Abstract
The cyclic guanosine monophosphate (cGMP)/cGMP-dependent protein kinase type I (cGKI) pathway regulates many cellular functions. The current study shows that 8-Br-cGMP stimulates the number of attached primary but not that of subcultured murine vascular smooth muscle cells (VSMCs). These effects of 8-Br-cGMP require the presence of cGKI. In agreement with previous studies, cGKI inhibited the number of cells in repeatedly passaged murine VSMCs. Activation of the cGMP/cGKI pathway in freshly isolated primary VSMCs slightly decreased apoptosis and strongly increased cell adhesion. The stimulation of cell adhesion by cGKI involves an inhibition of the RhoA/Rho kinase pathway and increased exposure of β1 and β3 integrins on the cell surface. Together, these results identify a novel proadhesive function of cGMP/cGKI signaling in primary VSMCs and suggest that the opposing effects of this pathway on VSMC number depend on the phenotypic context of the cells.
INTRODUCTION
The nitric oxide (NO)/cyclic guanosine monophosphate (cGMP)/cGMP-dependent protein kinase type I (cGKI) pathway regulates important functions in many cell types, including vascular smooth muscle cells (VSMCs) (Feil et al., 2003; Lohmann and Walter, 2005; Hofmann et al., 2006; Lincoln et al., 2006). In addition to its potent vasorelaxant properties, it has been associated with vascular proliferative diseases, such as atherosclerosis and restenosis (von der Leyen et al., 1995; Janssens et al., 1998; Varenne et al., 1998; von der Thusen et al., 2004). Several studies (an excellent summary is given in Lincoln et al., 2006) suggested that activation of cGKI-dependent pathways has antimitogenic effects in smooth muscle cells (SMCs) in culture (Garg and Hassid, 1989; Boerth et al., 1997; Chiche et al., 1998; Brophy et al., 2002) and might be vasculoprotective in the intact animal (Anderson et al., 2000; Sinnaeve et al., 2002). Despite a large body of evidence that active cGKI acts antiproliferative, Hassid and coworkers reported that NO/cGMP amplified the proliferative response of fibroblast growth factor-2 in primary VSMCs (Hassid et al., 1994). These results were supported by a report that NO and cGMP analogues activated rather than inhibited the mitogen-activated protein (MAP) kinase pathway in freshly isolated rat aortic VSMCs (Komalavilas et al., 1999). An increased MAP kinase activity has been associated with an increase in cell proliferation. The antiproliferative effect of cGMP/cGKI signaling could not be confirmed by a study on atherosclerosis in an in vivo mouse model (Wolfsgruber et al., 2003). This study suggested a proatherogenic role of VSMC cGKI. Furthermore, the recent analysis of several murine models for restenosis did not show significant effects of the presence or absence of cGKI in VSMCs on restenosis (Lukowski et al., 2008).
Previous in vitro and in vivo studies led to the hypothesis that cGKI may affect different functions in primary and cultured VSMCs. A loss or reduction of cGKI, as it may occur during passage of VSMCs, is associated with the development of a synthetic phenotype (reviewed in Lincoln et al., 2006). Because the inhibitory effects of cGKI on cell number were observed predominantly in passaged VSMCs, whereas the stimulatory effect of cGKI was found in primary VSMCs (Wolfsgruber et al., 2003), the contrary findings might depend on the phenotype of the VSMCs, i.e., synthetic versus contractile smooth muscle cells.
In the present study, we analyzed the modulatory effects of cGMP on cell number in primary and passaged aortic VSMCs from control and cGKI-deficient mice (Wegener et al., 2002). We conclude that cGKI acts antiproliferative on passaged VSMCs, whereas it promotes the cell number of freshly isolated VSMCs primarily via stimulation of cell adhesion.
MATERIALS AND METHODS
Materials
8-Bromoguanosine-3′, 5′-cyclic monophosphate was purchased from Biolog (Hayward, CA), U-46619 was from Alexis Laboratories (San Diego, CA), and H1152 was from Calbiochem (San Diego). Blocking antibodies against β1 and β3 integrins (Schultz and Armant, 1995; Ridger et al., 2001) were purchased from Biolegend (San Diego, CA) (CD29, CD61). All other reagents and chemicals were purchased from Roth (Karlsruhe, Germany), Invitrogen (Carlsbad, CA), or Sigma-Aldrich (St. Louis, MO).
Mouse Breeding
Wild-type, heterozygous, and homozygous cGKI knockout mice were obtained as described previously (Pfeifer et al., 1998; Wegener et al., 2002; Weber et al., 2007). The animals used in this work were generated from heterozygous cGKI+/L− mice on a 129/Sv genetic background (Wegener et al., 2002). VSMCs isolated from wild-type (cGKI+/+) and heterozygous (cGKI+/L−) mice showed identical results, and they were thus used as controls (ctr). Animals were handled according to German animal protection law.
Cell Culture
Aortic VSMCs were isolated from control mice (cGKI+/+ and cGKI+/L−) and cGKI knockout (ko) mice (cGKIL−/L−) by enzymatic digestion and grown in DMEM (Invitrogen) supplemented with 10% fetal calf serum (FCS) (Invitrogen) and 1% Pen/Strep (Invitrogen) at 37°C and 6% CO2 as described previously (Kuhbandner et al., 2000; Wolfsgruber et al., 2003). To establish subcultures, primary VSMCs were grown to 80–90% confluence, trypsinized, counted, and replated at a density of 200,000 cells/100-mm cell culture dish for further passaging.
Determination of Cell Number
To determine the cell number of attached cells, VSMCs were plated in 96-well plates (primary cells at 20,000 cells/well and passaged cells at 5000 cells/well) and cultured for 3 d in the absence and presence of drugs. If not stated otherwise, drugs were added before plating. The number of attached cells was determined by the CellTiter 96 AQ–NonRadioactive Cell Proliferation Assay (MTS assay) (Promega, Madison, WI), which is an indirect method of cell quantification and involves the bioreduction of a tetrazolium salt by living cells to a colored formazan product. The assay was used according to the manufacturer's protocol. Briefly, after cells were washed once with serum-free medium, 100 μl of serum-free medium was added to each well followed by 20 μl of MTS solution and incubation at 37°C and 6% CO2. The OD495 was measured after 30 and 60 min by using a plate reader (Titertek Multiscan MCC/340; Titertek, Huntsville, AL). To validate the results obtained with the MTS assay, the toluidine blue (TB) assay was performed subsequently to the MTS assay by using the same wells. Staining of cells with TB is a more direct measure of cell number compared with the MTS assay. Cells were fixed and stained with TB solution (0.5% TB, 2% formaldehyde, and 0.2% glutaraldehyde in phosphate-buffered saline [PBS]) for 10 min at room temperature. Then, excessive TB solution was removed by five successive washing steps with PBS. Finally, the cells were destained in 100 μl of 1% SDS, and the OD620 was measured. Both assays gave comparable results, supporting the significance of our experiments. To analyze integrin-mediated adhesion, integrin blocking antibodies were added to the cells before plating. For each experiment a 24-well plate was treated accordingly to confirm the obtained values by microscopy.
