vitro and in vivo. These functions include 1) CD44-mediated signaling and activation of a multigenic invasion program (Entwistle et al., 1996; Lamb et al., 1997) and tumor growth (Bartolazzi et al., 1994), 2) capture of growth factors and chemokines (Radotra and McCormick, 1997), 3) facilitation of detachment and CD44 cleavage (Friedl, 1997), e.g., by matrix-metalloproteinases (Okamoto et al., 1999), 4) CD44-mediated degradation of HA and removal of tissue barriers (Cultry et al., 1994), 5) enhanced transendothelial migration and extravasation (Koochekpour et al., 1995), 6) protection against immune defense by assembling a pericellular HA-containing matrix coat (Knudson, 1998), and 7) the prevention of apoptosis (Yu et al., 1997). In conclusion, CD44, in contrast to β1 integrins, is unlikely to mediate physically integrated receptor–ligand interactions in the migratory action.
Migration was inhibited by adhesion-blocking mAbs against the β1-chain as well as the α2-chain, confirming the contribution of both integrin chains to bind not only unpolymerized collagen, as used in many studies, but also multimeric fibrillar collagen exhibiting fiber diameters of up to 0.5 μm. The difference between mAb 6F1- and P1E6-mediated inhibition of migration correlates with their capacity to block cell adhesion to collagen. Both mAbs bind to the I-domain of the α2 chain comprising residues 173–259 (Kamata et al., 1994), which was described as a ligand recognition and adhesion-promoting region (Kamata et al., 1994). Although exact epitope mapping as well as the relative binding efficiency to fibrillar rather than monomeric collagen remain to be established, mAb P1E6-induced inhibition of cell adhesion to the collagen-coated surface is incomplete (Carter et al., 1990) and appears less efficient if compared with mAb 6F1 (Coller et al., 1989; Kamata et al., 1994).
MV3 cells were shown to additionally express the alternative collagen receptors a3β1 and avβ3 integrins, potentially contributing to migration in 3-D collagen lattices (Haas and Plow, 1994; Mitjans et al., 1995; Lauer et al., 1998); however, the central function of α2β1 integrin in MV3 cell migration dominated over these other integrins. First, no additional effect on residual motility was seen if all relevant integrin α chains were simultaneously blocked, and second, blocking of α2 integrins by a combination of two independent blocking mAbs was as pronounced as seen in blocking the common β1 chain, thus excluding alternative migration-enhancing functions of these integrins. As a further possibility, interaction of CD44 with type I collagen, which was described as an alternative, low-affinity ligand for CD44 (Wayner and Carter, 1987; Knudson, 1998), was proposed to support growth-dependent penetration of mammary epithelial cell clusters into collagen gels (Iida and Bourguignon, 1997); however, using high-resolution time-lapse videomicroscopy, a promigratory function of CD44–collagen interaction was excluded in spontaneously migrating as well as immobilized MV3 cells after blocking or activation of β1 integrins in native collagen matrices, excluding binding of CD44 to collagen as a promigratory mechanism in this model. In conclusion, the mechanism by which integrins—however not CD44—promote MV3 cell migration through 3-D collagen and multicomponent lattices resides in their adhesive and force-generating function, both of which were antagonized by adhesion-perturbing mAb.
We also tested whether the migration of dedifferentiated and highly invasive melanoma cells follows a similar, tightly regulated migration program of intermediate adhesion as proposed for non-neoplastic fibroblasts (Regen and Horwitz, 1992; Lauffenburger and Horwitz, 1996; Cox and Huttenlocher, 1998) or whether alternative, potentially less coordinated and more versatile mechanisms are used. The haptokinetic migration model mostly established for β1 integrins predicts maximal migration rates at an intermediate level of cell-substratum adhesion formed at the leading edge and maintained throughout the cell body (Schmidt et al., 1993; Cox and Huttenlocher, 1998). To achieve cell translocation, attachment must be followed by coordinated detachment from the trailing edge. In MV3 cells, both blocking of adhesion by mAb 4B4 (resulting in spherical cell shape via loss of cell–collagen fiber interaction) as well as induction of a high-affinity binding state of β1 integrins by mAb 8A2 (leading to cell immobilization via long-term maintenance of multipolar interaction to collagen fibers—hence a loss of polarized detachment) led to a strong decrease in locomotor activity. This confirms that both sufficient adhesion strength coupled to pseudopod protrusion as well as polar detachment (Regen and Horwitz, 1992; Palecek et al., 1997) are rate-limiting processes for MV3 cell migration in 3-D collagen lattices that are not compensated by CD44.
