Abstract
Tendon cells are specialized cells of the insect epidermis that connect basally attached muscle tips to the cuticle on their apical surface via prominent arrays of microtubules. Tendon cells of Drosophila have become a useful genetic model system to address questions with relevance to cell and developmental biology. Here, we use light, confocal, and electron microscopy to present a refined model of the subcellular organization of tendon cells. We show that prominent arrays of F-actin exist in tendon cells that fully overlap with the microtubule arrays, and that type II myosin accumulates in the same area. The F-actin arrays in tendon cells seem to represent a new kind of actin structure, clearly distinct from stress fibers. They are highly resistant to F-actin–destabilizing drugs, to the application of myosin blockers, and to loss of integrin, Rho1, or mechanical force. They seem to represent an important architectural element of tendon cells, because they maintain a connection between apical and basal surfaces even when microtubule arrays of tendon cells are dysfunctional. Features reported here and elsewhere for tendon cells are reminiscent of the structural and molecular features of support cells in the inner ear of vertebrates, and they might have potential translational value.
INTRODUCTION
In most invertebrates, muscles are antagonized by an exoskeleton on the outer ecto/epidermal surface, rather than interior bones. In this scenario, muscles have to establish links to the basal surface of ecto/epidermal cells, the apices of which are anchored to the exoskeletal matrix (cuticle). Such body wall cells have to establish adhesive contacts and cytoskeletal elements able to withstand the mechanical (shearing and pulling) forces created between contracting muscles and the antagonizing exoskeleton. Cytoskeletal elements ideal to resist such forces are intermediate filaments (Howard, 2001), and, accordingly, muscle-attached hypodermal cells in Caenorhabditis elegans are highly enriched in intermediate filaments, as is similarly the case for keratinocytes in the mammalian skin. Failure of such intermediate filaments to form or anchor appropriately, has dire effects on the cells' integrity (Jonkman, 1999; Bosher et al., 2003). In Drosophila, intermediate filaments are virtually absent (Bartnik and Weber, 1989; Adams et al., 2000; Goldstein and Gunawardena, 2000), and alternative cytoskeletal solutions have to be in place. In epidermal muscle attachment cells of Drosophila (hereafter referred to as tendon cells), the solution to this problem is the development of dense arrays of microtubules. The minus ends of these microtubules are anchored via apical cell junctions to the exoskeletal matrix (cuticle), and their plus ends via integrin-dependent basal cell junctions to tendon matrix. This tendon matrix is also associated with the tips of muscles, thus constituting the myo-epidermal link (Reedy and Beall, 1993; Prokop et al., 1998a,b; Tucker et al., 2004; Bökel et al., 2005).
During the last few decades, Drosophila tendon cells have become exciting model systems to be used for various venues of research. For example, in the context of tendon cells, complex signaling events have been unraveled (Volk, 1999, 2006). Tendon cells provide spatial cues attracting motile tips of developing muscles. Conversely, these muscles reinforce differentiation of the targeted tendon cells. Furthermore, many cellular assembly processes have been addressed, including the differentiation of cell junctions on basal and apical cell surfaces, as well as the establishment of the cytoskeletal architecture and its molecular links to the cell surface (e.g., Prokop et al., 1998a,b; Tucker et al., 2004; Bökel et al., 2005). Apart from integrins, several components have been identified, all of them known to be important in mammalian cell biology. Among these are integrin-linked kinase (Ilk; Zervas et al., 2001), focal adhesion kinase (FAK; Palmer et al., 1999), Paxillin (Yagi et al., 2001), PINCH (Clark et al., 2003), Talin (Brown et al., 2002), Tensin (Blistery) (Torgler et al., 2004), and the BPAG1/ACF7 orthologue Short stop (Röper et al., 2002).
Apart from providing an excellent model system for the study of cell signaling and assembly processes, tendon cells may also provide a representative model for certain cell types in vertebrates. An example of this is the similarity between Drosophila tendon cells and the Deiter's and pillar cells in the organ of Corti of the inner ear. These cells provide rigidity and support for sensory hair cells so that they are able to pivot when deflected by basilar membrane movement relative to the tectorial membrane (Forge and Wright, 2002). Hence, these cells also function in an environment of considerable mechanical challenge. Most interestingly, they share some of the properties of Drosophila tendon cells: 1) they contain apico-basal arrays of microtubules (Forge and Wright, 2002), 2) these microtubules display the unusual diameter of 15 rather than 13 protofilaments (Saito and Hama, 1982; Mogensen and Tucker, 1990), and 3) both of them are rich in Spectraplakin proteins (mammalian BPAG1 vs. its close Drosophila orthologue Short stop, Shot) (Leonova and Lomax, 2002; Röper et al., 2002). This suggests that tendon cells may not be an insect-specific cell type, but they have structurally related counterparts in vertebrates. However, in contrast to current descriptions of tendon epithelial cells, Deiter's and pillar cells have been reported to contain arrays of anti-parallel actin filaments lying interspersed with the microtubule arrays, both of which are heavily cross-linked (Slepecky and Chamberlain, 1983; Arima et al., 1986). These actin fibers have been suggested to convey stability rather than dynamic morphogenetic properties to the support cells (Slepecky and Chamberlain, 1987).
Here, we show that prominent F-actin arrays also exist in Drosophila tendon cells. Based on refined imaging approaches and a number of molecular markers we show that these fibers project apico-basally, fully overlap with the microtubule arrays, and are associated with type II myosin. Our data suggest that the F-actin arrays of tendon cells are additional architectural elements of tendon cells, but they display properties very distinct from stress fibers.
MATERIALS AND METHODS
Immunohistochemistry and Imaging
Antibodies used were anti-Zipper (rabbit, 1:500; kindly provided by D. Kiehart (Duke University, Durham, NC); Kiehart and Feghali, 1986), anti-mCD8 (rat, 1:10; Invitrogen, Carlsbad, CA), anti-Disk large (Dlg) (rabbit, 1:1000; courtesy of U. Thomas, Institute for Neurobiology, Magdeburg, Germany; Woods and Bryant, 1991), anti-βPS-integrin (CF6G11 ascites; mouse, 1:300; courtesy of E. Martin-Blanco, Instituto de Biologia Molecular de Barcelona, Barcelona, Spain; Brower et al., 1984); FAK (rabbit, 1:1000; courtesy of R. H. Palmer, Umea University, Umea, Sweden; Grabbe et al., 2004); anti-c-Paxillin (catalog no. 610051; mouse, 1:100; BD Biosciences Transduction Laboratories, Lexington, KY); anti-α-actinin (MAC276 ascites; rat, 1:10; courtesy of B. Bullard; Lakey et al., 1990); anti-Enabled (5G2; mouse, 1:20; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), anti-tubulin (mouse, 1:1000; T-9026; Sigma Chemical. Poole, Dorset, United Kingdom), anti-tyrosinated tubulin (MAB1864; rat, 1:500; Millipore Bioscience Research Reagents, Temecula, CA), anti-glu tubulin (rabbit, 1:200; Millipore Bioscience Research Reagents), anti-Short stop (guinea pig; 1:100; courtesy of T. Volk; Strumpf and Volk, 1998), and preabsorbed secondary antibodies (donkey; 1:200; Jackson ImmunoResearch, West Grove, PA). Cyanine (Cy)3- or Cy5-coupled phalloidin was diluted 1:500 or 1:100, respectively, and applied for 1 h.
Dissection of late stage 17 embryos (stages according to Campos-Ortega and Hartenstein, 1997) or of late L3 larvae was carried out in phosphate-buffered saline (PBS) followed by 1-h fixation in 4% paraformaldehyde and 1 h wash in PBS plus 0.3% Triton X-100, with the following two exceptions: anti-α-actinin and anti-c-paxillin stainings were carried out on larvae fixed for 1 min in 60°C PBS, and EB1::green fluorescent protein (GFP)-expressing animals were treated for 4 min in −20°C methanol. For live imaging (Figure 7), embryos were manually devitellinized and mounted in PBS under a coverslip. Imaging was carried out with an AxioCam (Carl Zeiss, Jena, Germany) on a BX50WI fluorescent microscope (Olympus, Tokyo, Japan) or with an SP5 confocal microscope (Leica, Wetzlar, Germany).
Figure 7.
