Abstract
G-protein coupled receptors activate heterotrimeric G proteins at the plasma membrane in which most of their effectors are intrinsically located or transiently associated as the external signal is being transduced. This paradigm has been extended to the intracellular compartments by studies in yeast showing that trafficking of Gα activates phosphatidylinositol 3-kinase (PI3K) at endosomal compartments, suggesting that vesicle trafficking regulates potential actions of Gα and possibly Gβγ at the level of endosomes. Here, we show that Gβγ interacts with Rab11a and that the two proteins colocalize at early and recycling endosomes in response to activation of lysophosphatidic acid (LPA) receptors. This agonist-dependent association of Gβγ to Rab11a-positive endosomes contributes to the recruitment of PI3K and phosphorylation of AKT at this intracellular compartment. These events are sensitive to the expression of a dominant-negative Rab11a mutant or treatment with wortmannin, suggesting that Rab11a-dependent Gβγ trafficking promotes the activation of the PI3K/AKT signaling pathway associated with endosomal compartments. In addition, RNA interference-mediated Rab11a depletion, or expression of a dominant-negative Rab11a mutant attenuated LPA-dependent cell survival and proliferation, suggesting that endosomal activation of the PI3K/AKT signaling pathway in response to Gβγ trafficking, via its interaction with Rab11, is a relevant step in the mechanism controlling these fundamental events.
INTRODUCTION
Hundreds of different G protein-coupled receptors (GPCRs) exist in eukaryotes. They detect the presence of diverse extracellular stimuli, including hormones, neurotransmitters, lipids, and ions, and they activate intracellular heterotrimeric G proteins by promoting the binding of guanosine triphosphate (GTP) to the Gα subunit and its dissociation from Gβγ. Both the GTP-bound Gα and the liberated Gβγ subunits interact with a selected set of effectors, including phospholipases, adenylyl cyclases, ion channels, phosphoinositide 3-kinases, and guanine exchange factors (Gilman, 1989; Clapham and Neer, 1997; Ford et al., 1998; Gautam et al., 1998; Albert and Robillard, 2002; Welch et al., 2002; Preininger and Hamm, 2004; Rosenfeldt et al., 2004). The signal is then propagated within the cells by diffusible second messengers that modulate protein–protein interactions and enzymatic activities. Current views place heterotrimeric G protein signaling to the plasma membrane in which activated receptors promote the exchange of guanosine diphosphate for GTP in the Gα subunit resulting in its dissociation from Gβγ. The cycle ends when Gα hydrolyzes GTP and will reassociates with Gβγ. Recent findings in yeast have extended this model by the demonstration that GTP-loaded Gα associates with endosomes as part of the mechanism of yeast PI3-kinase (Vps34p) activation (Slessareva et al., 2006). Whether receptor-activated mammalian Gα or Gβγ subunits can follow an intracellular trafficking pathway or contribute to further signaling or desensitization is not known. The initial phase of GPCR activation is frequently followed by posttranslational modifications of the receptor, leading to endocytosis and desensitization (Pierce et al., 2002). However, endocytosis can also define novel signaling outputs by presenting different downstream effectors at endocytic compartments compared with the plasma membrane (von Zastrow and Sorkin, 2007). For example, it has been suggested that protein kinase A-phosphorylated β2-adrenergic receptors associate with β-arrestins and acquire novel coupling specificities to activate the extracellular signal-regulated kinase (ERK) signaling cascade (Daaka et al., 1997a; Lefkowitz et al., 2002). Previous studies on trafficking of Gβγ after its synthesis and assembly revealed its association with the plasma membrane via the carboxy-terminal isoprenyl as a result of covalent modification of the CAAX sequence present in Gγ. These studies also showed that exit of Gβγ from the endoplasmic reticulum and trafficking to the plasma membrane require the expression and acylation of Gα (Michaelson et al., 2002; Takida and Wedegaertner, 2003). However, current knowledge restricts Gβγ actions to the plasma membrane, either by modulating the activity of effectors intrinsically located there, such as G protein-coupled inwardly rectifying potassium channels (Reuveny et al., 1994; Sadja et al., 2003) or adenylyl cyclases (Tang and Gilman, 1991; Willoughby and Cooper, 2007), or by recruiting some effectors, such as phospholipase Cβ2 (Rhee and Bae, 1997), G protein-coupled receptor kinase-2 (Daaka et al., 1997b), PI3Kγ (Brock et al., 2003), or P-Rex1 (Barber et al., 2007). Recent evidence using fluorescently tagged Gβγ has shown that, once at the plasma membrane, Gβγ released from heterotrimeric G proteins can be translocated to intracellular membranes via a yet undefined molecular mechanism(s). Such examples include internalization of Gβγ in response to the action of β-adrenergic receptors (Novotny et al., 1995; Hynes et al., 2004; Saini et al., 2007). Gilman and colleagues suggested that a pool of free Gβγ is available to permit receptor-mediated endocytosis (Lin et al., 1998). Activities attributed to internalized Gβγ include the activation of protein kinase D, causing remodeling of the Golgi apparatus (Jamora et al., 1999; Diaz Anel and Malhotra, 2005). Again, how Gβγ reaches and is activated at these subcellular locations is not known.