In conclusion, the mechanisms used by MV3 cells for migration within a 3-D matrix partially correspond to the integrin-dependent locomotory action of non-neoplastic cells such as fibroblasts on planar surfaces, following the paradigm of intermediate adhesion (Huttenlocher et al., 1996; Lauffenburger and Horwitz, 1996; Palecek et al., 1997) and transient high-affinity binding (Cox and Huttenlocher, 1998); however, in such a 3-D biomechanically demanding multicomponent ECM, not only the biophysical architecture but also the forces required to overcome biophysical matrix resistance may greatly differ from 2-D haptokinetic migration models. Although some adhesivity mediated by CD44 on immobilized HA ligand may favor motility across surfaces, migration in 3-D tissues may follow more complex principles, suggesting that careful reevaluation of promigratory adhesion receptors, which were initially established for 2-D migration models, may yield a different order of hierarchy for cell migration through 3-D interstitial tissue.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge N. L. Kovach (University of Washington, Seattle, WA), B. S. Coller (Mount Sinai Medical Center, New York, NY), and S. Jalkanen (University of Turku, Turku, Finland) for the generous supply of antibodies. We also thank Martina Joβberger and Margit Ott for excellent technical assistance, and Rebekka Topp and Andreas Christmann (Institute of Statistics, University of Dortmund, Dortmund, Germany) for great advice and support in statistical analysis. This work was supported by the Deutsche Forschungsgemeinschaft (KL 510/3-2) and the Wilhelm-Sander-Foundation (No. 96.030.01); K.S.Z. was supported by the Fritz-Bender-Foundation, Germany.
Abbreviations used:
- CS
chondroitin sulfate
- ECM
extracellular matrix
- HA
hyaluronan
- LRSC
lissamine-rhodamine sulfonyl chloride
- 3-D
three-dimensional
Footnotes
Online version of this article contains video material. Online version available at www.molbiolcell.org.
REFERENCES
- Arroyo AG, Sanchez-Mateos P, Campanero MR, Martin- Padura I, Dejana E, Sanchez-Madrid F. Regulation of the VLA integrin-ligand interactions through the beta1 subunit. J Cell Biol. 1992;117:659–670. doi: 10.1083/jcb.117.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Aznavoorian S, Stracke ML, Parsons J, McClanahan J, Liotta LA. Integrin αvβ3 mediates chemotactic and haptotactic motility in human melanoma cells through different signaling pathways. J Biol Chem. 1996;271:3247–3254. doi: 10.1074/jbc.271.6.3247. [DOI] [PubMed] [Google Scholar]
- Bartolazzi A, Peach R, Aruffo A, Stamenkovic I. Interaction between CD44 and hyaluronate is directly implicated in the regulation of tumor development. J Exp Med. 1994;180:53–66. doi: 10.1084/jem.180.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer JS, Schreiner CL, Giancotti FG, Ruoslahti E, Juliano RL. Motility of fibronectin receptor-deficient cells on fibronectin and vitronectin: collaborative interactions among integrins. J Cell Biol. 1992;116:477–487. doi: 10.1083/jcb.116.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch M, Mitchell S, Hart IR. Isolation and characterization of human melanoma cell variants expressing high and low levels of CD44. Cancer Res. 1991;51:6660–6667. [PubMed] [Google Scholar]
- Bretscher MS. Moving membrane up to the front of migrating cells. Cell. 1996;85:465–467. doi: 10.1016/s0092-8674(00)81246-5. [DOI] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–519. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Carter WG, Wayner EA, Bouchard TS, Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990;110:1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cidadao AJ. Interactions between fibronectin, glycosaminoglycans and native collagen fibrils: an EM study in artificial three-dimensional extracellular matrices. Eur J Cell Biol. 1989;48:303–312. [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Coller BS, Beer JH, Scudder LE, Steinberg MH. Collagen-platelet interactions: evidence for a direct interaction of collagen with platelet GPIa/IIa and an indirect interaction with platelet GPIIb/IIIa mediated by adhesive proteins. Blood. 1989;74:182–192. [PubMed] [Google Scholar]