Persistence of F-actin arrays in the absence of Rho1 or integrin function. Live images of embryos (wt, wild type; mys, myospheroid mutant; Rho-IR/RhoN19, tendon cell-specific expression of Rho1 iRNA/RhoN19) with tendon-cell specific expression of GFP-tagged proteins (act, actin; tub, tubulin); anterior up in A–C and left in D–F′. Cytoskeletal arrays are hardly affected by loss of Rho1 function (C and D) and are arranged into rings in myospheroid mutant embryos (arrowheads in D′, E′, and F′). Most pictures are taken of indirect muscle attachments in the dorsal muscle field, where the cytoskeletal arrays form one anterior and one posterior cytoskeletal belt (arrows in D). Bar, 14 μm.
Fly Stocks
The following fly stocks were used: UAS-GFP-a-tub84B (courtesy of C. Boekel, Dresden Technical University, Dresden, Germany; Grieder et al., 2000), UAS-ena::GFP (courtesy of T. Millard; Gates et al., 2007), UAS-Shot-PA::GFP (courtesy of P. Kolodziej, Vanderbilt University Medical Center, Nashville, TN, deceased; Lee and Kolodziej, 2002), UAS-EB1::GFP (courtesy of P. Kolodziej; unpublished), UAS-actin::GFP (UAS-Act5C.T:GFP127.37.2 and UAS-Act5C.T:GFP127.18.4; Bloomington Stock Center, Indiana University, Bloomington, IN; Kelso et al., 2002), UAS-mCD8-GFP (courtesy of L. Luo, Stanford University, Stanford, CA; Lee and Luo, 1999), sqhAX3; P[w+ sqh-gfp]42 in which the transgene is the only source of regulatory light chain (courtesy of E. Martin-Blanco; Royou et al., 2002), UAS-Rho1-dsRNA8.2, UAS-Rho1-dsRNA8.1/TM3,Sb1, UAS-Rho1.N192.1 (Bloomington Stock Center), shotsf20 (Prokop et al., 1998b); shot3 (courtesy of P. Kolodziej; Lee et al., 2000), stripe-Gal4 (courtesy of T. Volk; Subramanian et al., 2003), Neurexin IV::GFP (line CA06597 obtained from FlyTrap), myospheroid (mysXG43; courtesy of N. Brown, University of Cambridge, Cambridge, United Kingdom; Bunch et al., 1992), UAS-shot-dsRNA34 (courtesy of T. Volk, Weizmann Institute, Rehovot, Israel; Subramanian et al., 2003), integrin-linked kinase::GFP (IlkZCL3111; FlyTrap line, Bloomington Stock Center), collagen IV::GFP (vkgG454; FlyTrap line G00454; courtesy of E. Martin-Blanco; Morin et al., 2001), and enaGC1 and ena23 (Bloomington Stock Center; Wills et al., 1999).
Application of Toxins
Toxins were applied in PBS to cultured fillet preparations of late stage 17 or late L3 larvae in PBS or commercial Schneider's medium (Invitrogen) by using the following concentrations and incubation times: 1–2.5 h in 1–100 μM nocodazole (made up from a 5 mM stock in dimethyl sulfoxide [DMSO]); 1 mg/ml collagenase IV (Sigma Chemical) for 5–8 min (muscle detachment monitored under dissection microscope); 30 min in commercial trypsin-EDTA 1× solution for cell culture (Lonza 17-161, Lonza Verviers SPRL, Verviers, Belgium; 500 mg/l trypsin); 1–2.5 h in 4 μM cytochalasin D (made up from a frozen 2 mM stock solution in DMSO) and/or 6 μM latrunculin A; and 1 h in 50 μM or 1 mmol of Y-27632 (Sigma Chemical; made up from a 29 mM stock in water; NIH3T3 fibroblast cultured were treated for 1 h in 30 μM Y-27632 in DMEM medium). Collagenase IV or trypsin treatments were followed by at least 15 min in PBS or Schneider's medium.
Electron Microscopy
Electron microscopy was performed as described previously (Budnik and Ruiz-Cañada, 2006). For the ultrastructural analyses of collagenase IV-treated animals, St.17/L1 animals were filleted on Sylgard as described previously (Budnik and Ruiz-Cañada, 2006), treated for 5–6 min in 1 mg/ml collagenase IV (Sigma Chemical) in saline, briefly washed in pure saline, and then fixed for 1 h in 2.5% GDA in 0.05 M phosphate buffer, followed by further standard procedures (Budnik and Ruiz-Cañada, 2006).
RESULTS
Visualizing Microtubule Arrays of Larval Tendon Cells
Structural features of tendon cells have been well described at the ultrastructural level. However, existing light microscopic descriptions, as a more practical platform for genetic and pharmacological studies of the structure, function, and development of these cells, are less well understood with respect to subcellular resolution (Subramanian et al., 2003). Because microtubules are the most prominent structural feature of tendon cells (see Introduction), we started our light microscopic analyses with targeted expression of UAS-tubulin::GFP by using the tendon cell-specific driver line stripe-Gal4 (Gal4/UAS-system described in Duffy, 2002). At indirect muscle attachments, fluorescence is arranged into speckled or dashed fields of 30–40 μm in diameter, which we interpret as primarily vertically oriented arrays of microtubules (Supplemental Figure 1F). At direct muscle attachments, similar speckled or dashed fields also exist (Figure 1, B and C4). However, if the muscles are sufficiently stretched, part of these fibers is arranged into dense fluorescent bands measuring 2.5 to 5 μm in width, depending on the level of stretch. We interpret these bands (hereafter referred to as “cytoskeletal belts”) as oblique microtubules arranged in parallel to the axis of the pulling muscles (between arrow heads in Figures 1 and 2). This interpretation of the cytoskeletal arrays is in full agreement with all further observations made during this project.
Figure 1.
Resolving the apico-basal axis of tendon cells in the confocal microscope. Late larval tendon cells are shown in various views, as indicated bottom right in all images (anterior to the left): vertical views are presented in frontal (f) or sagittal (s) orientation, horizontal views are presented in either of three focal plains (b, basal; i, intermediate; a, apical), as illustrated by red arrows in the cartoon in B; alternatively horizontal views are shown as maximal projections (p), or as partial projections (b/i, basal plus intermediate; i/a, intermediate plus apical). (A) Direct muscle attachment (asterisk) shown as a maximal projection in horizontal view; the cell surface marker mCD8::GFP (CD8) demonstrates the tendon cell's shape and its close association with the phalloidin-stained muscle (Phal; white dot); Dlg illustrates the cell perimeter (curved white arrow) at the level of intraepidermal septate junctions. (B) Model of tendon cell in sagittal and two horizontal views explaining its overall organization and introducing symbols used throughout this paper: asterisk, tendon cell; white dot, muscle; white/black arrow, distal/proximal end of MTJ; white/black arrow heads, basal/apical end of cytoskeletal arrays; used abbreviations: bm, basement membrane; ca, cytoskeletal array (tubulin in green, F-actin in red); cu, cuticle; ep, normal epidermal cell; mtj, myotendinous junction; sj, septate junction. All other images show different views (bottom right) of phalloidin-labeled muscles colabeled with tendon cell-specific tubulin::GFP (Tub; C1–C4), GFP-tagged Neurexin (Nrx, labels septate junctions; D1–D4), or Viking::GFP (Vkg, labels basement membrane; E1–E4); symbols as in B. Bar, 22 μm (A) and 25 μm (C–E).
Figure 2.
Properties of microtubule arrays in tendon cells. Horizontal views of muscles (white dots) and tendon cells (asterisks) taken in intermediate (i) or basal (b) focal planes, according to Figure 1B. Usual networks of microtubules at the surface of muscles and tendon cells (A1) are severely reduced or abolished upon nocodazole treatment (B1); curved arrows point at nerves reaching into the image. In control animals, cytoskeletal arrays are labeled with tubulin::GFP (magenta), whereas anti-tubulin staining mostly fails to penetrate deep into tendon cells (A2); in nocodazole-treated animals the cytoskeletal belts are perfectly labeled by both anti-tubulin and tubulin::GFP (B2). Cytoskeletal belts can also be labeled with other anti-tubulin antibodies (E; Glu, anti-glu-tubulin; Tyr, anti-tyrosinated tubulin) or tubulin-associated proteins: Short stop::GFP (ShotGFP; F), antibody staining against C-terminal Short stop (ShotC; G) and EB1::GFP (EB1GFP; H). Bar is 10 μm (A and B) and 17 μm (E–H).