For the analysis of cell attachment kinetics, freshly isolated VSMCs from control and cGKI-deficient animals were seeded at a density of 125,000 cells/ml in a μ-Dish35 mm, low with Grid-500 (ibidi) in the absence and presence of 100 μM 8-Br-cGMP. Pictures were taken 4 h after seeding the cells and subsequently every 24 h. Five representative fields of view were analyzed for each condition and point in time.
Time-Lapse Microscopy
Freshly isolated VSMCs (125,000) were cultured in a 24 well plate for each condition in 1 ml of CO2-free medium (Invitrogen) supplemented with 10% FCS, 2 mM l-glutamine, and 1% Pen/Strep. Cell kinetics was recorded for 72 h in the absence and presence of 1 mM 8-Br-cGMP. Recording was started 4 h after seeding to allow sedimentation of the cells. The microscope stage and the surrounding were kept at 37°C.
RhoA Activity Assay
RhoA activity was determined by the G-Lisa assay (Cytoskeleton, Denver, CO) according to the manufacturer's manual. To stimulate RhoA activity, cells were treated with the thromboxane mimetic U-46619 at 3 μM for 5 min before lysis.
Flow Cytometry
To detect apoptotic cells, a human annexin V fluorescein isothiocyanate (FITC) kit (Bender MedSystems, Vienna, Austria) was used according to the manufacturer's protocol. Briefly, freshly isolated primary VSMCs were incubated in a Falcon tube (200,000 cells/ml) in DMEM supplemented with 10% FCS at 37°C and 6% CO2 in the absence and presence of 1 mM 8-Br-cGMP. Immediately after isolation, and after 4, 6, 10, and 24 h, aliquots of 100,000 cells were taken, stained with FITC-labeled annexin V and propidium iodide (PI), and subjected to flow cytometry by using a FACSCalibur (BD Biosciences, San Jose, CA). Cells (10,000) of each sample were counted, and data were analyzed with Cell Quest Pro (4.62). Annexin V-positive and PI-negative cells were considered apoptotic and used for statistical analysis.
To analyze integrin exposure on the cell surface, freshly isolated primary VSMCs were incubated for 24 h in DMEM supplemented with 10% FCS at 37°C and 6% CO2 in the absence and presence of 1 mM 8-Br-cGMP. Then, 150,000 cells were stained with antibodies against β1 or β3 integrins followed by a secondary FITC-labeled antibody and flow cytometry (in 5% FCS in PBS, pH 7.4). Dead cells were excluded by PI staining. Cells (10,000) per sample were counted, and data were analyzed with CellQuest Pro 4.62 (BD Biosciences).
Immunocytochemistry
Cells were plated on glass coverslips in a 24-well plate at a cell density of 100,000 cells/well. After 2 to 3 d of culture, cells were washed twice with PBS and fixed in 3.7% Formalin in PBS. Subsequently, cells were permeabilized with ice-cold (−20°C) acetone for 5 min, washed with 1% bovine serum albumin in PBS and incubated with 5% normal goat serum in PBS for 10 min. VSMCs were stained for vinculin (Sigma-Aldrich) for 45 min. After three washing steps, cells were incubated with an appropriate fluorescence-labeled secondary antibody (Alexa 488) for 30 min. For F-actin staining, cells were stained with rhodamine-phalloidin (Invitrogen). After staining, the cells were embedded in Mowiol (Calbiochem) with p-phenylendiamine (Sigma-Aldrich). Photomicrographs were taken with a confocal microscope (TCS NT [Leica, Wetzlar, Germany] or LSM 510 [Carl Zeiss, Jena, Germany]).
Western Blot
To analyze the phosphorylation of vasodilator-stimulated phosphoprotein (VASP), 100,000 cells/well were seeded in a six-well culture plate. Cells were grown to 80–90% confluence, and then they were serum starved for 48 h and treated with different compounds for 30 min in serum-free medium. Cells were harvested with lysis buffer (21 mM Tris HCl, pH 8.0, 0.7% SDS, 1.7% β-mercaptoethanol, and 0.2 mM phenylmethylsulfonyl fluoride) followed by Western blot analysis using antibodies for cGKI (Pfeifer et al., 1998), β-actin (Abcam, Cambridge, MA), and VASP (Alexis Laboratories). For detection of RhoA (Santa Cruz Biotechnology, Santa Cruz, CA) expression, primary VSMCs were cultured for 3 d in the absence and presence of 100 μM 8-Br-cGMP.
Statistics
If not otherwise reported, experiments were reproduced at least three times with similar results. Data are presented as mean ± SEM (n = 6–8) per experiment. Most data were analyzed with Origin Pro 6.1 software (OriginLab, Northampton, MA). To compare groups, an unpaired Student's t test was used. Apoptosis was analyzed by two-way analysis of variance by using the Prism 4.0 software (GraphPad Prism Software, San Diego, CA). Significance levels were ns, not significantly different; and *, **, and *** denote significant differences at p < 0.05, p < 0.01, and p < 0.001, respectively.
RESULTS
cGKI Promotes Recovery of Primary VSMCs in Culture
Murine primary aortic VSMCs were incubated under standard culture conditions. The number of cells was compared after 72-h incubation in the presence of different compounds. The number of viable attached cells was determined by using a colorimetric NonRadioactive Cell Proliferation Assay (CellTiter 96 AQ; Promega) and toluidine blue staining. Quantitative results were confirmed by microscopy (e.g., Figure 2B), which could exclude increase in cell size as major cause for increased cell number. Concentrations of 0.1 and 1 mM 8-Br-cGMP were chosen for cell treatment according to a dose–response curve of 8-Br-cGMP on cell attachment (Supplemental Figure 1).
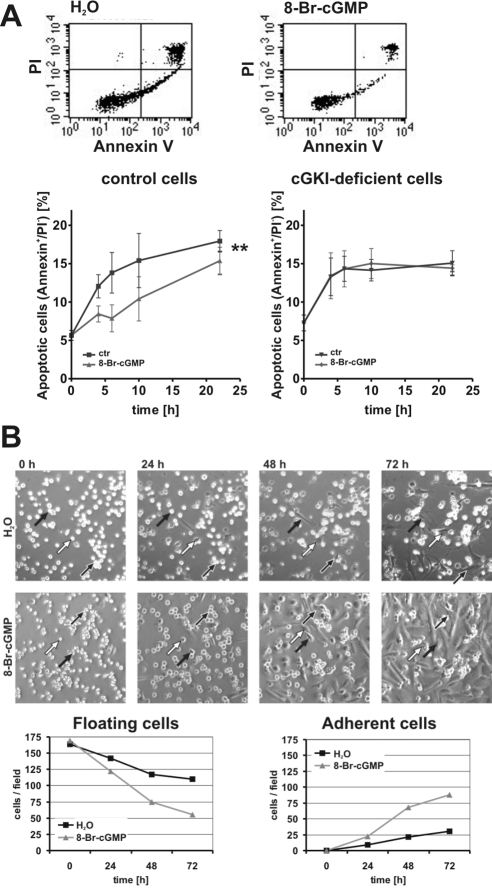
Figure 2.