- Comper WD, Laurent TC. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978;1:255–315. doi: 10.1152/physrev.1978.58.1.255. [DOI] [PubMed] [Google Scholar]
- Cox EA, Huttenlocher A. Regulation of integrin- mediated adhesion during migration. Microsc Res Tech. 1998;43:412–419. doi: 10.1002/(SICI)1097-0029(19981201)43:5<412::AID-JEMT7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Cultry M, Shizari M, Thompson EW, Underhill CB. Binding and degradation of HA by human breast cancer cell lines expressing different forms of CD44: correlation with invasive potential. J Cell Physiol. 1994;160:275–286. doi: 10.1002/jcp.1041600209. [DOI] [PubMed] [Google Scholar]
- Danen EHJ, van Muijen GNP, van de Wiel-van Kemenade E, Jansen KJF, Ruiter DJ, Figdor CG. Regulation of integrin-mediated adhesion to laminin and collagen in human melanocytes and in nonmetastatic and highly metastatic human melanoma cells. Int J Cancer. 1993;54:315–321. doi: 10.1002/ijc.2910540225. [DOI] [PubMed] [Google Scholar]
- Driessens MH, Stroeken PJ, Rodriguez Erena NF, van der Valk MA, van Rijthoven EA, Roos E. Targeted disruption of CD44 in MDAY-D2 lymphosarcoma cells has no effect on subcutaneous growth or metastatic capacity. J Cell Biol. 1995;131:1849–1855. doi: 10.1083/jcb.131.6.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entwistle J, Hall CL, Turley EA. HA receptors: regulators of signaling to the cytoskeleton. J Cell Biochem. 1996;61:569–577. doi: 10.1002/(sici)1097-4644(19960616)61:4<569::aid-jcb10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Etoh T, Byers HR, Mihm MC., Jr Integrin expression in malignant melanoma and their role in cell attachment and migration on extracellular matrix proteins. J Dermatol. 1992;19:841–846. doi: 10.1111/j.1346-8138.1992.tb03794.x. [DOI] [PubMed] [Google Scholar]
- Fessler JH. A structural function of mucopolysaccharide in connective tissue. Biochem J. 1960;76:124–132. doi: 10.1042/bj0760124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Bröcker E-B. Cancer cell interactions with the extracellular matrix involved in tissue invasion: motility mechanisms beyond the single cell paradigm. In: Heine H, Rimpler M, editors. Extracellular Matrix and Groundregulation System in Health and Disease. Stuttgart: Gustav Fischer; 1997. pp. 7–18. [Google Scholar]
- Friedl, P., and Bröcker, E.-B. (1999). The biology of cell locomotion within three-dimensional extracellular matrix. Cell. Mol. Life Sci. (in press). [DOI] [PMC free article] [PubMed]
- Friedl P, Bröcker E-B, Zänker KS. Integrins, cell matrix interactions and cell migration strategies: fundamental differences in leukocytes and tumor cells. Cell Adh Commun. 1998a;6:225–236. doi: 10.3109/15419069809004478. [DOI] [PubMed] [Google Scholar]
- Friedl P, Entschladen F, Conrad C, Niggemann B, Zänker KS. T lymphocytes migrating in 3-D collagen lattices lack focal adhesions and utilize β1 integrin-independent strategies for polarization, interaction with collagen fibers, and migration. Eur J Immunol. 1998b;28:2331–2343. doi: 10.1002/(SICI)1521-4141(199808)28:08<2331::AID-IMMU2331>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Friedl P, Maaser K, Klein CE, Niggemann B, Zänker KS. Migration of highly aggressive MV3 melanoma cells in 3-D collagen lattices results in local matrix reorganisation and shedding of beta1 integrins and CD44. Cancer Res. 1997;57:2061–2070. [PubMed] [Google Scholar]
- Friedl P, Noble PB, Zänker KS. Lymphocyte locomotion in three-dimensional collagen gels. Comparison of three quantitative methods for analyzing cell trajectories. J Immunol Methods. 1993;165:157–165. doi: 10.1016/0022-1759(93)90341-4. [DOI] [PubMed] [Google Scholar]
- Friedl P, Noble PB, Zänker KS. Lymphocyte locomotion in a three-dimensional collagen matrix: expression and function of cell adhesion molecules. J Immunol. 1995;154:4973–4985. [PubMed] [Google Scholar]