To confirm our findings made with tubulin::GFP, we carried out stainings with distinct anti-tubulin antibodies (Figure 2, A1, E, and E′) and tested the localization of the microtubule-binding proteins EB1 (Figure 2H) and Short stop (Shot; Figure 2, F and G), which have been shown to be expressed in tendon cells (e.g., Subramanian et al., 2003). All of these markers show enrichment at the cytoskeletal belt (Figure 2, E–H) and, among them, Shot displays a more elaborate pattern within the cytoskeletal belt: two prominent bands of protein accumulation run in parallel to the muscle tip on the proximal and distal side of the cytoskeletal belt. In between these bands, Shot expression is lower and often occurs in fiber-like arrangements (Figure 2, F and G).
Two predictions can be made on the basis of the above-mentioned observation. First, because Shot has been shown previously to stabilize microtubules (Lee and Kolodziej, 2002), it could be acting here to stabilize the microtubule arrays in tendon cells. Second, the cytoskeletal belt can be spatially subdivided, suggesting that the two bands of prominent Shot::GFP localization correspond to the apical and basal ends of the microtubule arrays. This would mean that apical and basal surfaces of tendon cells can be distinguished at the light microscopic level, with obvious implications for future studies. The following experiments were performed to test these two hypotheses.
Tubulin Arrays Are Highly Stable and Can Be Resolved in the Apicobasal Axis
To test for a potential stability of microtubules in tendon cells, we cultured fillet preparations of larvae for 2–2.5 h in medium containing high concentrations (10–100 μM) of nocodazole. Nonarray microtubules in these tendon cells are vastly eliminated, demonstrating the strength of the treatment (Figure 2B). In contrast, the microtubule arrays are fully protected (Figure 2B′). Thus, the arrays contain stable microtubules, and Shot is a potential factor mediating this property.
To explore the apicobasal resolution of the cytoskeletal belts, we studied the localization of known marker molecules by using either antibodies or fly lines carrying GFP-tagged versions of endogenous proteins. Neurexin IV localizes to septate junctions along the lateral side of epidermal cells (Baumgärtner et al., 1996; Knust, 2000). Accordingly, Neurexin IV::GFP delineates all epidermal cells, including the tendon cells. Analyses of such specimens reveal that, in contrast to the planar arrangement of most other epidermal cells, tendon cells take on a step-like shape in sagittal view (Figure 1, B and D2), most likely imposed by the attached muscle. When viewed as a frontal section through a confocal image stack (Figure 1D1), the cytoskeletal belt (visualized with phalloidin, as explained in the next chapter) is framed on each side by lines of Neurexin IV::GFP expression (Figure 1D1). Similar observations were made with anti-Dlg staining, a further marker for septate junctions (Knust, 2000; Figure 1A). These observations clearly demonstrate that the cytoskeletal belts span tendon cells in an apico-basal direction. To confirm our finding further, we probed marker molecules known to be associated with myotendinous junctions, i.e., expected to localize to the basal end of the cytoskeletal arrays. Indeed, stainings for βPS-integrin, Ilk::GFP, FAK, or Paxillin all localized to the basal end of the cytoskeletal arrays (shown for Ilk; Figure 3C1).
Figure 3.
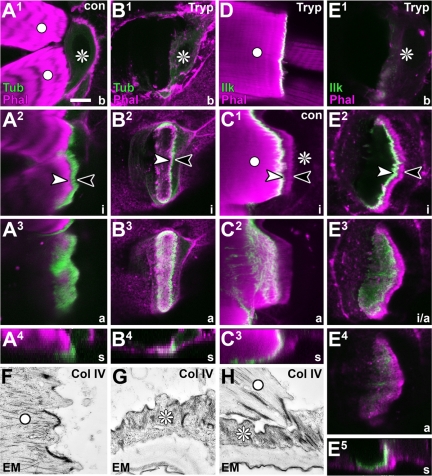
Refining localization studies via enzymatic muscle detachment. (A1–E5) Different views of control (con) or trypsin-treated (Tryp) direct muscle attachments; planes of view (bottom right) and symbols as explained in Figure 1B. Specimens are labeled with phalloidin (Phal) together with either tendon cell-specific tubulin::GFP (Tub) or GFP-tagged endogenous Ilk. The organization of tubulin into a cytoskeletal belt (between arrow heads in A2) and dashed/stippled fields (A3) is maintained in tendon cells after muscle detachment (B2 and B3), i.e., the muscle leaves behind its footprint in the orphan tendon cell (B4). Ilk::GFP is restricted to the immediate contact zone of muscle and tendon cells, decorating only the basal end of cytoskeletal arrays (C1). On muscle detachment, Ilk::GFP is found at the muscle tip (D), but also on the basal surface of the cytoskeletal arrays (E1–E5). Electron micrographs taken from collagenase IV-treated (Col IV) embryos show a detached muscle (F), an orphan tendon cell (G), and a direct muscle attachment in the process of detachment (H); in all cases, junctional surfaces and their intracellular linkage to the cytoskeleton seem fully intact. Bar, 10 μm (A–D), 6.1 μm (E), and 900 nm (F–H).
However, the close apposition and interdigitation of tendon cell and muscle tip membranes (Figure 4, B and D) makes it impossible to differentiate epithelial and muscular surfaces at the junction by using fluorescence or confocal microscopes. Therefore, staining could theoretically be localizing to the muscle tip alone. To solve this problem, we developed a strategy based on incubation of larval fillets with collagenase IV or trypsin proteinases (see Materials and Methods). Such strategy leads to a clean extracellular detachment of muscles, as revealed by ultrastructural inspection of treated specimens (Figure 3, F–H). The tendon cells seem unharmed by this treatment, as demonstrated by their integrity, even upon culturing for another 2–3 h after detachment in enzyme-free medium (Figure 5D). In most cases the detached muscles leave behind prominent footprints in the orphan tendon cells, reflected in their continued step-like appearances and display of a cytoskeletal belt for at least 30 min after detachment (Figure 3, B4 and E5). Via this muscle detachment strategy, muscle- and tendon cell-associated fractions of proteins, identified by antibody stainings, can be clearly assigned, as illustrated here for Ilk::GFP. Ilk::GFP precisely overlaps with the area of the cytoskeletal arrays but restricts to the basal tendon cell surface, as would be consistent with the findings from intact muscle attachments (Figure 3, C–E5, and Supplemental Figure 2).
Figure 4.
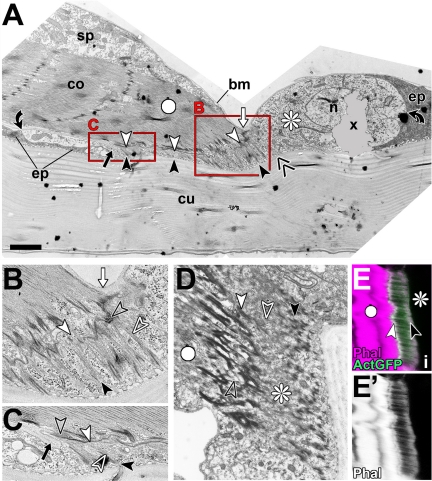
Ultrastructural features of late larval direct muscle attachments. (A–C) Image of a direct muscle attachment in sagittal view (A) and two close-ups (B and C; as boxed in A). In this preparation, the tendon cell (asterisk) occurs more translucent than surrounding epidermal cells (ep), making its maximal extension (between black curved arrows) easy to visualize. Arrays of microtubules (between black and white arrowheads; open white arrows point at microtubules) are longer toward the distal tip of the myotendinous junction, and the inner layer of the cuticle is bent upward in this area (double chevron in A). Basement membrane reaches to the distal (white arrows in A and B) and proximal (black arrows in A and C) end of the myotendinous junction, but it seems not to penetrate the myotendinous cleft (open black arrows). (D–E′) The cytoskeletal array of another tendon cell showing a relatively broad band of densely coated finger like indentations apically (black arrowhead in D) and the undulating band of the hemi-adherens junction basally (white arrowhead in D); these electron-dense specializations are likely to be the structural equivalents to the apical and basal bands labeled by actin::GFP (ActGFP) or phalloidin in confocal images of tendon cells (arrowheads in E). Bar, 2 μm (A), 0.8 μm (B and C), 1.5 μm (D), and 7.4 μm (E–E′).