Analysis of apoptosis and cell attachment kinetics. (A) Analysis of apoptosis of primary control and cGKI-deficient VSMCs. Representative original measurements of primary control VSMCs after 6 h in suspension in the absence (H2O) and presence of 8-Br-cGMP (1 mM). Cells were labeled with Annexin V and PI. The time course of the appearance of apoptotic cells (Annexin V-positive and PI-negative) in control and cGKI-deficient cell preparations is shown in the bottom diagrams. (B) Analysis of cell number and attachment by time-lapse videomicroscopy. VSMCs were plated in the absence (H2O) and presence of 8-Br-cGMP (1 mM) and monitored for 72 h. Representative pictures are shown at the indicated times. Arrows highlight individual cells during the time of observation. The number of adherent and floating cells in the absence and presence of 8-Br-cGMP was determined for each microscopic field (bottom).
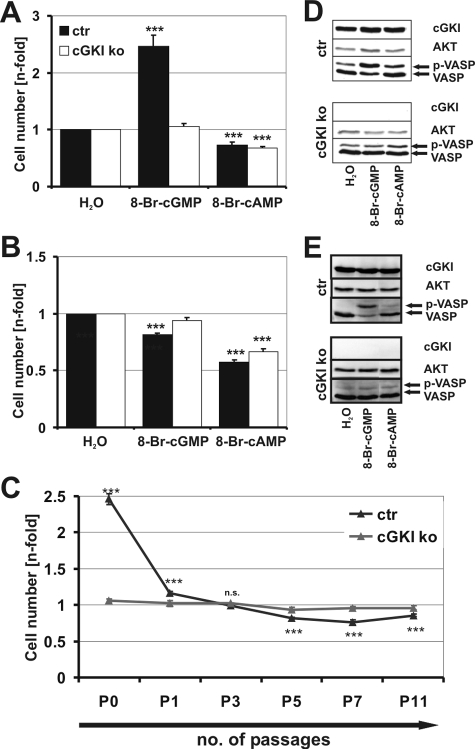
The number of murine primary aortic VSMCs was not affected by the presence or absence of cGKI under standard culture conditions (Supplemental Figure 2), indicating that cGKI is dispensable for basal adhesion. Addition of the membrane-permeable cGMP analogue 8-Br-cGMP significantly increased the number of primary VSMCs in the presence but not in the absence of cGKI (Figure 1A). Similar results were obtained when 8-Br-cGMP was replaced by the cGMP analogues 8-Br-PET-cGMP or 8-pCPT-cGMP (data not shown). In contrast, 8-Bromoadenosine-3′, 5′-cyclic monophosphate (8-Br-cAMP), a stimulator of cAMP-dependent protein kinase (cAK), reduced the number of primary and subcultured VSMCs in a cGKI-independent manner (Figure 1, A and B). In line with previous reports (Garg and Hassid, 1989; Boerth et al., 1997; Chiche et al., 1998; Brophy et al., 2002), 8-Br-cGMP acts antiproliferative on passaged murine VSMCs in a cGKI-dependent manner (Figure 1B). The inhibitory effect of cGKI in subcultured murine cells was relatively weak and became statistically significant after five passages of the VSMCs (Figure 1, B and C). Interestingly, the strong cGKI-dependent increase in cell number in primary cells is already lost with the first passage (Figure 1C). The differential effects of 8-Br-cGMP on cell number of primary versus subcultured murine VSMCs was not due to a change in cGKI expression or enzyme activity during subculture as monitored by Western blot analysis of cGKI and phosphorylation of its substrate VASP (Figure 1, D and E, and Supplemental Figure 3). The phosphorylation of VASP, which is also a substrate of cAK, in response to 8-Br-cAMP was weak compared with 8-Br-cGMP-induced phosphorylation in control cells. This could be explained by the chosen stimulatory conditions that were optimized for cGKI-induced phosphorylation of VASP.
Figure 1.
Cell culture of control (ctr) and cGKI-deficient (ko) primary and subcultured VSMCs. Number of attached cells of primary (A) and subcultured passage 5 (B) VSMCs. Cell number was measured in the absence (H2O) and presence of 8-Br-cGMP (100 μM) or 8-Br-cAMP (100 μM) and normalized to control conditions (H2O). (C) cGKI-mediated growth of VSMCs in culture. Growth performance of primary (P0) up to passage 11 (P11) VSMCs in response to 8-Br-cGMP (100 μM) (MTS assay). Cell number was normalized to control. (D and E) Phosphorylation of VASP in response to 8-Br-cGMP (100 μM) and 8-Br-cAMP (100 μM) in primary (D) and subcultured passage 5 (E) VSMCs. An antibody against cGKI was used to demonstrate the presence and absence of cGKI protein. Akt was used as loading control. See Materials and Methods for further details.
Because there is a plethora of literature describing a loss of cGKI expression during passaging (Cornwell and Lincoln, 1989; Boerth et al., 1997), the obtained results for murine VSMCs were compared with VSMCs derived from rat and human to exclude an species specific effect (Supplemental Figure 4). Subcultured rat (Supplemental Figure 4A) as well as subcultured human (Supplemental Figure 4B) SMCs expressed cGKI. Furthermore, as revealed by phosphorylation of VASP, cGKI was still active.
cGKI Promotes Cell Number of Primary VSMCs by Increasing Adhesion
The above-mentioned experiments clearly demonstrated that an activation of cGKI increased the number of attached viable cells after 3 d in primary culture (Figure 1A). This effect of cGKI could be caused by several mechanisms, including 1) an antiapoptotic effect of cGKI; 2) a promitogenic effect, i.e., a proliferative response; and/or 3) an increased number of cells adhering to the culture dish. Activation of cGKI slightly but significantly decreased apoptosis during the initial time period (24 h) after isolation of the cells from the aorta (Figure 2A). This finding was unexpected, because a proapoptotic effect of cGKI has been reported in SMCs (Chiche et al., 1998). However, the observed antiapoptotic effect was too small to explain the two- to threefold increase in the number of primary VSMCs attached to the culture dish after 72 h in the presence of 8-Br-cGMP (Figure 1A). To dissect the potential effects of cGMP on cell adhesion and/or proliferation, the attachment of freshly isolated VSMCs was monitored by time-lapse microscopy during the first 72 h after plating. Whereas dividing cells were not observed during this period, treatment with 8-Br-cGMP strongly increased the fraction of adherent cells (Figure 2B). Note that equal amounts of cells were plated in the absence and presence of 8-Br-cGMP (Figure 2B). To proof the cGKI-mediated increase in cell adhesion in control cells and to rule out the effect in cGKI-deficient VSMCs, the time-lapse experiment was repeated in an alternative approach. Freshly isolated VSMCs from control and cGKI-deficient animals were seeded in a μ-Dish, and attachment of cells was documented by taking photomicrographs every 24 h (Supplemental Figure 5). The detailed analysis of this experiment confirmed a cGKI-mediated increased adhesion; hence, the absence of a proadhesive effect in response to 8-Br-cGMP in the cGKI-deficient cells (Supplemental Figure 5). The identical results of these two alternative approaches exclude the possibility that the observed effect is caused by the chosen method.