- Friedl P, Zänker KS, Bröcker E-B. Cell migration strategies in 3-D extracellular matrix: differences in morphology, cell matrix interactions, and integrin function. Microsc Res Tech. 1998c;43:369–378. doi: 10.1002/(SICI)1097-0029(19981201)43:5<369::AID-JEMT3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Goebeler M, Kaufmann D, Bröcker E-B, Klein CE. Migration of highly aggressive melanoma cells on hyaluronic acid is associated with functional changes, increased turnover and shedding of CD44 receptors. J Cell Sci. 1996;109:1957–1964. doi: 10.1242/jcs.109.7.1957. [DOI] [PubMed] [Google Scholar]
- Guo Y, Ma J, Wang J, Che X, Narula J, Bigby M, Wu M, Sy MS. Inhibition of human melanoma growth and metastasis in vivo by anti-CD44 monoclonal antibody. Cancer Res. 1994;54:1561–1565. [PubMed] [Google Scholar]
- Haas TA, Plow EF. Integrin-ligand interactions: a year in review. Curr Opin Cell Biol. 1994;6:656–662. doi: 10.1016/0955-0674(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Ginsberg MH, Horwitz AF. Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand-binding affinity. J Cell Biol. 1996;134:1551–1562. doi: 10.1083/jcb.134.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Iida N, Bourguignon LY. Coexpression of CD44 variant (v10/ex14) and CD44S in human mammary epithelial cells promotes tumorigenesis. J Cell Physiol. 1997;171:152–160. doi: 10.1002/(SICI)1097-4652(199705)171:2<152::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Jalkanen S, Bargatze RF, de los Toyos J, Butcher EC. Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85–95-kDa glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol. 1987;105:983–990. doi: 10.1083/jcb.105.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata T, Puzon W, Takada Y. Identification of putative ligand binding sites within I domain of integrin alpha 2 beta 1 (VLA-2, CD49b/CD29) J Biol Chem. 1994;269:9659–9663. [PubMed] [Google Scholar]
- Kincade PW, Zheng Z, Katoh S, Hanson L. The importance of cellular environment to function of the CD44 matrix receptor. Curr Opin Cell Biol. 1997;9:635–642. doi: 10.1016/s0955-0674(97)80116-0. [DOI] [PubMed] [Google Scholar]
- Klein CE, Dressel D, Steinmayer T, Mauch C, Eckes B, Krieg T, Bankert RB, Weber L. Integrin alpha 2 beta 1 is up-regulated in fibroblasts and highly aggressive melanoma cells in three-dimensional collagen lattices and mediates the reorganization of collagen I fibrils. J Cell Biol. 1991a;115:1427–1436. doi: 10.1083/jcb.115.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein CE, Steinmayer T, Kaufmann D, Weber L, Bröcker EB. Identification of a melanoma progression antigen as integrin VLA-2. J Invest Dermatol. 1991b;96:281–284. doi: 10.1111/1523-1747.ep12464485. [DOI] [PubMed] [Google Scholar]
- Klominek J, Sumitran Karuppan S, Hauzenberger D. Differential motile response of human malignant mesothelioma cells to fibronectin, laminin and collagen type IV: the role of beta1 integrins. Int J Cancer. 1997;72:1034–1044. doi: 10.1002/(sici)1097-0215(19970917)72:6<1034::aid-ijc19>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Knudson W. Tumor-associated HA. Providing an extracellular matrix that facilitates invasion. Am J Pathol. 1996;148:1721–1726. [PMC free article] [PubMed] [Google Scholar]
- Knudson W. The role of CD44 as a cell surface HA receptor during tumor invasion of connective tissue. Front Biosci. 1998;3:604–615. doi: 10.2741/a305. [DOI] [PubMed] [Google Scholar]
- Knudson W, Knudson CB. Overproduction of HA in the tumor stroma. In: Adany R, editor. Tumor Matrix Biology. Boca Raton, FL: CRC Press; 1995. pp. 55–79. [Google Scholar]
- Koochekpour S, Pilkington GJ, Merzak A. Hyaluronic acid/CD44H interaction induces cell detachment and stimulates migration and invasion of human glioma cells in vitro. Int J Cancer. 1995;63:450–454. doi: 10.1002/ijc.2910630325. [DOI] [PubMed] [Google Scholar]
- Kovach NL, Carlos TM, Yee E, Harlan JM. A monoclonal antibody to beta 1 integrin (CD29) stimulates VLA-dependent adherence of leukocytes to human umbilical vein endothelial cells and matrix components. J Cell Biol. 1992;116:499–509. doi: 10.1083/jcb.116.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladeda V, Aguirre GJ, Bal dKJ. Function and expression of CD44 during spreading, migration, and invasion of murine carcinoma cells. Exp Cell Res. 1998;242:515–527. doi: 10.1006/excr.1998.4094. [DOI] [PubMed] [Google Scholar]