Figure 5.
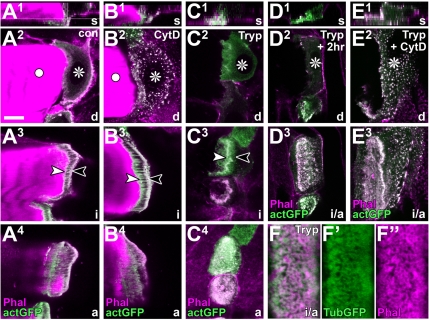
Properties of F-actin arrays in tendon cells. (A1–E3) Different views of control (con), trypsin-(Tryp), and/or cytochalasin D-treated (CytD) direct muscle attachment sites; planes of view (bottom right) and symbols as explained in Figure 1B. Specimens are labeled with phalloidin (Phal) together with tendon cell-specific actin::GFP (actGFP). The organization of actin into a cytoskeletal belt (between arrowheads in A3) and dashed/stippled fields (A4) clearly resembles tubulin::GFP-labeled specimens (compare Figure 3A). Cytochalasin D destroys most actin in these cells (dots in B2 and E2), but it has little or no effect on the F-actin arrays (B3 and B4), even in orphan tendon cells (E3). Muscle detachment does not significantly affect F-actin arrays in cytoskeletal belt (C3) and stippled fields (C4), and F-actin arrays can still be identified, if detached tendon cells are cultured for another 2 h (D3). (F–F″) Close-up of a detached tendon cell labeled with tubulin::GFP and phalloidin showing the closely intermingled nature of both cytoskeletal fractions. Bar, 10 μm (A–E), 2.8 μm (F′–F″).
As a final step to confirm our model deduced from confocal analyses, we carried out electron microscopy on sagittal sections of late larval tendon cells. Such images show the predicted step-like arrangement of tendon cells (Figure 4A), in which the microtubules are long at the distal ends of myotendinous junctions (Figure 4B) and short proximally (Figure 4C). Furthermore, these analyses demonstrate that basement membrane seems to be excluded from myotendinous junctions (arrows in Figure 4, B and C), consistent with findings in late embryonic specimens (Prokop et al., 1998a). We tested this aspect at the confocal level using a GFP-tagged version of the integral basement membrane component collagen IV (Viking::GFP). The Viking::GFP localization pattern clearly confirmed the exclusion of basement membrane from myotendinous junctions (arrows in Figure 1E), further demonstrating the spatial resolution of the confocal analysis and its precise correlation with ultrastructural findings.
In summary, we have refined strategies which enable us to carry out more detailed microscopic analyses of tendon cells, resolving cytoskeletal bands in the apico-basal axis and resolving the tendon cell versus muscle side at muscle attachments. Applying these strategies, we investigated further properties of tendon cells.
Tendon Cells Contain Prominent Arrays of Stable F-Actin
Support cells in the Organ of Corti show arrays of highly cross-linked actin filaments, intermingled with the prominent arrays of microtubules (Slepecky and Chamberlain, 1983; Arima et al., 1986). This tempted us to investigate the presence of similar structures in tendon cells. To this end, we targeted expression of actin::GFP to these cells. To our surprise, actin::GFP is strongly expressed in all areas of the cytoskeletal arrays and forms a fibrous cytoskeletal belt (Figure 5A3), very similar to that seen for tubulin::GFP (compare Figures 5A and 4E vs. 3A and 2A2). The only difference is that actin, but not tubulin, has a tendency to enrich apically and basally, most likely due to its incorporation into submembranous densities of hemi-adherens junctions at the apical and basal tendon cell surfaces (Figure 4, D and E). The current assumption, that microtubules are the sole stabilizing elements of tendon cells, needs to be reconsidered.
The phalloidin staining pattern in tendon cells is congruent to the localization pattern of actin::GFP (Figures 4E and 5A). This supports our findings and provides a simple labeling strategy for further experimentation. In double labeling experiments, phalloidin marks the same area as tubulin::GFP (Figure 1C3), and this becomes especially obvious upon muscle detachment (Figures 3B3 and 5F). Therefore, we propose that F-actin and microtubule arrays are intermingled. Such a scenario resembles the parallel arrangement of actin filaments and microtubules demonstrated previously for epidermal cells of developing Drosophila wings (Mogensen and Tucker, 1988). These wing epithelial cells share a variety of structural features with tendon cells and likewise have to withstand enormous mechanical challenges, especially when the wing blades unfold in the freshly hatched fly (Bökel et al., 2005).
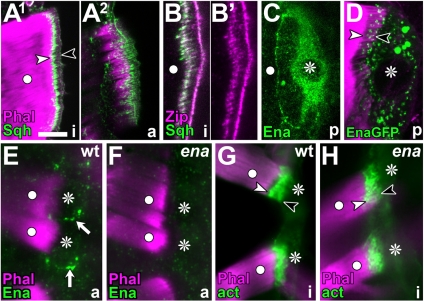
Ultrastructural studies using S1 decoration in support cells showed actin fibers to be anti-parallel (Slepecky and Chamberlain, 1983; Arima et al., 1986). To address this property, we monitored tendon cells for localization of Enabled, a molecule of the actin assembly machinery binding to barbed ends of F-actin (Krause et al., 2003). Although the staining for anti-Enabled was enriched in late larval tendon cells, compared with the surrounding epidermal cells, there was no obvious enrichment at the cytoskeletal arrays (Figure 6C). This was likewise the case when targeting Ena::GFP to late larval tendon cells (Figure 6D). In late embryos, Enabled staining was often enriched in dots along the lateral border of tendon cells, but it was never associated with the cytoskeletal belts (Figure 6E). Thus, these experiments do not provide any insights into the orientation of F-actin in tendon cells. However, they suggest that these fibers display low dynamism and might therefore not depend on an efficient F-actin polymerization machinery, as seen in other F-actin–rich structures such as filopodia of growth cones (Dwivedy et al., 2007; our unpublished results for Drosophila). In agreement with this notion, enaGC1/ena23 loss-of-function mutant embryos, which lack prominent Enabled expression in tendon cells (Figure 6F) and display severe CNS phenotypes (data not shown), fail to display obvious phenotypes of the F-actin arrays (Figure 6H). Low dynamism of F-actin arrays may imply that they are stable. We tested this possibility by treating tendon cells with F-actin destabilizing drugs. Such treatment causes actin to aggregate into dots dispersed throughout tendon cells, demonstrating the effectiveness of the treatment, whereas the F-actin arrays were fairly unaffected (Figure 5B). Even 2.5-h incubation with a cocktail of 4 μM cytochalasin D together with 6 μM latrunculin A, failed to abolish the F-actin arrays (data not shown). This resistance to destabilizing drugs is in agreement with the observation that the apicobasal actin fiber arrays in support cells of the Organ of Corti are stable (Slepecky and Chamberlain, 1987; Oshima et al., 1992).
Figure 6.
Further properties of tendon cells. Direct muscle attachments (white dots, muscles; asterisks, and tendon cells), labeled as indicated bottom left: Sqh, Spaghetti squash; Zip, Zipper; Ena, Enabled; EnaGFP, Enabled::GFP; act, actin::GFP; Phal, Phalloidin; anterior is left. (A–B′) Sqh::GFP localizes to the cytoskeletal belt (between arrow heads in A1) and the apical fields of cytoskeletal arrays (A2); Zipper colocalizes with Sqh at the cytoskeletal belt (B). (C) Enabled is enriched in late larval tendon cells (asterisk), but it does not concentrate at cytoskeletal arrays. (D) Enabled::GFP is also absent from cytoskeletal arrays. (E–H) In late embryonic tendon cells, Ena accumulates at the tendon cell periphery of wild type (white arrows in E) but not enaGC1/23 mutant embryos (F); absence of Enabled has no obvious effect on the F-actin arrays (G vs. H). Bar, 10 μm (A, C, and D) 6 μm (B), and 7 μm (E–H).