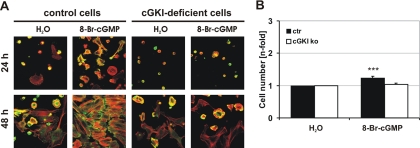
The proadhesive effect of 8-Br-cGMP was accompanied by the occurrence of stress fibers and focal adhesions as visualized by staining of fixed cells for F-actin and vinculin, respectively. These cytoskeletal changes in response to 8-Br-cGMP could not be observed in cGKI-deficient cells (Figure 3A). To exclude a proliferative effect and to strengthen the observed effect of cGKI on cell adhesion, VSMCs were allowed to attach in the absence of 8-Br-cGMP for 3 d. Subsequently, adherent cells were exposed to 8-Br-cGMP for further 3 d. A slight, although significant increase in cell number could be observed (Figure 3B), confirming a previous observation (Wolfsgruber et al., 2003). These results indicated that the cGMP/cGKI-stimulated increase in cell number at 72 h of primary culture was mainly due to an increase in the efficiency of cell adhesion rather than to an effect on cell proliferation or apoptosis.
Figure 3.
Analysis of cell adhesion and proliferation. (A) Cytoskeletal staining of primary control and cGKI-deficient VSMCs. Cells were grown on glass coverslips for 24 and 48 h in the absence (H2O) or presence of 8-Br-cGMP (1 mM). VSMCs were stained for F-Actin (red) and vinculin (green) as marker for focal adhesions. Photographs were taken with a confocal microscope (TCS NT; Leica). (B) Primary VSMCs were grown for 72 h under control conditions. Subsequently, 8-Br-cGMP (100 μM) was added for additional 72 h.
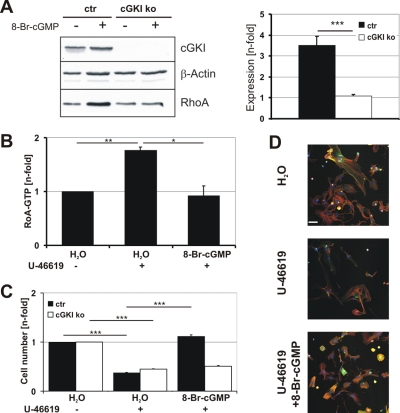
Adhesion of Primary VSMCs Is Mediated by Inhibition of the RhoA/Rho Kinase Pathway
Cell adhesion is regulated by a number of signaling pathways, including the RhoA/Rho kinase pathway (Ridley and Hall, 1992; Rottner et al., 1999; Worth et al., 2004). The small GTPase RhoA is a known substrate of cGKI (Sauzeau et al., 2003; Rolli-Derkinderen et al., 2005). As expected from a previous report (Sauzeau et al., 2003), treatment of primary VSMCs with 8-Br-cGMP increased the amount of total RhoA protein in control but not in cGKI-deficient cells (Figure 4A), indicating that activation of cGKI increased the RhoA concentration. This effect on the RhoA protein level could be mediated via phosphorylation of RhoA at Ser188, thereby possibly affecting RhoA activity by stabilization of an inactive, cytosolic RhoA-guanosine triphosphate (GTP)/GDP dissociation inhibitor (GDI) complex (Sauzeau et al., 2000; Rolli-Derkinderen et al., 2005). Indeed, activation of RhoA via the thromboxane receptor agonist U-46619 (Wettschureck and Offermanns, 2005; Rieken et al., 2006) was efficiently blocked in the presence of 8-Br-cGMP (Figure 4B), as shown previously (Sauzeau et al., 2000; Seko et al., 2003). In line with a role of RhoA in VSMC adhesion, the number of adhering primary cells was suppressed by U-46619. Importantly, 8-Br-cGMP prevented the inhibitory effect of U-46619 in control but not cGKI-deficient cells (Figure 4C). The antiadhesive effect of U-46619 treatment was also reflected by a strong reduction in F-actin and vinculin staining (Figure 4D). These results support the notion that the proadhesive effect of cGMP/cGKI is mediated, at least in part, via inhibition of RhoA signaling.
Figure 4.
Adhesion of VSMCs is mediated by inhibition of RhoA. (A) Western blot analysis of total RhoA protein. Primary control (ctr) and cGKI-deficient (ko) VSMCs were grown in the absence (H2O) and presence of 8-Br-cGMP (100 μM) for 72 h. Cells were harvested, and protein lysates were subjected to Western blot (left). β-Actin was used as loading control. RhoA expression was quantified using the AIDA software by normalizing each RhoA band to its respective loading control (n = 7 ctr; n = 3 ko). The ratio of the values obtained after 8-Br-cGMP treatment to those after H2O treatment is expressed as Expression [n-fold] (right). (B) G-Lisa RhoA assay. RhoA activity (RhoA-GTP) was determined in primary control VSMCs that were grown for 72 h in the absence (H2O) or presence of 8-Br-cGMP (100 μM). Cells were treated with U-46619 (3 μM) 5 min before lysis to increase RhoA-GTP levels as indicated. All values (n = 3 for each condition) were normalized to control (H2O). (C) Primary control (ctr) and cGKI-deficient (ko) VSMCs were grown for 72 h in the absence (H2O) and presence of 8-Br-cGMP (100 μM) and/or U-46619 (3 μM) as indicated. The number of cells was determined by the MTS assay. (D) Primary control cells were grown for 72 h in the absence (H2O) or presence of 8-Br-cGMP (100 μM) or U-46619 (3 μM) and then stained for F-actin (red), vinculin (green), and DNA (Hoechst). White bar, 50 μm. Photographs were taken with a confocal microscope (LSM 510; Carl Zeiss).
A well characterized downstream target of RhoA is Rho kinase. Inhibition of this kinase has been reported to increase cell adhesion (Koga et al., 2006). Addition of the Rho kinase inhibitor H1152 to the primary VSMCs mimicked the effects of 8-Br-cGMP on cell number and morphology (Figure 5). Similar results were obtained with Y27632, another well known Rho kinase inhibitor (data not shown). The observations that coapplication of 8-Br-cGMP and H1152 did not cause an additive effect (Figure 6C) and that H1152 also led to an increase in cell number of cGKI-deficient cells (Figure 5A) suggested that cGKI and Rho kinase are components of the same pathway and that Rho kinase acts downstream of cGKI.
Figure 5.
Adhesion of VSMCs is mediated by inhibition of Rho kinase. (A) Cell number of primary control (ctr) and cGKI-deficient (ko) VSMCs in the absence (H2O) and presence of 8-Br-cGMP (100 μM) or H1152 (0.3 μM). (B) Photographs of primary control cells were taken with a confocal microscope (LSM 510; Carl Zeiss) after 72 h in culture in the absence (H2O) and presence of 8-Br-cGMP (100 μM) or H1152 (0.3 μM). Cells were stained for F-actin (red), vinculin (green), and DNA (Hoechst). The merge of F-actin and vinculin staining is yellow. White bar, 50 μm.