Intracellular trafficking of diverse signal transduction proteins is coordinated by a variety of Rab GTPases that regulate the budding and fission of vesicles from compartments in which these Rab proteins are specifically located (Sonnichsen et al., 2000; Zerial and McBride, 2001; Miaczynska and Zerial, 2002). Rab11, one of the 40 known Rab GTPases, controls the transport of membrane receptors, including some GPCRs, from early endosomes to perinuclear slowly recycling endosomes (Ullrich et al., 1996; Seachrist and Ferguson, 2003). Interestingly, trafficking and recycling of GPCRs has been linked to PI3-kinases (Hunyady et al., 2002; Miaczynska and Zerial, 2002; Houle and Marceau, 2003; Kalia et al., 2006), raising the possibility that GPCRs provoke the activation of endosome-associated PI3-kinase(s).
Here, we demonstrate that lysophosphatidic acid (LPA) receptors induce intracellular trafficking of Gβγ and promote its interaction with Rab11a and the activation of the PI3K/AKT signaling pathway associated with Rab11a-positive endosomes. We propose that Rab11a directs Gβ1γ2 into endosomal pathways, thereby switching GPCR signaling from the plasma membrane to endosomal compartments. Endosomal AKT activation in response to Rab11a-directed Gβ1γ2 may contribute and fine-tune the proliferative and antiapoptotic effects of LPA receptor stimulation.
MATERIALS AND METHODS
DNA Constructs
pEF-His6-Gβ1, pEF-His6-Gγ2, and pCDNA3-Gαi2 were kindly provided by Dr. Silvio Gutkind (National Institute of Dental and Craniofacial Research, National Institutes of Health). pCEFL3XFlag phosducin-like protein 1 (PhLP1) was subcloned from pCDNA3-PhLP1 kindly provided by Dr. Sheryl Craft (University of Southern California Keck School of Medicine). The enhanced green fluorescent protein (EGFP)-tagged wild-type and mutant Rab GTPases have been generated by Dr. Robert Lodge (University of Quebec) as described previously (Hunyady et al., 2002). The short hairpin RNA (shRNA) for Rab11a was kindly provided by Dr. Yoshiyuki Wakabayashi (National Institute of Child Health and Human Development, National Institutes of Health) (Wakabayashi et al., 2005).
Yeast Two-Hybrid
Gβ1 cDNA was subcloned into pGB3 plasmid in frame with the sequence coding for the Gal4 DNA binding domain, generating the pGB3-Gβ1 that was used as the bait for the yeast two-hybrid system. A human brain cDNA library (Clontech, Palo Alto, CA) was screened with Gβ1 by using the Matchmaker system 3 (Clontech), following the manufacturer's instructions, with some modifications (Vazquez-Prado et al., 2004). Putative interacting clones were obtained by selecting transformants in media lacking Ade/His/Leu/Trp and checked for α-galactosidase expression by the X α-Gal assay. The specificity of the interaction was determined using pGBKT7-p53 and pGB3 empty vector as control.
Cell Lines and Transfections
Human embryonic kidney (HEK)-293T cells were routinely cultured in DMEM (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum and 1% glutamine, penicillin, and streptomycin at 37°C in a 5% CO2 atmosphere. Cells were transfected using Lipofectamine Plus reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's indications. For transient transfections, cells were plated onto poly-d-lysine–coated tissue culture dishes. Four micrograms of total plasmid DNA was used to transfect cells grown to 60–70% confluence in 10-cm-diameter dishes. After 5 h of initiated the transfection, 5 ml of complete DMEM was added, the assays were performed 48 h after transfection.