Nonmuscle Myosin Type II Associates with Cytoskeletal Arrays
The presence of prominent arrays of actin fibers together with reports of several typical focal adhesion components at the myotendinous junction (see Introduction and Figure 3C), tempted us to speculate that these F-actin structures might represent stress fibers, such as seen in migrating cells or myofibroblasts (Hinz and Gabbiani, 2003). Stress fibers contain α-Actinin and nonmuscle Myosin type II (Lo et al., 2004; Lele and Kumar, 2007). Support cells in the Organ of Corti also seem to contain these proteins (Drenckhahn et al., 1982; Slepecky and Chamberlain, 1985; Donaudy et al., 2004). Therefore, we investigated the presence of these factors in Drosophila tendon cells.
Antisera against Drosophila α-actinin reveal strong staining in Z-discs of the muscle and at the myotendinous junction (data not shown), but no α-actinin was detectable on the cytoskeletal arrays of tendon cells or on their basal surfaces upon muscle detachment (data not shown). However, on the basis of these results, the presence of α-actinins in tendon cells cannot be fully excluded because a paralogue, α-actinin3, is reported in FlyBase (Drysdale et al., 2005), for which no antibody is available.
To address the presence of myosin II, we used a previously published fly line expressing Sqh::GFP (a GFP-tagged version of type II myosin regulatory light chain, Spaghetti squash) under the control of the sqh promoter in a sqh mutant background (Royou et al., 2002). In these larvae, only tendon cells, but not any other epidermal cells, showed cytoplasmic enrichment of Sqh::GFP to various degrees (data not shown). Most importantly, most tendon cells show prominent localization of Sqh::GFP to the cytoskeletal arrays (Figure 6, A1 and A2). We confirmed this finding by staining against endogenous Zipper protein, the nonmuscle Myosin II heavy chain (Young et al., 1993). Anti-Zipper staining was enriched particularly in tendon cells, in which it colocalizes with Sqh::GFP at the cytoskeletal belts (Figure 6B).
Arrays of F-Actin in Tendon Cells Form in the Absence of Rho1, Rock, or Integrin Activity
Encouraged by our finding with myosin II, we tested whether essential regulators of stress fibers, such as Rho1, its effector Rock, and integrins (Schoenwaelder and Burridge, 1999), are involved in the regulation of F-actin arrays in tendon cells. First, we down-regulated Rho1 activity via tendon cell-specific expression of Rho1-specific antisense RNA or of Rho1N19, a dominant-negative version of Rho1. Both treatments caused lethality at the late L2/early L3 larval stage. However, in contrast to ordinary stress fibers in migrating cells (Schoenwaelder and Burridge, 1999), the cytoskeletal belts in such tendon cells clearly failed to vanish upon inhibition of Rho1 activity (Figure 7, B and C). We complemented these studies with experiments using Y-27632, a selective inhibitor for Rock. Rho1 is generally believed to activate myosin light chain through Rock (Govek et al., 2005), and this holds true in Drosophila (Verdier et al., 2006). Previously, Y-27632 has been successfully used in Drosophila and was shown to have a direct effect on Sqh::GFP localization (Royou et al., 2002). However, in our experiments, one hour incubations in Y-27632 at conventional or elevated doses (50 μM or 1 mmol) did not affect F-actin arrays in tendon cells or the localization of Sqh::GFP (data not shown), whereas parallel treatment of mouse NIH3T3 fibroblasts for the same period completely disassembled their stress fibers (data not shown).
To test a potential involvement of integrins in F-actin array formation or maintenance, we analyzed myospheroid (mysXG43) mutant embryos, which lack all integrin function at the myotendinous junction (Bunch et al., 1992). In mysXG43 mutant embryos expressing actin::GFP in tendon cells, potential F-actin arrays can be seen in only a subset of tendon cells where they occur in unusual ring structures (Figure 7D′). We observed the same phenomenon when using tubulin::GFP expression (Figure 7E′). At first sight, this seemed to contradict previous ultrastructural analyses showing that microtubules are present in mys mutant tendon cells. However, those electron microscopy studies also showed that microtubule arrays are dramatically shortened in mysXG43 mutant tendon cells (Prokop et al., 1998a). We therefore reasoned that subdetectable levels of actin::GFP or tubulin::GFP in many mysXG43 mutant tendon cells might be due to severe shortening, rather than the absence of the cytoskeletal arrays. In contrast to this finding, we predicted that molecular components of the myotendinous junction, such as Ilk::GFP, should be abundantly present in mys mutant embryos, based on the former report that hemiadherens junctions in mys mutant tendon cells are unaffected at the ultrastructural level (Prokop et al., 1998a). Indeed, Ilk::GFP was found to be reliably expressed in mysXG43 mutant tendon cells, where it forms the same ring structures as actin::GFP and tubulin::GFP (Figure 7F′). Hence, our light microscopic analyses are in accordance with previous electron microscopic studies, and we propose that only the length and shape, but not the principal formation, of F-actin arrays is dependent on integrin function.
In fibroblasts, stress fibers are positively regulated through mechanical force, partly mediated through type II myosin function (Bershadsky et al., 2006; Lele and Kumar, 2007). Such mechanical forces are abolished in mysXG43 mutant embryos where muscles detach from tendon cells (Prokop et al., 1998a). Therefore, the F-actin arrays we found to persist in mysXG43 mutant embryos suggest that mechanical force is not crucial for their formation. To test this hypothesis more directly, we detached muscles from tendon cells at the late larval stage via collagenase IV- or trypsin treatment and cultured these specimens for 2–3 h after detachment. However, under these conditions, neither intensity nor distribution of actin::GFP, tubulin::GFP, or Sqh::GFP seemed gravely affected (actin::GFP shown in Figure 5D). Application of 4 μM cytochalasin D during the culturing period did not suggest any noticeable increase in the vulnerability of the actin fibers (Figure 5E). These findings are consistent with our studies in integrin-deficient embryos.
We conclude from these studies that the F-actin fibers in tendon cells fail to display regulatory properties described for stress fibers, and the role of type II myosin in these fibers remains elusive.
F-Actin Arrays Maintain a Physical Apico-Basal Link When Microtubule Arrays Are Dysfunctional
We next tested the properties of F-actin arrays under conditions when the microtubule arrays are affected. For example, the actin–microtubule linker Shot of the Spectraplakin family is required for the formation, and/or maintenance, of the microtubule arrays in tendon cells (Gregory and Brown, 1998; Prokop et al., 1998b; Strumpf and Volk, 1998; Subramanian et al., 2003). We therefore analyzed the presence and stability of F-actin arrays upon loss of Shot function in tendon cells. When knocking down shot with a previously published interference RNA (iRNA) construct, we found significant elongation of tendon cells and their cytoskeletal belts (Figure 8C), as reported previously (Subramanian et al., 2003). F-actin arrays of such embryos treated for 1 h with 4 μM cytochalasin D, or with PBS as a control, were very similar in appearance (Figure 8D). Because knockdown in these animals might not be complete, we combined iRNA expression with one copy of the shot3 null allele or analyzed shotsf20/shot3 mutant embryos (the strongest known alleles). In either case, muscle tips are maximally retracted from the cuticle. However, in spite of this dramatic elongation of tendon cells, long thick F-actin cables maintain a link between cuticle and the severely retracted muscle tips (Figure 8, E–J). Expression of tubulin is very low in these structures (data not shown), in agreement with previous reports that tubulin levels are reduced in shot mutant embryos (Strumpf and Volk, 1998). When challenging the F-actin cables with cytochalasin D, latrunculin A, or a combination of both agents, treated and sham-treated embryos did not reveal any obvious differences (Figure 8, E–J).
Figure 8.
Persistence of F-actin arrays in the absence of Shot function. Flat dissected embryos (arrows, bottom left, indicate anterior) with tendon–cell-specific expression of actin-GFP (green); muscles are labeled with phalloidin (magenta); different genotypes (indicated on left) were treated for 90 min in either PBS (left column) or PBS with toxin (right column; LaA, 6 μM latrunculin A; CyD, 4 μM cytochalasin D). Arrowheads indicate elongated F-actin arrays. Bar, 19 μm.
We conclude that Shot is not required for F-actin array formation and stability in tendon cells. However, these data show that F-actin arrays can maintain an apico-basal link in the absence of functional microtubule arrays, and they demonstrate their architectural contribution to tendon cell integrity.
DISCUSSION
Novel Insight into Drosophila Tendon Cells
Tendon cells of Drosophila have been used as successful cellular models for a considerable time (Volk, 1999, 2006). Here, we have delivered refined light and laser microscopic approaches providing enhanced opportunities for future work in this system. Applying these strategies, we have refined insight into the organization of tendon cells, and we have uncovered new insight into their molecular composition.