Figure 6.
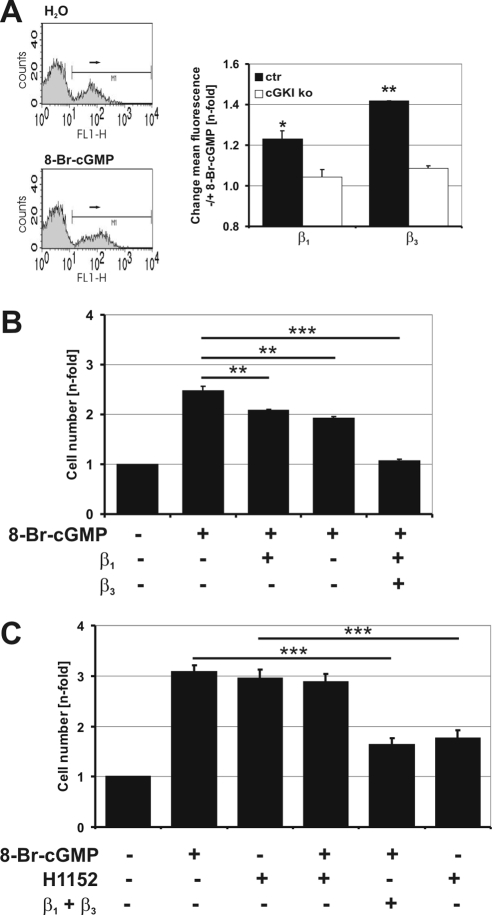
cGKI-dependent adhesion is mediated by β1 and β3 integrins. (A) Analysis of integrin surface exposure by flow cytometry. Labeling of β1 and β3 integrins on primary control VSMCs that were grown for 24 h in suspension in the absence (H2O) or presence of 8-Br-cGMP (1 mM). The control cells show an increased mean fluorescence signal for β3 integrins in response to 8-Br-cGMP (left). The statistic evaluation of the β1 and β3 integrin signal in control (ctr) and cGKI-deficient (ko) VSMCs in response to 8-Br-cGMP is shown (right). Data shown were from one out of three experiments with similar results. Each experiment consisted of six independent samples. Values represent the change in fluorescence in the presence of 8-Br-cGMP compared with control (H2O). (B) Integrin-mediated adhesion of primary control VSMCs in response to 8-Br-cGMP. The MTS-assay was performed with primary VSMCs that were grown for 72 h in the absence or presence of 8-Br-cGMP (1 mM) and integrin blocking antibodies (25 μg/ml) as indicated. Cell numbers were normalized to the value obtained in the absence of compounds. Increased adhesion in response to 8-Br-cGMP is significantly reduced upon treatment with either β integrin blocking antibody. (C) Comparison of integrin-mediated adhesion of primary control VSMCs in response to 8-Br-cGMP and H1152. Primary control VSMCs were grown for 72 h in the absence or presence of 8-Br-cGMP (1 mM) or H1152 (0.3 μM) and integrin blocking antibodies (25 μg/ml each) as indicated. Cell number was assayed by the TB method and normalized to the value obtained in the absence of compounds.
cGKI-stimulated Adhesion Is Mediated via Integrins
It is generally accepted that adhesion of cells is linked to integrins (Schwartz et al., 1995; Berrier and Yamada, 2007). To analyze whether integrins are also involved in cGKI-mediated adhesion, primary VSMCs derived from control and cGKI-deficient mice were labeled for β1 and β3 integrins, two integrins that are known to be important for adhesion. Both integrins could be detected on the cell surface by flow cytometry. Addition of 8-Br-cGMP increased the mean fluorescence signal for β1 and β3 integrins, indicating an increase in integrin presentation at the cell membrane. This effect could only be detected in control but not in cGKI-deficient cells (Figure 6A). To determine whether β1 and/or β3 integrins were functionally involved in cGKI-dependent adhesion of primary VSMCs, the number of attached cells was analyzed in the presence of β1 and/or β3 blocking antibodies. As shown in Figure 6B, 8-Br-cGMP–stimulated adhesion was reduced by antibodies against β1 as well as β3 integrin. Moreover, coadministration of both antibodies resulted in an additive effect and abolished the proadhesive effect of 8-Br-cGMP. Importantly, the integrin-blocking antibodies decreased the proadhesive stimulation elicited by the Rho kinase inhibitor H1152 to a similar extent (Figure 6C), corroborating the view that cGMP/cGKI stimulates integrin exposure and cell adhesion via inhibition of Rho kinase.
DISCUSSION
cGKI is an important regulator of many smooth muscle functions, including VSMC growth, adhesion, proliferation, and apoptosis (Hofmann et al., 2006; Lincoln et al., 2006). Many in vitro studies demonstrated an antiproliferative effect of cGMP and cGKI signaling in subcultured VSMCs (Garg and Hassid, 1989; Hassid et al., 1994; Li and Sun, 2005), VSMC-derived cell lines (Capey et al., 2007) or VSMCs that have been transfected with cGKI (Boerth et al., 1997; Browner et al., 2004; Dey et al., 2005). In agreement with these studies, we find that activation of the cGMP/cGKI pathway slightly inhibited proliferation of passaged murine VSMCs. In passaged murine VSMCs, the mechanism(s) leading to a reduction in cell number by cGMP is not clear. Inhibition was not associated with a loss of cGKI or its activity and in fact required the presence of cGKI. Furthermore, it is important to note that control and cGKI-deficient VSMCs showed similar basal properties and phenotypes under standard culture conditions (Supplemental Figure 2 and Figure 3A). These results suggest that the phenotypic modulation of VSMCs during passages requires unknown factors in addition to a potential change in cGKI expression level.
In contrast to passaged VSMCs, the present work clearly demonstrates that cGKI activation increases the number of attached primary, freshly isolated VSMCs. The cGMP/cGKI-dependent increase in cell number was mainly mediated via an increase in cell adhesion as shown by 1) time-lapse microscopy, 2) enhanced exposure of integrin β1 and β3 on the cell surface; and 3) inhibition of enhanced adhesion by antibodies that block β1 and β3 integrins. The proadhesive effect is the major cause of the cGKI-dependent increase in the number of primary VSMCs after 72 h in culture. The anti-apoptotic (Figure 2A) and proproliferative (Figure 3B) properties of the cGMP/cGKI pathway had a minor, although significant effect on the cell number.
The effect of cGMP/cGKI-signaling on apoptosis is discussed controversial. In SMCs, cGKI has been reported to have a proapoptotic effect (Pollman et al., 1996; Chiche et al., 1998). In contrast, an antiapoptotic effect of cGKI has been documented for neuronal cells (Fiscus, 2002; Ha et al., 2003). The different results reported for SMCs might be related to the major differences in the chosen cell system. In contrast to our study, Chiche et al. (1998) used subcultured cells that were transfected with cGKI. The newly identified role for cGKI to increase adhesion of primary VSMCs might represent a protective mechanism against anoïkis. It is well accepted that adhesion to the extracellular matrix is necessary for survival of differentiated adherent cells in the cardiovascular system, including endothelial cells, smooth muscle cells, fibroblasts, and cardiac myocytes (Michel, 2003). Therefore, it is likely that cGKI exerts different effects in primary and passaged VSMCs.
