Abstract
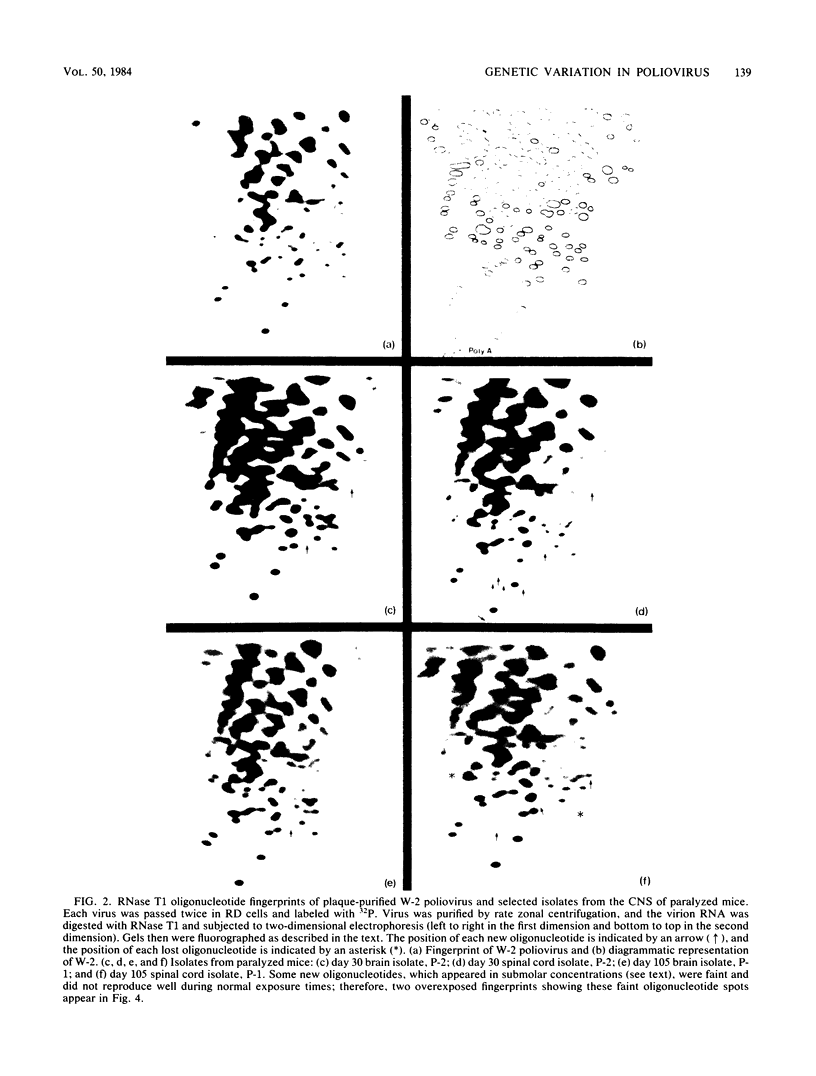
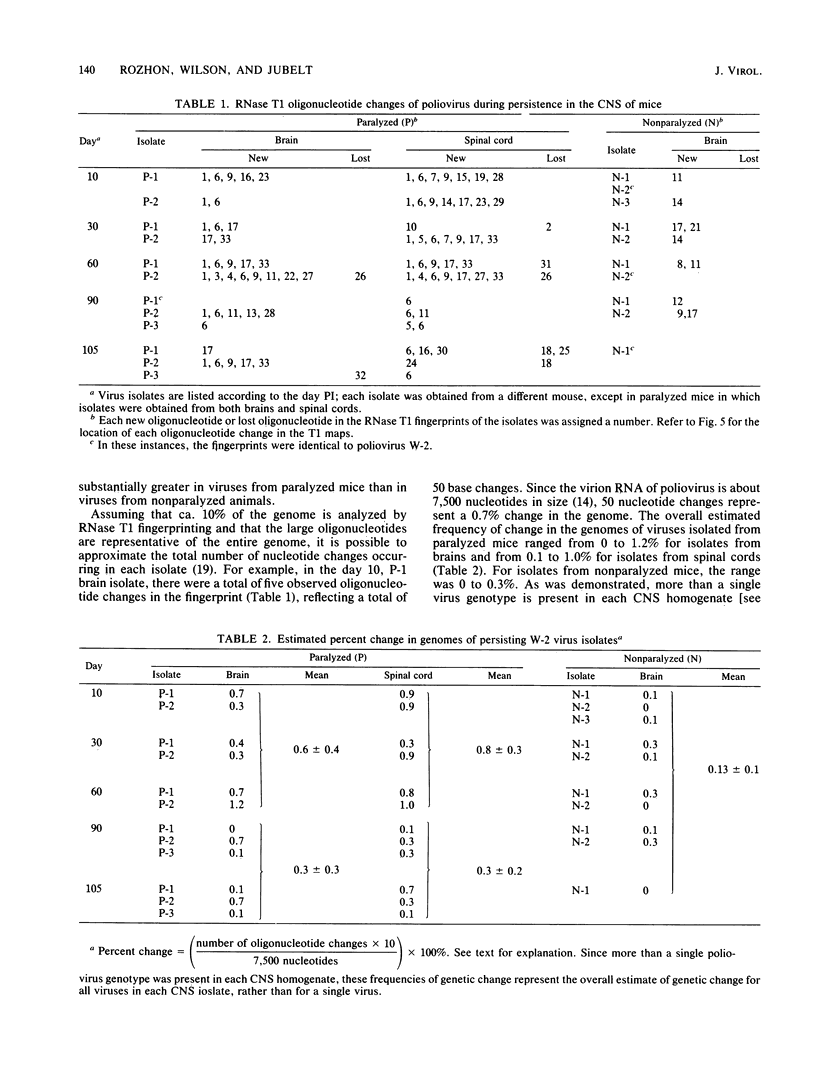
Genomic changes occurring in the attenuated W-2 strain of poliovirus 2 during persistent infection of the central nervous system of immunosuppressed mice were analyzed. The RNase T1 oligonucleotide fingerprints of 34 different viruses, isolated from the brains and spinal cords of paralyzed and nonparalyzed mice during a 105-day period, were used to quantitate and compare the mutations occurring in each isolate. Although mice were inoculated with plaque-purified virus, genetically distinct viruses were recovered from the central nervous system. The number of oligonucleotide changes occurring in isolates from paralyzed mice generally was greater than that observed in isolates from nonparalyzed mice. However, differences in the extent of mutation in isolates from the two groups of mice did not appear to be related to the level of virus replication. In paralyzed mice, the number of oligonucleotide changes on average was greater in viruses isolated during the first 60 days of the infection than in the last 45 days. The number of oligonucleotide changes was essentially constant throughout the infection, however, in viruses isolated from the brains of nonparalyzed mice. In addition, several specific oligonucleotide changes were found only in viruses isolated from paralyzed animals.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson R. P., Young J. F., Palese P. Oligonucleotide mapping: evaluation of its sensitivity by computer-simulation. Nucleic Acids Res. 1982 Jan 11;10(1):237–246. doi: 10.1093/nar/10.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BODIAN D. Emerging concept of poliomyelitis infection. Science. 1955 Jul 15;122(3159):105–108. doi: 10.1126/science.122.3159.105. [DOI] [PubMed] [Google Scholar]
- Boulger L. R., Arya S. C., Ahourai P., Marsden S. A. International comparison of species of monkey used for the neurovirulence test for oral poliomyelitis vaccine. J Biol Stand. 1978 Jul;6(3):233–242. doi: 10.1016/s0092-1157(78)80010-9. [DOI] [PubMed] [Google Scholar]