Affinity Precipitation
To assess in mammalian cells the interaction between Rab11a and Gβ1γ2, plasmids coding for EGFP-Rab11a, His6-Gβ1, and His6-Gγ2 were transfected into HEK-293T cells. In a set of experiments, cells were transfected with different amounts of either EGFP-Rab wild type or EGFP-Rab mutants as indicated in the figures. When appropriated, Flag-tagged PhLP1 or Gαi2 were cotransfected; the proportion of PhLP1/Rab11a or Gαi2/Rab11a, in terms of transfected plasmids, was 3:1. Two days after transfection, cell lysates were obtained with lysis buffer (50 mM Tris, pH 7.5, 0.15 M NaCl, 1% Triton X-100, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride), and incubated in a rocking platform for 2 h at 4°C with TALON resin (Clontech). The resin was collected by centrifugation and washed four times with ice-cold lysis buffer containing 5 mM imidazole. Bound proteins were eluted by boiling for 5 min in sample buffer (62.5 mM Tris, pH 6.8, 10% glycerol, 2% SDS, 5% β-mercaptoethanol, 0.1% bromphenol blue, and EDTA 5 mM) and fractionated on a 10% SDS-polyacrylamide gel electrophoresis (PAGE) gel and detected by Western blotting by using a mouse specific anti-green fluorescent protein (GFP) antibody (MMS-118R; Covance Research Products, Princeton, NJ), anti-histidines antibody (H1029; Sigma-Aldrich), anti Gαi2 (SC-7276; Santa Cruz Biotechnology, Santa Cruz, CA), anti-Flag antibody (F3165; Sigma-Aldrich) according to the experiment and revealed with the West Pico system (Pierce Chemical, Rockford, IL), or Immobilon Western chemiluminescent substrate (Millipore, Billerica, MA).
Rab11a GTPase Assay
His6-Rab11 was expressed and purified from Escherichia coli BL21 by using ProBond purification system (Invitrogen) and eluted with elution buffer (50 mM NaH2PO4, 300 mM NaCl, 5 mM EDTA, and 0.05% Tween 20, pH 8.0). His6-Gβ1 and His6-Gγ2 from transfected HEK-293T cells were purified using ProBond system (Invitrogen) and eluted with elution buffer. Proteins were dialyzed overnight against buffer containing 20 mM HEPES·KOH, pH 7.5, 150 mM KCl, 1 mM EDTA, 1 mM EGTA, and 0.5 mM dithiothreitol; protein concentration was determined by Bradford assay. GTPase activity was measured with a colorimetric GTPase assay kit from Innova Biosciences (Novus Biologicals, Littleton, CO) following the manufacturer's protocol. Briefly, different amounts of His6-Rab11 were incubated overnight with 0.1 μM of His6-Gβ1-His6-Gγ2; GTP was added and incubated 30 min at room temperature. Gold mix or colorimetric buffer (Pi ColorLock Gold and Accelerator) was incorporated to the mixture and incubated for 5 min at room temperature. Next, stabilizer buffer was included, and the reaction mix was incubated 30 min. Optical density was determined at 620 nm by using a MultisKan MCC/340 plate reader (Thermo Fisher Scientific, Waltham, MA).
Immunoprecipitation
The interaction between endogenous Rab11 and Gβ1γ2 was assessed in nontransfected HEK-293T cells that were either nonstimulated or stimulated with 10 μM LPA as indicated in the figures. Confluent cultures were washed with phosphate-buffered saline (PBS) followed by lysis at 4°C in lysis buffer; lysates were then centrifuged at 14,000 rpm at 4°C for 10 min. The supernatants were incubated for 12 h at 4°C with 600 ng/ml anti-Rab11 antibody (SC-9020; Santa Cruz Biotechnology). The immune complexes were recovered by incubation for 2 h at 4°C with protein A/G Plus-agarose (25 μl, SC-2003; Santa Cruz Biotechnology). Beads were washed four times with ice-cold lysis buffer and boiled in sample buffer. Immunoprecipitated proteins were fractionated on a 15% SDS-PAGE and detected by Western blotting using anti-Gβ antibody (SC-261; Santa Cruz Biotechnology) and anti-Rab11 antibody (SC-9020; Santa Cruz Biotechnology).