The cGKI is known to relax smooth muscle by several mechanisms, including inhibition of Ca2+ sensitization of contraction via inhibition of RhoA/Rho kinase signaling (Somlyo and Somlyo, 2003). This article adds a novel role to the interaction between the cGMP/cGKI and the RhoA/Rho kinase signaling pathways in murine VSMCs. Activation of cGKI inhibits RhoA/Rho kinase signaling, resulting in increased adhesion via β1 and β3 integrins of primary murine VSMCs. It is well accepted that RhoA/Rho kinase is involved in cell adhesion (Ridley and Hall, 1992; Burridge and Wennerberg, 2004). Here, we provide strong evidence that cGKI stimulates the attachment of primary, freshly isolated VSMCs by inhibition of the RhoA/Rho kinase pathway. The effects of 8-Br-cGMP and the Rho kinase inhibitor H1152 on cell number were not additive, and the effects of pharmacologic manipulation of RhoA and Rho kinase were independent of the genotype. In contrast, activation of cGKI efficiently suppressed RhoA activation by the thromboxane receptor agonist U-46619 as well as the U-46619-induced decrease of the number of attached cells. These results suggest that cGMP/cGKI and RhoA/Rho kinase are components of the same signaling pathway and converge in regulating cell adhesion. cGKI is downstream of the thromboxane receptor but acts upstream of RhoA and Rho kinase. Indeed, cGKI has been reported to phosphorylate and thereby stabilize the RhoA protein in an inactive RhoA-GTP/GDI complex, leading to an increase in total RhoA protein (Sauzeau et al., 2000; Rolli-Derkinderen et al., 2005).
Whether RhoA stimulates or inhibits adhesion seems to depend on the cell type and culture conditions (Ridley and Hall, 1992; Laudanna et al., 1996; Schoenwaelder et al., 2002; Worthylake and Burridge, 2003; Vielkind et al., 2005; Slotta et al., 2006). Increased adhesion after blockade of Rho kinase has also been described by others (Kim et al., 2005; Koga et al., 2006). Koga et al. (2006) demonstrated that inhibition of Rho kinase in human trabecular meshwork cells causes increased adhesion but that it had no influence on proliferation (Koga et al., 2006). The signaling networks linking RhoA and integrins vary according to the mode of cell growth, such as adhesion of a floating cell to the substratum, spreading, or migration (Schwartz and Shattil, 2000; Danen et al., 2002). For example, inhibition of RhoA/Rho kinase in leukocytes elevates integrin-mediated adhesion (Liu et al., 2002; Worthylake and Burridge, 2003). The present study indicates that a similar mechanism was observed in primary VSMCs as shown by 1) a reduction in the number of attached cells upon stimulation with the thromboxane receptor agonist U-46619 that activates RhoA, 2) stimulation of the attached cell number by inhibition of Rho kinase with H1152 or Y27632, and 3) a decrease of the attached cell number in response to 8-Br-cGMP or H1152 by blocking β1 and β3 integrins.
Integrins have a pivotal role in mediating processes such as adhesion, migration, and cell–cell contacts. To allow these different processes, the expression of integrins is tightly regulated during development. For example in adult human aortic VSMCs α1β1 integrin is highly expressed, representing a resting, contractile phenotype, and it is strongly reduced or lost, with the beginning of cell culture (Belkin et al., 1990). In line with these findings, a recent study using a conditional β1 integrin knockout model showed that the lack of β1 integrin in VSMCs causes a synthetic phenotype (Abraham et al., 2008). These data support the notion that integrins have major impact on the phenotype of VSMCs that may be modulated by cGKI.
In summary, we show that cGKI promotes integrin-mediated adhesion of primary VSMCs by inhibiting the RhoA/Rho kinase pathway.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Wolfgang Erl for kindly providing the SMCs of human and rat origin. We also thank Sabine Brummer, Teodora Kennel, and Barbara Böhlig, who were instrumental to many experiments. This work was supported by grants from the Deutsche Forschungsgemeinschaft and Fonds der Chemischen Industrie.
Abbreviations used:
- cAK
cAMP-dependent protein kinase
- cGKI
cGMP-dependent protein kinase type I
- VSMC
vascular smooth muscle cell.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-04-0370) on August 6, 2008.
REFERENCES
- Abraham S., Kogata N., Fassler R., Adams R. H. Integrin beta1 subunit controls mural cell adhesion, spreading, and blood vessel wall stability. Circ. Res. 2008;102:562–570. doi: 10.1161/CIRCRESAHA.107.167908. [DOI] [PubMed] [Google Scholar]
- Anderson P. G., Boerth N. J., Liu M., McNamara D. B., Cornwell T. L., Lincoln T. M. Cyclic GMP-dependent protein kinase expression in coronary arterial smooth muscle in response to balloon catheter injury. Arterioscler. Thromb. Vasc. Biol. 2000;20:2192–2197. doi: 10.1161/01.atv.20.10.2192. [DOI] [PubMed] [Google Scholar]
- Belkin V. M., Belkin A. M., Koteliansky V. E. Human smooth muscle VLA-1 integrin: purification, substrate specificity, localization in aorta, and expression during development. J. Cell Biol. 1990;111:2159–2170. doi: 10.1083/jcb.111.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrier A. L., Yamada K. M. Cell-matrix adhesion. J. Cell Physiol. 2007;213:565–573. doi: 10.1002/jcp.21237. [DOI] [PubMed] [Google Scholar]
- Boerth N. J., Dey N. B., Cornwell T. L., Lincoln T. M. Cyclic GMP-dependent protein kinase regulates vascular smooth muscle cell phenotype. J. Vasc. Res. 1997;34:245–259. doi: 10.1159/000159231. [DOI] [PubMed] [Google Scholar]
- Brophy C. M., Woodrum D. A., Pollock J., Dickinson M., Komalavilas P., Cornwell T. L., Lincoln T. M. cGMP-dependent protein kinase expression restores contractile function in cultured vascular smooth muscle cells. J. Vasc. Res. 2002;39:95–103. doi: 10.1159/000057758. [DOI] [PubMed] [Google Scholar]
- Browner N. C., Dey N. B., Bloch K. D., Lincoln T. M. Regulation of cGMP-dependent protein kinase expression by soluble guanylyl cyclase in vascular smooth muscle cells. J. Biol. Chem. 2004;279:46631–46636. doi: 10.1074/jbc.M408518200. [DOI] [PubMed] [Google Scholar]