- Clements J. E., D'Antonio N., Narayan O. Genomic changes associated with antigenic variation of visna virus. II. Common nucleotide sequence changes detected in variants from independent isolations. J Mol Biol. 1982 Jul 5;158(3):415–434. doi: 10.1016/0022-2836(82)90207-8. [DOI] [PubMed] [Google Scholar]
- Clements J. E., Pedersen F. S., Narayan O., Haseltine W. A. Genomic changes associated with antigenic variation of visna virus durig persistent infection. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4454–4458. doi: 10.1073/pnas.77.8.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewley J., Gentsch J., Bishop D. H. Three unique viral RNA species of snowshoe hare and La Crosse bunyaviruses. J Virol. 1977 May;22(2):459–468. doi: 10.1128/jvi.22.2.459-468.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. E., Bodian D., Price D., Butler I. J., Vickers J. H. Chronic progressive poliomyelitis secondary to vaccination of an immunodeficient child. N Engl J Med. 1977 Aug 4;297(5):241–245. doi: 10.1056/NEJM197708042970503. [DOI] [PubMed] [Google Scholar]
- Gudnadóttir M. Visna-maedi in sheep. Prog Med Virol. 1974;18(0):336–349. [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Jubelt B., Gallez-Hawkins G., Narayan O., Johnson R. T. Pathogenesis of human poliovirus infection in mice. I. Clinical and pathological studies. J Neuropathol Exp Neurol. 1980 Mar;39(2):138–148. doi: 10.1097/00005072-198003000-00003. [DOI] [PubMed] [Google Scholar]
- Jubelt B., Narayan O., Johnson R. T. Pathogenesis of human poliovirus infection in mice. II. Age-dependency of paralysis. J Neuropathol Exp Neurol. 1980 Mar;39(2):149–159. doi: 10.1097/00005072-198003000-00004. [DOI] [PubMed] [Google Scholar]
- Kew O. M., Nottay B. K., Hatch M. H., Nakano J. H., Obijeski J. F. Multiple genetic changes can occur in the oral poliovaccines upon replication in humans. J Gen Virol. 1981 Oct;56(Pt 2):337–347. doi: 10.1099/0022-1317-56-2-337. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Kennedy S. I. Semliki forest virus persistence in mouse L929 cells. Virology. 1980 Jan 15;100(1):141–155. doi: 10.1016/0042-6822(80)90560-7. [DOI] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Chase J. Antigenic shift of visna virus in persistently infected sheep. Science. 1977 Jul 22;197(4301):376–378. doi: 10.1126/science.195339. [DOI] [PubMed] [Google Scholar]