The essential advance in microscopic analyses is the clear demonstration that tendon cells can be resolved in the apicobasal axis at the position of the cytoskeletal belt and that subcellular markers, such as Paxillin, Ilk, integrin, or Neurexin IV, can be mapped along this axis. The proteinase-based detachment assay provides a quick means to confirm potential localization of proteins to basal surfaces of tendon cells. By showing that apical and basal ends of cytoskeletal belts can be distinguished, these prominent structures have become helpful landmarks in their own right, which can be easily visualized via phalloidin staining. From the studied markers in conjunction with new ultrastructural data, we are confident that our model of the subcellular organization of tendon cells at the light microscopic level (Figure 1B) provides a reliable readout with good spatial resolution. Via these strategies, it is now possible to assign proteins rapidly to subcellular compartments, making us less dependent on complex immunoelectron microscopic localization studies. For example, by using a GFP-tagged version of endogenous collagen IV (Vkg), we could establish effortlessly that basement membrane components are absent from the myotendinous junction (Figure 1E), whereas previously we were unable to answer this question unequivocally using detailed ultrastructural studies (Prokop et al., 1998a; arrows in Figure 4).
Shared Features between Tendon Cells and Support Cells in the Inner Ear of Vertebrates
In addition to providing refined descriptions of the organization of tendon cells, we have reported new molecular features for this cell type. Their microtubular arrays are resistant to nocodazole, and they display prominent arrays of stable F-actin that seem to be associated with type II myosin (Sqh and Zipper). These observations extend the catalogue of properties shared between Drosophila tendon cells and support cells in the inner ear of vertebrates. Both display apico-basal arrays of microtubules, which are stable and contain the unusual number of 15 protofilaments (Slepecky et al., 1995), both display apicobasal arrays of stable actin filaments, and both seem to express type II myosin and Spectraplakins. This leads us to speculate that the organization of both cell types could be analogous or even homologous, and work in tendon cells might become a source of inspiration with potential translational value, e.g., in the context of age-related deafness caused by decay of support cells (Saha and Slepecky, 2000; Forge and Wright, 2002). However, it needs to be pointed out that α-actinin, which is present in support cells of the inner ear, might be absent in Drosophila tendon cells.
Shared and Distinct Features of Hemi-adherens Junctions and Focal Adhesions
The existence of F-actin arrays in tendon cells sheds new light into focal adhesion components which are prominently expressed at the basal surface of tendon cells (see Introduction and Figure 3). Such components are best understood in migrating cells in culture, in which they assemble into integrin-dependent membrane-associated complexes called focal adhesions (Zaidel-Bar et al., 2004). Focal adhesions anchor and regulate actin stress fibers, contractile cell-spanning structures associated with factors such as type II myosin and α-actinin (Lo et al., 2004; Lele and Kumar, 2007). In addition, focal adhesions are also targeted by microtubules (Bershadsky et al., 2006), the second prominent cytoskeletal component attached to myotendinous junctions in tendon cells. However, there are clear differences between both adhesion complexes. Loss of integrin function correlates with loss of focal adhesions and stress fibers (Schoenwaelder and Burridge, 1999). In contrast, the hemi-adherens junctional complex of tendon cells and cytoskeletal arrays are, in principle, formed and maintained in the absence of integrins and muscle adhesion, as demonstrated ultrastructurally elsewhere (Prokop et al., 1998a) and here by the maintained localization of various markers, such as actin, tubulin, and the focal adhesion component Ilk (Figure 7, D–F′). Down-regulation of Rho or Rock is known to reduce focal adhesions and stress fibers (Schoenwaelder and Burridge, 1999), which does not hold true for F-actin arrays in tendon cells (Figure 7, A–C). Similarly, microtubules negatively influence focal adhesions (Bershadsky et al., 2006), whereas they are stably anchored at the myotendinous junctional complex of tendon cells. Thus, although both adhesion complexes contain similar molecular players, their systemic output is clearly distinct. It will be very interesting to carry out comparative studies of these proteins to appreciate the breadth of their functions in the context of cell migration versus integrity. For example, it might not be unrealistic to speculate that a simple molecular switch, such as Vinculin (Humphries et al., 2007), could turn a Rho-dependent into a Rho-independent constellation. The strategies and F-actin arrays reported here provide an improved experimental platform for such analyses in tendon cells.
The Potential Role of F-Actin Arrays
A very likely role for F-actin arrays is the provision of additional stability, enabling tendon cells to withstand forces built up between contracting muscles and the rigid cuticle. In general, microtubules are ideal architectural elements to provide stability against shear forces (Howard, 2001), an obvious challenge tendon cells are exposed to. Microtubules are strong in resisting tensile force. However, they are likely to be intolerant to overstretching, and they would be expected to break when elongated >1% of their length (Howard, personal communication). The F-actin arrays may be an ideal complementary system in this situation. Their architectural importance is best demonstrated in situations where microtubule arrays are significantly impaired, as is the case in shot mutant embryos (Figure 8). Initially, the enormous lengthening of F-actin observed under these conditions seems surprising given that actin filaments tend to break when overstretched, even more when under torsion (Tsuda et al., 1996). However, F-actin in cellular networks displays vastly enhanced elastic properties through cross-linking factors and myosin association (Gardel et al., 2006). Furthermore, length adaptation through active polymerization might prevent a force overload of the array system.
Actin fibers might also play a role during development. Thus, work in cultured tendon cells has suggested that microtubules in developing tendon cells nucleate at the apical surface and extend toward the basal side where their plus ends are precisely targeted to pre-existing cortical capturing sites provided by the hemi-adherens junction (Tucker et al., 2004). Potentially pre-existing actin fibers could guide these microtubule targeting events by serving as tracks for their plus ends. A similar scenario has been proposed for the targeting of microtubules to focal adhesions (Kodama et al., 2004). However, this possibility can only be tested once we understand the mechanisms underlying F-actin array formation.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to numerous colleagues mentioned in Materials and Methods, the Vienna and Bloomington Stock Centers, and the Developmental Studies Hybridoma Bank for providing stocks and antibodies. We thank Mark Travis, Koyuki Kondo, and Melanie Klein for assistance with some experiments; Adele Hill for critical reading; John Howard and Christoph Ballestrem for helpful discussions; and the anonymous referee for suggesting the use of trypsin. This work was funded by grants of the Biotechnology and Biological Sciences Research Council (BB/C515998/1 and BB/E009085/1) and the German Israeli Foundation (I 073-203.05/98) to A. P. and Erasmus fellowships to I. H. and O. H. The Bioimaging Facility confocal microscopes used in this study were purchased with grants from the BBSRC, the Wellcome Trust, and the University of Manchester Strategic Fund.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-02-0182) on July 30, 2008.