- Burridge K., Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Capey S., Mosedale J. G., van den Berg C. W. Characterisation of the complement susceptibility of the rat aortic smooth muscle cell line A7r5. Mol. Immunol. 2007;44:608–614. doi: 10.1016/j.molimm.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Chiche J. D., Schlutsmeyer S. M., Bloch D. B., de la Monte S. M., Roberts J. D., Jr, Filippov G., Janssens S. P., Rosenzweig A., Bloch K. D. Adenovirus-mediated gene transfer of cGMP-dependent protein kinase increases the sensitivity of cultured vascular smooth muscle cells to the antiproliferative and pro-apoptotic effects of nitric oxide/cGMP. J. Biol. Chem. 1998;273:34263–34271. doi: 10.1074/jbc.273.51.34263. [DOI] [PubMed] [Google Scholar]
- Cornwell T. L., Lincoln T. M. Regulation of intracellular Ca2+ levels in cultured vascular smooth muscle cells. Reduction of Ca2+ by atriopeptin and 8-bromo-cyclic GMP is mediated by cyclic GMP-dependent protein kinase. J. Biol. Chem. 1989;264:1146–1155. [PubMed] [Google Scholar]
- Danen E. H., Sonneveld P., Brakebusch C., Fassler R., Sonnenberg A. The fibronectin-binding integrins alpha5beta1 and alphavbeta3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J. Cell Biol. 2002;159:1071–1086. doi: 10.1083/jcb.200205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey N. B., Foley K. F., Lincoln T. M., Dostmann W. R. Inhibition of cGMP-dependent protein kinase reverses phenotypic modulation of vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 2005;45:404–413. doi: 10.1097/01.fjc.0000157455.38068.12. [DOI] [PubMed] [Google Scholar]
- Feil R., Lohmann S. M., de Jonge H., Walter U., Hofmann F. Cyclic GMP-dependent protein kinases and the cardiovascular system: insights from genetically modified mice. Circ. Res. 2003;93:907–916. doi: 10.1161/01.RES.0000100390.68771.CC. [DOI] [PubMed] [Google Scholar]
- Fiscus R. R. Involvement of cyclic GMP and protein kinase G in the regulation of apoptosis and survival in neural cells. Neurosignals. 2002;11:175–190. doi: 10.1159/000065431. [DOI] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J. Clin. Invest. 1989;83:1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha K. S., Kim K. M., Kwon Y. G., Bai S. K., Nam W. D., Yoo Y. M., Kim P. K., Chung H. T., Billiar T. R., Kim Y. M. Nitric oxide prevents 6-hydroxydopamine-induced apoptosis in PC12 cells through cGMP-dependent PI3 kinase/Akt activation. FASEB J. 2003;17:1036–1047. doi: 10.1096/fj.02-0738com. [DOI] [PubMed] [Google Scholar]
- Hassid A., Arabshahi H., Bourcier T., Dhaunsi G. S., Matthews C. Nitric oxide selectively amplifies FGF-2-induced mitogenesis in primary rat aortic smooth muscle cells. Am. J. Physiol. 1994;267:H1040–H1048. doi: 10.1152/ajpheart.1994.267.3.H1040. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Feil R., Kleppisch T., Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol. Rev. 2006;86:1–23. doi: 10.1152/physrev.00015.2005. [DOI] [PubMed] [Google Scholar]
- Janssens S., Flaherty D., Nong Z., Varenne O., van Pelt N., Haustermans C., Zoldhelyi P., Gerard R., Collen D. Human endothelial nitric oxide synthase gene transfer inhibits vascular smooth muscle cell proliferation and neointima formation after balloon injury in rats. Circulation. 1998;97:1274–1281. doi: 10.1161/01.cir.97.13.1274. [DOI] [PubMed] [Google Scholar]
- Kim Y. B., Yu J., Lee S. Y., Lee M. S., Ko S. G., Ye S. K., Jong H. S., Kim T. Y., Bang Y. J., Lee J. W. Cell adhesion status-dependent histone acetylation is regulated through intracellular contractility-related signaling activities. J. Biol. Chem. 2005;280:28357–28364. doi: 10.1074/jbc.M412608200. [DOI] [PubMed] [Google Scholar]
- Koga T., Koga T., Awai M., Tsutsui J., Yue B. Y., Tanihara H. Rho-associated protein kinase inhibitor, Y-27632, induces alterations in adhesion, contraction and motility in cultured human trabecular meshwork cells. Exp. Eye Res. 2006;82:362–370. doi: 10.1016/j.exer.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Komalavilas P., Shah P. K., Jo H., Lincoln T. M. Activation of mitogen-activated protein kinase pathways by cyclic GMP and cyclic GMP-dependent protein kinase in contractile vascular smooth muscle cells. J. Biol. Chem. 1999;274:34301–34309. doi: 10.1074/jbc.274.48.34301. [DOI] [PubMed] [Google Scholar]
- Kuhbandner S., Brummer S., Metzger D., Chambon P., Hofmann F., Feil R. Temporally controlled somatic mutagenesis in smooth muscle. Genesis. 2000;28:15–22. doi: 10.1002/1526-968x(200009)28:1<15::aid-gene20>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Laudanna C., Campbell J. J., Butcher E. C. Role of Rho in chemoattractant-activated leukocyte adhesion through integrins. Science. 1996;271:981–983. doi: 10.1126/science.271.5251.981. [DOI] [PubMed] [Google Scholar]
- Li S. J., Sun N. L. Regulation of intracellular Ca2+ and calcineurin by NO/PKG in proliferation of vascular smooth muscle cells. Acta Pharmacol. Sin. 2005;26:323–328. doi: 10.1111/j.1745-7254.2005.00049.x. [DOI] [PubMed] [Google Scholar]
- Lincoln T. M., Wu X., Sellak H., Dey N., Choi C. S. Regulation of vascular smooth muscle cell phenotype by cyclic GMP and cyclic GMP-dependent protein kinase. Front. Biosci. 2006;11:356–367. doi: 10.2741/1803. [DOI] [PubMed] [Google Scholar]
- Liu L., Schwartz B. R., Lin N., Winn R. K., Harlan J. M. Requirement for RhoA kinase activation in leukocyte de-adhesion. J. Immunol. 2002;169:2330–2336. doi: 10.4049/jimmunol.169.5.2330. [DOI] [PubMed] [Google Scholar]
- Lohmann S. M., Walter U. Tracking functions of cGMP-dependent protein kinases (cGK) Front. Biosci. 2005;10:1313–1328. doi: 10.2741/1621. [DOI] [PubMed] [Google Scholar]
- Lukowski R., et al. Role of smooth muscle cGMP/cGKI signaling in murine vascular restenosis. Arterioscler. Thromb. Vasc. Biol. 2008;28:1244–1250. doi: 10.1161/ATVBAHA.108.166405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel J. B. Anoikis in the cardiovascular system: known and unknown extracellular mediators. Arterioscler. Thromb. Vasc. Biol. 2003;23:2146–2154. doi: 10.1161/01.ATV.0000099882.52647.E4. [DOI] [PubMed] [Google Scholar]
- Pfeifer A., et al. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J. 1998;17:3045–3051. doi: 10.1093/emboj/17.11.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollman M. J., Yamada T., Horiuchi M., Gibbons G. H. Vasoactive substances regulate vascular smooth muscle cell apoptosis. Countervailing influences of nitric oxide and angiotensin II. Circ. Res. 1996;79:748–756. doi: 10.1161/01.res.79.4.748. [DOI] [PubMed] [Google Scholar]