- Roos R. P., Richards O. C., Ehrenfeld E. Analysis of Theiler's virus isolates from persistently infected mouse nervous tissue. J Gen Virol. 1983 Mar;64(Pt 3):701–706. doi: 10.1099/0022-1317-64-3-701. [DOI] [PubMed] [Google Scholar]
- Roos R. P., Richards O. C., Green J., Ehrenfeld E. Characterization of a cell culture persistently infected with the DA strain of Theiler's murine encephalomyelitis virus. J Virol. 1982 Sep;43(3):1118–1122. doi: 10.1128/jvi.43.3.1118-1122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozhon E. J., Kratochvil J. D., Lipton H. L. Analysis of genetic variation in Theiler's virus during persistent infection in the mouse central nervous system. Virology. 1983 Jul 15;128(1):16–32. doi: 10.1016/0042-6822(83)90315-x. [DOI] [PubMed] [Google Scholar]
- Rozhon E. J., Lipton H. L., Brown F. Characterization of Theiler's murine encephalomyelitis virus RNA. J Gen Virol. 1982 Aug;61(Pt 2):157–165. doi: 10.1099/0022-1317-61-2-157. [DOI] [PubMed] [Google Scholar]
- Spindler K. R., Horodyski F. M., Holland J. J. High multiplicities of infection favor rapid and random evolution of vesicular stomatitis virus. Virology. 1982 May;119(1):96–108. doi: 10.1016/0042-6822(82)90068-x. [DOI] [PubMed] [Google Scholar]
- Wilfert C. M., Buckley R. H., Mohanakumar T., Griffith J. F., Katz S. L., Whisnant J. K., Eggleston P. A., Moore M., Treadwell E., Oxman M. N. Persistent and fatal central-nervous-system ECHOvirus infections in patients with agammaglobulinemia. N Engl J Med. 1977 Jun 30;296(26):1485–1489. doi: 10.1056/NEJM197706302962601. [DOI] [PubMed] [Google Scholar]
- Wyatt H. V. Poliomyelitis in hypogammaglobulinemics. J Infect Dis. 1973 Dec;128(6):802–806. doi: 10.1093/infdis/128.6.802. [DOI] [PubMed] [Google Scholar]
- Yoneyama T., Hagiwara A., Hara M., Shimojo H. Alteration in oligonucleotide fingerprint patterns of the viral genome in poliovirus type 2 isolated from paralytic patients. Infect Immun. 1982 Jul;37(1):46–53. doi: 10.1128/iai.37.1.46-53.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]