REFERENCES
- Adams M. D., et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Arima T., Uemura T., Yamamoto T. Cytoskeletal organization in the supporting cell of the guinea pig organ of Corti. Hear. Res. 1986;24:169–175. doi: 10.1016/0378-5955(86)90061-4. [DOI] [PubMed] [Google Scholar]
- Bartnik E., Weber K. Widespread occurrence of intermediate filaments in invertebrates; common principles and aspects of diversion. Eur. J. Cell Biol. 1989;50:17–33. [Google Scholar]
- Baumgärtner S., Littleton J. T., Broadie K., Bhat M. A., Harbecke R., Lengyel J. A., Chiquet-Ehrismann R., Prokop A., Bellen H. J. A Drosophila neurexin is required for the formation and function of septate junctions. Cell. 1996;87:1059–1068. doi: 10.1016/s0092-8674(00)81800-0. [DOI] [PubMed] [Google Scholar]
- Bershadsky A. D., Ballestrem C., Carramusa L., Zilberman Y., Gilquin B., Khochbin S., Alexandrova A. Y., Verkhovsky A. B., Shemesh T., Kozlov M. M. Assembly and mechanosensory function of focal adhesions: experiments and models. Eur J. Cell Biol. 2006;85:165–173. doi: 10.1016/j.ejcb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Bökel C., Prokop A., Brown N. H. Papillote and Piopio: Drosophila ZP-domain proteins required for cell adhesion to the apical extracellular matrix and microtubule organization. J. Cell Sci. 2005;118:633–642. doi: 10.1242/jcs.01619. [DOI] [PubMed] [Google Scholar]
- Bosher J. M., Hahn B. S., Legouis R., Sookhareea S., Weimer R. M., Gansmuller A., Chisholm A. D., Rose A. M., Bessereau J. L., Labouesse M. The Caenorhabditis elegans vab-10 spectraplakin isoforms protect the epidermis against internal and external forces. J. Cell Biol. 2003;161:757–768. doi: 10.1083/jcb.200302151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower D. L., Wilcox M., Piovant M., Smith R. J., Reger L. A. Related cell-surface antigens expressed with positional specificity in Drosophila imaginal discs. Proc. Natl. Acad. Sci. USA. 1984;81:7485–7489. doi: 10.1073/pnas.81.23.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. H., Gregory S. L., Rickoll W. L., Fessler L. I., Prout M., White R. A., Fristrom J. W. Talin is essential for integrin function in Drosophila. Dev. Cell. 2002;3:569–579. doi: 10.1016/s1534-5807(02)00290-3. [DOI] [PubMed] [Google Scholar]
- Budnik V., Ruiz-Cañada C., editors. The fly neuromuscular junction: structure and function. San Diego, CA: Elsevier Academic Press; 2006. [Google Scholar]
- Bunch T. A., Salatino R., Engelsgjerd M. C., Mukai L., West R. F., Brower D. L. Characterization of mutant alleles of myospheroid, the gene encoding the beta subunit of the Drosophila PS integrins. Genetics. 1992;132:519–528. doi: 10.1093/genetics/132.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Ortega J. A., Hartenstein V. The embryonic development of Drosophila melanogaster. Berlin, Germany: Springer Verlag; 1997. [Google Scholar]
- Clark K. A., McGrail M., Beckerle M. C. Analysis of PINCH function in Drosophila demonstrates its requirement in integrin-dependent cellular processes. Development. 2003;130:2611–2621. doi: 10.1242/dev.00492. [DOI] [PubMed] [Google Scholar]
- Donaudy F., et al. Nonmuscle myosin heavy-chain gene MYH14 is expressed in cochlea and mutated in patients affected by autosomal dominant hearing impairment (DFNA4) Am. J. Hum. Genet. 2004;74:770–776. doi: 10.1086/383285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn D., Kellner J., Mannherz H. G., Groschel-Stewart U., Kendrick-Jones J., Scholey J. Absence of myosin-like immunoreactivity in stereocilia of cochlear hair cells. Nature. 1982;300:531–532. doi: 10.1038/300531a0. [DOI] [PubMed] [Google Scholar]
- Drysdale R. A., et al. FlyBase: genes and gene models. Nucleic Acids Res. 2005;33:D390–D395. doi: 10.1093/nar/gki046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J. B. GAL4 system in Drosophila: a fly geneticist's Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- Dwivedy A., Gertler F. B., Miller J., Holt C. E., Lebrand C. Ena/VASP function in retinal axons is required for terminal arborization but not pathway navigation. Development. 2007;134:2137–2146. doi: 10.1242/dev.002345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forge A., Wright T. The molecular architecture of the inner ear. Br. Med. Bull. 2002;63:5–24. doi: 10.1093/bmb/63.1.5. [DOI] [PubMed] [Google Scholar]
- Gardel M. L., Nakamura F., Hartwig J., Crocker J. C., Stossel T. P., Weitz D. A. Stress-dependent elasticity of composite actin networks as a model for cell behavior. Phys. Rev. Lett. 2006;96 doi: 10.1103/PhysRevLett.96.088102. 088102. [DOI] [PubMed] [Google Scholar]
- Gates J., Mahaffey J. P., Rogers S. L., Emerson M., Rogers E. M., Sottile S. L., Van Vactor D., Gertler F. B., Peifer M. Enabled plays key roles in embryonic epithelial morphogenesis in Drosophila. Development. 2007;134:2027–2039. doi: 10.1242/dev.02849. [DOI] [PubMed] [Google Scholar]
- Goldstein L. S., Gunawardena S. Flying through the Drosophila cytoskeletal genome. J. Cell Biol. 2000;150:F63–F68. doi: 10.1083/jcb.150.2.f63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govek E. E., Newey S. E., Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- Grabbe C., Zervas C. G., Hunter T., Brown N. H., Palmer R. H. Focal adhesion kinase is not required for integrin function or viability in Drosophila. Development. 2004;131:5795–5805. doi: 10.1242/dev.01462. [DOI] [PubMed] [Google Scholar]
- Gregory S. L., Brown N. H. kakapo, a gene required for adhesion between cell layers in Drosophila, encodes a large cytoskeletal linker protein related to plectin and dystrophin. J. Cell Biol. 1998;143:1271–1282. doi: 10.1083/jcb.143.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieder N. C., de Cuevas M., Spradling A. C. The fusome organizes the microtubule network during oocyte differentiation in Drosophila. Development. 2000;127:4253–4264. doi: 10.1242/dev.127.19.4253. [DOI] [PubMed] [Google Scholar]
- Hinz B., Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Curr. Opin. Biotechnol. 2003;14:538–546. doi: 10.1016/j.copbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Howard J. Mechanics of Motorproteins and the Cytoskeleton. Sunderland, MA: Sinauer Associates; 2001. [Google Scholar]
- Humphries J. D., Wang P., Streuli C., Geiger B., Humphries M. J., Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J. Cell Biol. 2007;179:1043–1057. doi: 10.1083/jcb.200703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman M. F. Hereditary skin diseases of hemidesmosomes. J. Dermatol. Sci. 1999;20:103–121. doi: 10.1016/s0923-1811(99)00017-1. [DOI] [PubMed] [Google Scholar]
- Kelso R. J., Hudson A. M., Cooley L. Drosophila Kelch regulates actin organization via Src64-dependent tyrosine phosphorylation. J. Cell Biol. 2002;156:703–713. doi: 10.1083/jcb.200110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehart D. P., Feghali R. Cytoplasmic myosin from Drosophila melanogaster. J. Cell Biol. 1986;103:1517–1525. doi: 10.1083/jcb.103.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E. Control of epithelial cell shape and polarity. Curr. Opin. Genet. Dev. 2000;10:471–475. doi: 10.1016/s0959-437x(00)00115-5. [DOI] [PubMed] [Google Scholar]
- Kodama A., Lechler T., Fuchs E. Coordinating cytoskeletal tracks to polarize cellular movements. J. Cell Biol. 2004;167:203–207. doi: 10.1083/jcb.200408047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M., Dent E. W., Bear J. E., Loureiro J. J., Gertler F. B. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu. Rev. Cell Dev. Biol. 2003;19:541–564. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- Lakey A., Ferguson C., Labeit S., Reedy M., Larkins A., Butcher G., Leonard K., Bullard B. Identification and localization of high molecular weight proteins in insect flight and leg muscle. EMBO J. 1990;9:3459–3467. doi: 10.1002/j.1460-2075.1990.tb07554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Harris K.-L., Whitington P. M., Kolodziej P. A. short stop is allelic to kakapo, and encodes rod-like cytoskeletal-associated proteins required for axon extension. J. Neurosci. 2000;20:1096–1108. doi: 10.1523/JNEUROSCI.20-03-01096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Kolodziej P. A. Short stop provides an essential link between F-actin and microtubules during axon extension. Development. 2002;129:1195–1204. doi: 10.1242/dev.129.5.1195. [DOI] [PubMed] [Google Scholar]