- Ridger V. C., Wagner B. E., Wallace W. A., Hellewell P. G. Differential effects of CD18, CD29, and CD49 integrin subunit inhibition on neutrophil migration in pulmonary inflammation. J. Immunol. 2001;166:3484–3490. doi: 10.4049/jimmunol.166.5.3484. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Rieken S., Herroeder S., Sassmann A., Wallenwein B., Moers A., Offermanns S., Wettschureck N. Lysophospholipids control integrin-dependent adhesion in splenic B cells through G(i) and G(12)/G(13) family G-proteins but not through G(q)/G(11) J. Biol. Chem. 2006;281:36985–36992. doi: 10.1074/jbc.M605287200. [DOI] [PubMed] [Google Scholar]
- Rolli-Derkinderen M., Sauzeau V., Boyer L., Lemichez E., Baron C., Henrion D., Loirand G., Pacaud P. Phosphorylation of serine 188 protects RhoA from ubiquitin/proteasome-mediated degradation in vascular smooth muscle cells. Circ. Res. 2005;96:1152–1160. doi: 10.1161/01.RES.0000170084.88780.ea. [DOI] [PubMed] [Google Scholar]
- Rottner K., Hall A., Small J. V. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr. Biol. 1999;9:640–648. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- Sauzeau V., Le Jeune H., Cario-Toumaniantz C., Smolenski A., Lohmann S. M., Bertoglio J., Chardin P., Pacaud P., Loirand G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J. Biol. Chem. 2000;275:21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- Sauzeau V., Rolli-Derkinderen M., Marionneau C., Loirand G., Pacaud P. RhoA expression is controlled by nitric oxide through cGMP-dependent protein kinase activation. J. Biol. Chem. 2003;278:9472–9480. doi: 10.1074/jbc.M212776200. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder S. M., Hughan S. C., Boniface K., Fernando S., Holdsworth M., Thompson P. E., Salem H. H., Jackson S. P. RhoA sustains integrin alpha IIbbeta 3 adhesion contacts under high shear. J. Biol. Chem. 2002;277:14738–14746. doi: 10.1074/jbc.M200661200. [DOI] [PubMed] [Google Scholar]
- Schultz J. F., Armant D. R. β1- and β3-Class integrins mediate fibronectin binding activity at the surface of developing mouse peri-implantation blastocysts. Regulation by ligand-induced mobilization of stored receptor. J. Biol. Chem. 1995;270:11522–11531. doi: 10.1074/jbc.270.19.11522. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A., Schaller M. D., Ginsberg M. H. Integrins: emerging paradigms of signal transduction. Annu. Rev. Cell Dev. Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A., Shattil S. J. Signaling networks linking integrins and rho family GTPases. Trends Biochem. Sci. 2000;25:388–391. doi: 10.1016/s0968-0004(00)01605-4. [DOI] [PubMed] [Google Scholar]
- Seko T., Ito M., Kureishi Y., Okamoto R., Moriki N., Onishi K., Isaka N., Hartshorne D. J., Nakano T. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ. Res. 2003;92:411–418. doi: 10.1161/01.RES.0000059987.90200.44. [DOI] [PubMed] [Google Scholar]
- Sinnaeve P., Chiche J. D., Gillijns H., Van Pelt N., Wirthlin D., Van De Werf F., Collen D., Bloch K. D., Janssens S. Overexpression of a constitutively active protein kinase G mutant reduces neointima formation and in-stent restenosis. Circulation. 2002;105:2911–2916. doi: 10.1161/01.cir.0000018169.59205.ca. [DOI] [PubMed] [Google Scholar]
- Slotta J. E., Braun O. O., Menger M. D., Thorlacius H. Fasudil, a Rho-kinase inhibitor, inhibits leukocyte adhesion in inflamed large blood vessels in vivo. Inflamm. Res. 2006;55:364–367. doi: 10.1007/s00011-006-6013-2. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Varenne O., Pislaru S., Gillijns H., Van Pelt N., Gerard R. D., Zoldhelyi P., Van de Werf F., Collen D., Janssens S. P. Local adenovirus-mediated transfer of human endothelial nitric oxide synthase reduces luminal narrowing after coronary angioplasty in pigs. Circulation. 1998;98:919–926. doi: 10.1161/01.cir.98.9.919. [DOI] [PubMed] [Google Scholar]
- Vielkind S., Gallagher-Gambarelli M., Gomez M., Hinton H. J., Cantrell D. A. Integrin regulation by RhoA in thymocytes. J. Immunol. 2005;175:350–357. doi: 10.4049/jimmunol.175.1.350. [DOI] [PubMed] [Google Scholar]
- von der Leyen H. E., Gibbons G. H., Morishita R., Lewis N. P., Zhang L., Nakajima M., Kaneda Y., Cooke J. P., Dzau V. J. Gene therapy inhibiting neointimal vascular lesion: in vivo transfer of endothelial cell nitric oxide synthase gene. Proc. Natl. Acad. Sci. USA. 1995;92:1137–1141. doi: 10.1073/pnas.92.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Thusen J. H., Fekkes M. L., Passier R., van Zonneveld A. J., Mainfroid V., van Berkel T. J., Biessen E. A. Adenoviral transfer of endothelial nitric oxide synthase attenuates lesion formation in a novel murine model of postangioplasty restenosis. Arterioscler. Thromb. Vasc. Biol. 2004;24:357–362. doi: 10.1161/01.ATV.0000114235.51044.92. [DOI] [PubMed] [Google Scholar]
- Weber S., et al. Rescue of cGMP kinase I knockout mice by smooth muscle specific expression of either isozyme. Circ. Res. 2007 doi: 10.1161/CIRCRESAHA.107.154351. [DOI] [PubMed] [Google Scholar]
- Wegener J. W., Nawrath H., Wolfsgruber W., Kuhbandner S., Werner C., Hofmann F., Feil R. cGMP-dependent protein kinase I mediates the negative inotropic effect of cGMP in the murine myocardium. Circ. Res. 2002;90:18–20. doi: 10.1161/hh0102.103222. [DOI] [PubMed] [Google Scholar]
- Wettschureck N., Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol. Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- Wolfsgruber W., Feil S., Brummer S., Kuppinger O., Hofmann F., Feil R. A proatherogenic role for cGMP-dependent protein kinase in vascular smooth muscle cells. Proc. Natl. Acad. Sci. USA. 2003;100:13519–13524. doi: 10.1073/pnas.1936024100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worth N. F., Campbell G. R., Campbell J. H., Rolfe B. E. Rho expression and activation in vascular smooth muscle cells. Cell Motil. Cytoskeleton. 2004;59:189–200. doi: 10.1002/cm.20036. [DOI] [PubMed] [Google Scholar]
- Worthylake R. A., Burridge K. RhoA and ROCK promote migration by limiting membrane protrusions. J. Biol. Chem. 2003;278:13578–13584. doi: 10.1074/jbc.M211584200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.