Fluorescence Microscopy
For immunofluorescence experiments, HEK-293T cells were grown on coverslips precoated with 20 μg/ml fibronectin (Calbiochem, La Jolla, CA) and transiently transfected with 0.75 μg of plasmids coding EGFP-Rab (either wild type or mutant as indicated in the figures) and 0.375 μg each of His6-Gβ1 and His6-Gγ2 coding plasmids. Two days after transfection, cells were fixed with 4% paraformaldehyde in PBS, pH 7.4, for 20 min at room temperature, washed five times with PBS and permeabilized with 100% methanol for 6 min at −20°C. Then, cells were incubated with anti-histidines antibody (H1029; Sigma-Aldrich) for 1 h at 37°C, washed, and incubated with rhodamine-conjugated anti-mouse antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) for 45 min at room temperature. For immunofluorescence experiments detecting endogenous proteins, nontransfected cells were grown on coverslips precoated with 20 μg/ml fibronectin, washed three times with PBS, and stimulated with 10 μM LPA for indicated times. Cells were then fixed and incubated with 20 μg/ml anti-Rab11 antibody (71-5300; Zymed Laboratories, South San Francisco, CA) followed by incubation with fluorescein-conjugated anti-rabbit antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) for 40 min at room temperature. The coverslips were then saturated with peroxidase-conjugated anti-rabbit antibody, washed three times with PBS and incubated with anti-Gβ antibody (SC-62; Santa Cruz Biotechnology) for 60 min at 37°C, washed, and incubated with rhodamine-conjugated anti-rabbit antibody (Jackson ImmunoResearch Laboratories). After washing, cells on coverslips were mounted on glass slides using ProLong Antifade (Invitrogen). Images were acquired with a DMIRE2 confocal laser-scanning microscope (Leica Microsystems, Deerfield, IL) by the use of a 63×, numerical aperture 1.4 oil immersion objective and a zoom of 3. The colocalization analysis was measured using the public domain NIH program (developed at National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/), expressed as a percentage of the total number of pixels.
Separation of Early Endosomal Fraction on a Flotation Step Gradient
For each experimental condition, early endosomal fraction was prepared from HEK-293T cells grown on 10-cm-diameter dishes. Subcellular fractionation was performed by centrifugation of cell lysates in sucrose gradients as described previously (Gorvel et al., 1991; Kobayashi et al., 2002). Briefly, four plates of cells grown to 80% confluence were incubated for 15 h in serum-free DMEM and washed three times with PBS followed by incubation with 10 μM LPA (in PBS) for the indicated times. In some experiments, cells were left unstimulated or either incubated with 300 nM wortmannin (Wm; Sigma-Aldrich) for 1 h in PBS or with 100 ng/ml pertussis toxin (PTX) for 20 h before LPA stimulation. Cells were then washed twice with PBS and scraped from the dishes with the edge of a rubber policeman and centrifuged at 1200 rpm for 5 min at 4°C in a Sorvall RT 6000 centrifuge. The cell pellet was resuspended in 3 ml of the homogenization buffer (250 mM sucrose and 3 mM imidazole, pH 7.4) and centrifuged at 3000 rpm for 10 min at 4°C. Cells were then resuspended in 0.5 ml of homogenization buffer containing protease inhibitors (10 μg/ml aprotinin, 10 μg/ml pepstatin, 1 μg/ml trypsin inhibitor, and 0.5 mM EDTA) and homogenized at 4°C by seven passages through a 22-gauge 1 1/4 needle fitted on a 1-ml plastic syringe. The homogenate was centrifuged for 10 min at 3000 rpm at 4°C, and the postnuclear supernatant (PNS) was collected. The PNS was then brought to 40.6% sucrose by adding 62% sucrose, 3 mM imidazole, pH 7.4, 1 mM EDTA, and loaded at the bottom of SW 60 tubes. A gradient consisting of three steps was then poured (1.5 ml of 35% sucrose, 3 mM imidazole, pH 7.4, 1 mM EDTA; 1 ml of 25% sucrose, 3 mM imidazole, pH 7.4, 1 mM EDTA; and 0.5 ml of homogenization buffer). The gradient was centrifuged at 35,000 rpm for 1 h at 4°C. The early endosomal fraction was collected at the interface formed between 25 and 35% sucrose. The late endosomal fraction was collected at the interface formed between homogenization buffer and 25% sucrose. The endosomal preparation was dispersed by sonication and proteins were separated on a 10% SDS-PAGE and detected by Western blotting using the following antibodies: anti-Rab11 (SC-9020; Santa Cruz Biotechnology), anti-Rab5B (SC-598; Santa Cruz Biotechnology), anti-early endosomal antigen (EEA)1 (BD 610457; BD Biosciences, San Jose, CA), anti-Rab7 (SC-10767; Santa Cruz Biotechnology), PI3-kinase (p110γ, 4252; Cell Signaling Technology, Danvers, MA), anti-phospho-AKT (SC-7985-R; Santa Cruz Biotechnology), and anti-AKT (P-2482; Sigma-Aldrich).