- Lee T., Luo L. Mosaic analysis with a repressible neurotechnique cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Lele T. P., Kumar S. Brushes, cables, and anchors: recent insights into multiscale assembly and mechanics of cellular structural networks. Cell Biochem. Biophys. 2007;47:348–360. doi: 10.1007/s12013-007-0013-x. [DOI] [PubMed] [Google Scholar]
- Leonova E. V., Lomax M. I. Expression of the mouse Macf2 gene during inner ear development. Brain Res. Mol. Brain Res. 2002;105:67–78. doi: 10.1016/s0169-328x(02)00394-7. [DOI] [PubMed] [Google Scholar]
- Lo C. M., Buxton D. B., Chua G. C., Dembo M., Adelstein R. S., Wang Y. L. Nonmuscle myosin IIb is involved in the guidance of fibroblast migration. Mol. Biol. Cell. 2004;15:982–989. doi: 10.1091/mbc.E03-06-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen M. M., Tucker J. B. Intermicrotubular actin filaments in the transalar cytoskeletal arrays of Drosophila. J. Cell Sci. 1988;91:431–438. doi: 10.1242/jcs.91.3.431. [DOI] [PubMed] [Google Scholar]
- Mogensen M. M., Tucker J. B. Taxol influences control of protofilament number at microtubule-nucleating sites in Drosophila. J. Cell Sci. 1990;97:101–107. doi: 10.1242/jcs.97.1.101. [DOI] [PubMed] [Google Scholar]
- Morin X., Daneman R., Zavortink M., Chia W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc. Natl. Acad. Sci. USA. 2001;98:15050–15055. doi: 10.1073/pnas.261408198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima T., Okabe S., Hirokawa N. Immunocytochemical localization of 205 kDa microtubule-associated protein (205 kDa MAP) in the guinea pig organ of Corti. Brain Res. 1992;590:53–65. doi: 10.1016/0006-8993(92)91081-o. [DOI] [PubMed] [Google Scholar]
- Palmer R. H., Fessler L. I., Edeen P. T., Madigan S. J., McKeown M., Hunter T. DFak56 is a novel Drosophila melanogaster focal adhesion kinase. J. Biol. Chem. 1999;274:35621–35629. doi: 10.1074/jbc.274.50.35621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A., Martín-Bermudo M. D., Bate M., Brown N. Absence of PS integrins or laminin A affects extracellular adhesion, but not intracellular assembly, of hemiadherens and neuromuscular junctions in Drosophila embryos. Dev. Biol. 1998a;196:58–76. doi: 10.1006/dbio.1997.8830. [DOI] [PubMed] [Google Scholar]
- Prokop A., Uhler J., Roote J., Bate M. C. The kakapo mutation affects terminal arborisation and central dendritic sprouting of Drosophila motor neurons. J. Cell Biol. 1998b;143:1283–1294. doi: 10.1083/jcb.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedy M. C., Beall C. Ultrastructure of developing flight muscle in Drosophila. II. Formation of the myotendon junction. Dev. Biol. 1993;160:466–479. doi: 10.1006/dbio.1993.1321. [DOI] [PubMed] [Google Scholar]
- Röper K., Gregory S. L., Brown N. H. The ‘Spectraplakins’: cytoskeletal giants with characteristics of both spectrin and plakin families. J. Cell Sci. 2002;115:4215–4225. doi: 10.1242/jcs.00157. [DOI] [PubMed] [Google Scholar]
- Royou A., Sullivan W., Karess R. Cortical recruitment of nonmuscle myosin II in early syncytial Drosophila embryos: its role in nuclear axial expansion and its regulation by Cdc2 activity. J. Cell Biol. 2002;158:127–137. doi: 10.1083/jcb.200203148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., Slepecky N. B. Age-related changes in microtubules in the guinea pig organ of Corti. Tubulin isoform shifts with increasing age suggest changes in micromechanical properties of the sensory epithelium. Cell Tissue Res. 2000;300:29–46. doi: 10.1007/s004419900163. [DOI] [PubMed] [Google Scholar]
- Saito K., Hama K. Structural diversity of microtubules in the supporting cells of the sensory epithelium of guinea pig organ of Corti. J. Electron Microsc. 1982;31:278–281. [PubMed] [Google Scholar]
- Schoenwaelder S. M., Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Curr. Opin. Cell Biol. 1999;11:274–286. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- Slepecky N., Chamberlain S. C. Distribution and polarity of actin in inner ear supporting cells. Hear. Res. 1983;10:359–370. doi: 10.1016/0378-5955(83)90098-9. [DOI] [PubMed] [Google Scholar]
- Slepecky N., Chamberlain S. C. Immunoelectron microscopic and immunofluorescent localization of cytoskeletal and muscle-like contractile proteins in inner ear sensory hair cells. Hear. Res. 1985;20:245–260. doi: 10.1016/0378-5955(85)90029-2. [DOI] [PubMed] [Google Scholar]
- Slepecky N., Chamberlain S. C. Tropomyosin co-localizes with actin microfilaments and microtubules within supporting cells of the inner ear. Cell Tissue Res. 1987;248:63–66. doi: 10.1007/BF01239963. [DOI] [PubMed] [Google Scholar]
- Slepecky N. B., Henderson C. G., Saha S. Post-translational modifications of tubulin suggest that dynamic microtubules are present in sensory cells and stable microtubules are present in supporting cells of the mammalian cochlea. Hear. Res. 1995;91:136–147. doi: 10.1016/0378-5955(95)00184-0. [DOI] [PubMed] [Google Scholar]
- Strumpf D., Volk T. Kakapo, a novel Drosophila protein, is essential for the restricted localization of the neuregulin-like factor, Vein, at the muscle-tendon junctional site. J. Cell Biol. 1998;143:1259–1270. doi: 10.1083/jcb.143.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A., Prokop A., Yamamoto M., Sugimura K., Uemura T., Betschinger J., Knoblich J. A., Volk T. Shortstop recruits EB1/APC1 and promotes microtubule assembly at the muscle-tendon junction. Curr. Biol. 2003;13:1086–1095. doi: 10.1016/s0960-9822(03)00416-0. [DOI] [PubMed] [Google Scholar]
- Torgler C. N., Narasimha M., Knox A. L., Zervas C. G., Vernon M. C., Brown N. H. Tensin stabilizes integrin adhesive contacts in Drosophila. Dev. Cell. 2004;6:357–369. doi: 10.1016/s1534-5807(04)00055-3. [DOI] [PubMed] [Google Scholar]
- Tsuda Y., Yasutake H., Ishijima A., Yanagida T. Torsional rigidity of single actin filaments and actin-actin bond breaking force under torsion measured directly by in vitro micromanipulation. Proc. Natl. Acad. Sci. USA. 1996;93:12937–12942. doi: 10.1073/pnas.93.23.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker J. B., Mackie J. B., Cottam D. M., Rogers-Bald M. M., Macintyre J., Scarborough J. A., Milner M. J. Positioning and capture of cell surface-associated microtubules in epithelial tendon cells that differentiate in primary embryonic Drosophila cell cultures. Cell Motil. Cytoskeleton. 2004;57:175–185. doi: 10.1002/cm.10167. [DOI] [PubMed] [Google Scholar]
- Verdier V., Guang Chao C., Settleman J. Rho-kinase regulates tissue morphogenesis via non-muscle myosin and LIM-kinase during Drosophila development. BMC Dev. Biol. 2006;6:38. doi: 10.1186/1471-213X-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk T. Singling out Drosophila tendon cells: a dialogue between two distinct cell types. Trends Genet. 1999;15:448–453. doi: 10.1016/s0168-9525(99)01862-4. [DOI] [PubMed] [Google Scholar]
- Volk T. Muscle attachment sites—where migrating muscles meet their match. In: Sink H., editor. Muscle development in Drosophilia. Georgetown, TX: Springer-Landes Bioscience; 2006. pp. 104–112. [Google Scholar]
- Wills Z., Bateman J., Korey C. A., Corner A., Van Vactor D. The tyrosine kinase Abl and its substrate enabled collaborate with the receptor phosphatase Dlar to control motor axon guidance. Neuron. 1999;22:301–312. doi: 10.1016/s0896-6273(00)81091-0. [DOI] [PubMed] [Google Scholar]
- Woods D. F., Bryant P. J. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- Yagi R., Ishimaru S., Yano H., Gaul U., Hanafusa H., Sabe H. A novel muscle LIM-only protein is generated from the paxillin gene locus in Drosophila. EMBO Rep. 2001;2:814–820. doi: 10.1093/embo-reports/kve178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P. E., Richman A. M., Ketchum A. S., Kiehart D. P. Morphogenesis in Drosophila requires nonmuscle myosin heavy chain function. Genes Dev. 1993;7:29–41. doi: 10.1101/gad.7.1.29. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R., Cohen M., Addadi L., Geiger B. Hierarchical assembly of cell-matrix adhesion complexes. Biochem. Soc. Trans. 2004;32:416–420. doi: 10.1042/BST0320416. [DOI] [PubMed] [Google Scholar]
- Zervas C. G., Gregory S. L., Brown N. H. Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J. Cell Biol. 2001;152:1007–1018. doi: 10.1083/jcb.152.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.