- Lamb RF, Hennigan RF, Turnbull K, Katsanakis KD, MacKenzie ED, Birnie GD, Ozanne BW. AP-1-mediated invasion requires increased expression of the HA receptor CD44. Mol Cell Biol. 1997;17:963–976. doi: 10.1128/mcb.17.2.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer JL, Gendron CM, Fields GB. Effect of ligand conformation on melanoma cell alpha3beta1 integrin- mediated signal transduction events: implications for a collagen structural modulation mechanism of tumor cell invasion. Biochemistry. 1998;37:5279–5287. doi: 10.1021/bi972958l. [DOI] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Lokeshwar VB, Fregien N, Bourguignon LY. Ankyrin-binding domain of CD44(GP85) is required for the expression of hyaluronic acid-mediated adhesion function. J Cell Biol. 1994;126:1099–1109. doi: 10.1083/jcb.126.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitjans F, Sander D, Adan J, Sutter A, Martinez JM, Jaggle CS, Moyano JM, Kreysch HG, Piulats J, Goodman SL. An antialpha v-integrin antibody that blocks integrin function inhibits the development of a human melanoma in nude mice. J Cell Sci. 1995;108:2825–2838. doi: 10.1242/jcs.108.8.2825. [DOI] [PubMed] [Google Scholar]
- Morimoto C, Letvin NL, Boyd AW, Hagan M, Brown HM, Kornacki MM, Schlossman SF. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985;134:3762–3769. [PubMed] [Google Scholar]
- Nikolai G, Niggemann B, Werner M, Zänker KS, Friedl P. Cross-linking of CD2 and CD3 in human CD4+ T lymphocytes results in rapid cytoskeletal engagement and the induction of migration. Immunology. 1998;95:62–68. doi: 10.1046/j.1365-2567.1998.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Yan W, Mukudai Y, Nakamura S, Nakamasu K, Kawata M, Kawamoto T, Noshiro M, Hamada T, Kato Y. Role of CS-HA interactions in the viscoelastic properties of extracellular matrices and fluids. Biochim Biophys Acta. 1998;1380:1–9. doi: 10.1016/s0304-4165(97)00119-0. [DOI] [PubMed] [Google Scholar]
- Okamoto I, Kawano Y, Tsuiki H, Sasaki J, Nakao M, Matsumoto M, Suga M, Ando M, Nakajima M, Saya H. CD44 cleavage induced by a membrane-associated metalloprotease plays a critical role in tumor cell migration [In Process Citation] Oncogene. 1999;18:1435–1446. doi: 10.1038/sj.onc.1202447. [DOI] [PubMed] [Google Scholar]
- Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- Peck D, Isacke CM. HA-dependent cell migration can be blocked by a CD44 cytoplasmic domain peptide containing a phosphoserine at position 325. J Cell Sci. 1998;111:1595–1601. doi: 10.1242/jcs.111.11.1595. [DOI] [PubMed] [Google Scholar]
- Radotra B, McCormick D. Glioma invasion in vitro is mediated by CD44-HA interactions. J Pathol. 1997;181:434–438. doi: 10.1002/(SICI)1096-9896(199704)181:4<434::AID-PATH797>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Regen CM, Horwitz AF. Dynamics of beta 1 integrin-mediated adhesive contacts in motile fibroblasts. J Cell Biol. 1992;119:1347–1359. doi: 10.1083/jcb.119.5.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riikonen T, Westermarck J, Koivisto L, Broberg A, Kahari VM, Heino J. Integrin α2β1 is a positive regulator of collagenase (MMP-1) and collagen α1(I) gene expression. J Biol Chem. 1995;270:13548–13552. doi: 10.1074/jbc.270.22.13548. [DOI] [PubMed] [Google Scholar]
- Rudzki Z, Jothy S. CD44 and the adhesion of neoplastic cells. Mol Pathol. 1997;50:57–71. doi: 10.1136/mp.50.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson C, Gustafson S. Studies on the interaction between HA and a rat colon cancer cell line. Glycoconj J. 1998;15:169–175. doi: 10.1023/a:1006920323095. [DOI] [PubMed] [Google Scholar]
- Schiro JA, Chan BM, Roswit WT, Kassner PD, Pentland AP, Hemler ME, Eisen AZ, Kupper TS. Integrin alpha 2 beta 1 (VLA-2) mediates reorganization and contraction of collagen matrices by human cells. Cell. 1991;67:403–410. doi: 10.1016/0092-8674(91)90191-z. [DOI] [PubMed] [Google Scholar]