Quantification of Apoptotic Cells by Flow Cytometry
Apoptotic cells were identified by using the Vybrant apoptosis assay kit 6 (composed of Biotin-X-AnnexinV/Alexa Fluor 350 streptavidin/propidium iodine) from Invitrogen following the manufacturer's protocol. Briefly, transfected cells were stimulated with LPA or left unstimulated. When appropriate, cells were incubated with 100 ng/ml PTX for 20 h. Cells were harvested, washed with ice-cold PBS, and subjected to Biotin-X-Annexin V and Fluor 350 streptavidin/propidium iodide staining in binding buffer (50 mM HEPES, 700 mM NaCl, and 12.5 mM CaCl2, pH 7.4) at room temperature for 30 min in the dark. Stained cells were analyzed by fluorescence-activated cell sorting (FACS Vantage; BD Biosciences) by using Cell Quest 3.3 software.
Proliferation Assay
HEK-293T cells transiently transfected with EGFP-Rab11 wild type, EGFP-Rab11 mutants, or Gαi2 were plated in 96-well flat-bottomed plates (15,000 cells/well) in 100 μl of DMEM. In some experiments, cells were incubated with 100 ng/ml PTX for 24 h. Cells were then stimulated in the same medium with LPA for 24 h or left unstimulated. Cell proliferation was measured using the 5-bromo-2′-deoxyuridine (BrdU) enzyme-linked immunosorbent assay from Roche (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions. For the last 16 h of the 24-h stimulation period, the cells were pulsed with BrdU. Absorbance at 405 and 492 nm was measured with a microplate reader (model 550; Bio-Rad, Hercules, CA).
Statistics
Statistical significance of the differences among data were determined by analysis of variance and Student-Newman-Keuls test or t test when appropriate using GraphPad Prism version 2.0 software (GraphPad Software, San Diego, CA). p < 0.05 was considered a statistically significant difference.
RESULTS
Identification of Rab11a as a Gβ1γ2-interacting Protein Influencing Gβ1γ2 Cellular Distribution
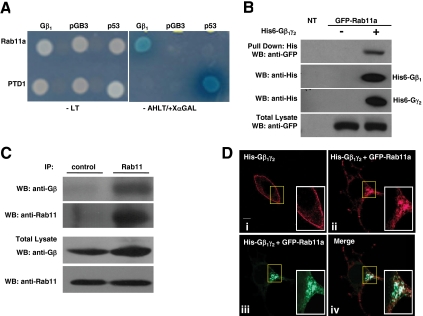
Gβγ can interact with Gα as well as with its diverse effectors at the plasma membrane. It has been recently documented that Gβγ relocates from the plasma membrane to internal membranes upon GPCR activation, via an unknown molecular mechanism (Hynes et al., 2004; Saini et al., 2007). To identify novel Gβγ-interacting proteins that might be involved in the intracellular trafficking of Gβγ, we screened a human brain cDNA library by using Gβ1 as the bait in a yeast two-hybrid system. Among the transformants obtained under high-stringency conditions (−Ade/−His/−Leu/−Trp), we identified a clone corresponding to Rab11a. Rab11a has interacted specifically with Gβ1 in the yeast two-hybrid assay (Figure 1A), as judged by the ability of the pair to support growth under highly stringent conditions and to promote the expression of α-galactosidase from an integrated reporter system. Association of Rab11a and Gβγ was also demonstrable in mammalian cells in pull-down experiments by using His-tagged Gβ1γ2 and a GFP-Rab11a construct both expressed in HEK-293T cells grown in DMEM supplemented with 10% fetal bovine serum (Figure 1B). To investigate whether the interaction between Gβ1γ2 and Rab11a occurs between endogenously expressed proteins, Rab11a immunoprecipitates were tested for the presence of Gβγ proteins from nontransfected cells grown in DMEM supplemented with 10% fetal bovine serum. Figure 1C shows that endogenous Gβγ was found in association with endogenous Rab11a.
Figure 1.
Gβ1γ2 interacts with Rab11a and they colocalize in HEK-293T cells. (A) A full-length clone of Rab11a was identified as a Gβ1 subunit interactor by yeast two-hybrid. The specificity of the interaction between Rab11a and Gβ1 was determined in the yeast two-hybrid system using pGB3 or p53 as negative controls. Yeast grew on media lacking leucine and tryptophan (−LT, which selects for the presence of plasmids; left), but only those displaying interactions grew in restrictive media lacking adenine, histidine, leucine, and tryptophan and were positive for the activity of α-galactosidase (−AHLT + X α-Gal; right). (B) Pull-down analysis in mammalian cells demonstrating the interaction between Gβγ and Rab11a. HEK-293T cells were transfected with GFP-Rab11a (expression detected in total lysates with GFP-specific antibodies; bottom) and His6-tagged Gβ1γ2, 2 d after transfection, His6-Gβ1γ2 was isolated from cells grown in DMEM supplemented with 10% fetal bovine serum by using Talon beads (top), and Rab11a was detected with GFP-specific antibodies. The presence of His-tagged Gβ1 and His-tagged Gγ2 in pull-downs (middle) was detected using an antibody recognizing the poly-histidine tag. NT, nontransfected cells. (C) Interaction between endogenous Gβγ and Rab11 in serum-stimulated HEK-293T. Rab11 was immunoprecipitated from cells grown to 80% of confluence in DMEM supplemented with 10% fetal bovine serum, the presence of Gβ in Rab11 immunoprecipitates and total cell lysate was detected by Western blot analysis (top and third panels, respectively). The presence of Rab11 in the immunoprecipitate and total lysate is shown in the second and fourth panels, respectively. Control refers to immunoprecipitation performed with a rabbit preimmune serum instead of the anti-Rab11 antibody. (D) Confocal images showing the distribution of His6-Gβ1γ2 and GFP-Rab11a in HEK-293 cells. Cells were transiently transfected with His6-Gβ1γ2 either in the absence or presence of GFP-Rab11a, fixed, and stained with anti-histidine antibody and analyzed by confocal microscopy. i, His6-Gβ1γ2 in the absence and in the presence (ii–iv) of GFP-Rab11a. ii, His6-Gβ1γ2 in the presence of GFP-Rab11a. iii, the same image showing GFP-Rab11a. iv, merged images showing colocalization of His6-Gβ1γ2 and GFP-Rab11a. Bar, 8 μm (i).
To determine the influence of Rab11a on the subcellular distribution of Gβ1γ2, His6-Gβ1γ2 heterodimers were transiently transfected into HEK-293T cells in the presence or absence of EGFP-Rab11a, and the cells were examined by confocal microscopy. When transfected in the absence of Rab11a, Gβ1γ2 was predominantly found at the plasma membrane (Figure 1D, i), consistent with a previous report (Evanko et al., 2001). However, expression of EGFP-Rab11a has changed the distribution of Gβ1γ2 that now mostly colocalized with Rab11a at endocytic structures (Figure 1D, ii–iv).