- Schmidt CE, Horwitz AF, Lauffenburger DA, Sheetz MP. Integrin-cytoskeletal interactions in migrating fibroblasts are dynamic, asymmetric, and regulated. J Cell Biol. 1993;123:977–991. doi: 10.1083/jcb.123.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JE. Extracellular matrix, supramolecular organization and shape. J Anat. 1995;187:259–269. [PMC free article] [PubMed] [Google Scholar]
- Scott JE, Chen Y, Brass A. Secondary and tertiary structures involving chondroitin and CSs in solution, investigated by rotary shadowing/electron microscopy and computer simulation. Eur J Biochem. 1992;209:675–680. doi: 10.1111/j.1432-1033.1992.tb17335.x. [DOI] [PubMed] [Google Scholar]
- Scott JE, Cummings C, Brass A, Chen Y. Secondary and tertiary structures of HA in aqueous solution, investigated by rotary shadowing-electron microscopy and computer simulation. HA is a very efficient network-forming polymer. Biochem J. 1991;274:699–705. doi: 10.1042/bj2740699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L, Sleeman J, Herrlich P, Ponta H. Hyaluronate receptors: key players in growth, differentiation, migration and tumor progression. Curr Opin Cell Biol. 1994;6:726–733. doi: 10.1016/0955-0674(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Sleeman JP, Arming S, Moll JF, Hekele A, Rudy W, Sherman LS, Kreil G, Ponta H, Herrlich P. Hyaluronate-independent metastatic behavior of CD44 variant-expressing pancreatic carcinoma cells. Cancer Res. 1996;56:3134–3141. [PubMed] [Google Scholar]
- Sonnenberg A, Daams H, Van der Valk MA, Hilkens J, Hilgers J. Development of mouse mammary gland: identification of stages in differentiation of luminal and myoepithelial cells using monoclonal antibodies and polyvalent antiserum against keratin. J Histochem Cytochem. 1986;34:1037–1046. doi: 10.1177/34.8.2426332. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- Sy MS, Mori H, Liu D. CD44 as a marker in human cancers. Curr Opin Oncol. 1997;9:108–112. doi: 10.1097/00001622-199701000-00017. [DOI] [PubMed] [Google Scholar]
- Thomas L, Byers HR, Vink J, Stamenkovic I. CD44H regulates tumor cell migration on hyaluronate-coated substrate. J Cell Biol. 1992;118:971–977. doi: 10.1083/jcb.118.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukira S, Oishi K, Sato N, Sagara J, Kawai A, Tsukita S. ERM family members as molecular linkers between cell surface glycoprotein CD44 and actin-based cytoskeleton. J Cell Biol. 1994;126:391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley EA. HA and cell locomotion. Cancer Metastasis Rev. 1992;11:21–30. doi: 10.1007/BF00047600. [DOI] [PubMed] [Google Scholar]
- Turley EA, Erickson CA, Tucker RP. The retention and ultrastructural appearances of various extracellular matrix molecules incorporated into three-dimensional hydrated collagen lattices. Dev Biol. 1985;109:347–369. doi: 10.1016/0012-1606(85)90461-0. [DOI] [PubMed] [Google Scholar]
- Van Muijen GN, Danen EH, Veerkamp JH, Ruiter DJ, Lesley J, van den Heuvel LP. Glycoconjugate profile and CD44 expression in human melanoma cell lines with different metastatic capacity. Int J Cancer. 1995;61:241–248. doi: 10.1002/ijc.2910610217. [DOI] [PubMed] [Google Scholar]
- Van Muijen GN, Jansen KF, Cornelissen IM, Smeets DF, Beck JL, Ruiter DJ. Establishment and characterization of a human melanoma cell line (MV3) which is highly metastatic in nude mice. Int J Cancer. 1991;48:85–91. doi: 10.1002/ijc.2910480116. [DOI] [PubMed] [Google Scholar]
- Wayner EA, Carter WG. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common β subunits. J Cell Biol. 1987;105:1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo T-Y, Nagy JA, Yeo K-T, Dvorak HF, Toole BP. Increased HA at sites of attachment to mesentery by CD44-positive mouse ovarian and breast tumor cells. Am J Pathol. 1996;148:1733–1740. [PMC free article] [PubMed] [Google Scholar]
- Yu BQ, Toole BO, Stamenkovic I. Induction of apoptosis of metastatic mammary carcinoma cells in vivo by disruption of tumor cell surface CD44 function. J Exp Med. 1997;186:1985–1996. doi: 10.1084/jem.186.12.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.