To investigate whether the interaction between endogenous Gβ1γ2 and Rab11a can be influenced by the activation of LPA receptors, Rab11a was immunoprecipitated from nonstimulated or LPA-stimulated serum-starved cells. Figure 2A shows that activation of LPA receptors promoted the association of endogenous Gβγ and Rab11a in a time-dependent manner. In addition, we also demonstrated the presence of AKT in the Gβ1γ2:Rab11a complex (Figure 2A). To determine the effect of GPCR stimulation on the subcellular distribution of endogenous Gβ1γ2, serum-starved HEK-293T cells were stimulated with LPA for the indicated times, and the localization of Gβ and Rab11 was examined by immunofluorescence by using confocal microscopy. In nonstimulated cells, Gβγ was predominantly found at the plasma membrane and in dispersed intracellular vesicles, whereas Rab11 was found in vesicles (Figure 2B, left, NS). However, after LPA stimulation, both proteins were mostly detected in colocalization at endocytic structures that concentrated in the juxtanuclear area (Figure 2B, left). Additionally, we analyzed the colocalization area between Gβ1γ2 and Rab11a; in nonstimulated cells, 9.05 ± 2.0% of the proteins colocalized, whereas in LPA-stimulated cells the overlap was extensive, 40.61 ± 2.68% for 5 min LPA-stimulated cells and 42.47 ± 2.59% for 15-min LPA-stimulated cells (Figure 2B, right). Collectively, these results indicated that the interaction between Gβ1γ2 and Rab11a revealed by the yeast two-hybrid screen and detected by pull-down experiments of transfected epitope-tagged proteins, also occurs between endogenous Gβ1γ2 and Rab11a proteins and is promoted by the activation of LPA receptors. They also suggested that Rab11a might link Gβγ to endosomal compartments regulating the cellular distribution of the heterodimer.
Figure 2.
Agonist-dependent interaction between endogenous Gβγ and Rab11 and effect on their subcellular localization. Rab11 was immunoprecipitated from serum-starved HEK-293T cells grown to 80% confluence and stimulated with 10 μM LPA or left without stimulus (NS) as indicated. (A) The presence of Gβ, Rab11, and AKT in Rab11 immunoprecipitates and total lysates was detected by Western blot analysis (IP:Rab11 and total lysates, respectively). Control refers to immunoprecipitation performed with anti-Grb2 antibody instead of the anti-Rab11 antibody. (B) Left, confocal images showing the distribution of Gβ and Rab11a upon LPA stimulation. HEK-293T cells were serum starved and stimulated with 10 μM LPA for the indicated times or left unstimulated (NS), fixed and stained with anti-Gβ antibody and anti-Rab11 antibody as described in Materials and Methods and analyzed by confocal microscopy. Confocal images show the distribution of Gβ in NS cells or at selected times after stimulation with 10 μM LPA. Gβ distribution in NS cells is consistent with its presence preferentially on the cell surface and in some Rab11-positive vesicles. Stimulation with LPA for 5 or 15 min promoted Gβ internalization, which was detected in Rab11-positive endosomes. Bars, 8 and 10 μm, respectively. Right, percentage of colocalization area was determined using the public domain NIH ImageJ (http://rsb.info.nih.gov/nih-image/). NS cells, gray bar. LPA-stimulated cells, black bars.
Constitutively Active Rab11a Mutant Promotes the Accumulation of Gβ1γ2 Associated with Large Endosomes
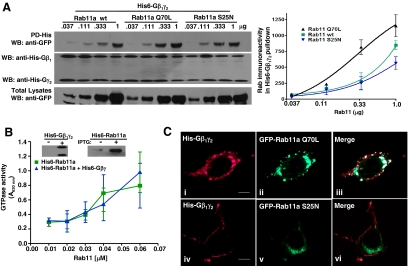
To investigate whether the activation status of Rab11a may affect its interaction with Gβ1γ2, we determined the interaction of His6-Gβ1γ2 with either wild type, constitutively active, or dominant-negative EGFP-tagged Rab11a mutants (Rab11a WT, Q70L, or S25N, respectively) by pull-down assays using lysates from HEK-293T cells transfected with increasing amounts of each Rab11a (Figure 3A). Although all three forms of Rab11 showed interaction with Gβ1γ2, the constitutively active Rab11a Q70L mutant clearly had a higher affinity for Gβ1γ2 as shown by its ability to interact more efficiently than wild-type Rab11a or Rab11a S25N in cells transfected with reduced amounts of the construct (Figure 3A). To investigate whether Gβ1γ2 could affect the catalytic status of Rab11a, an in vitro GTPase assay was used to determine the GTPase activity of recombinant His6-Rab11a in the presence or absence of His6-Gβ1γ2 isolated from HEK-293T cells. Figure 3B shows that Gβ1γ2 did not modify the GTPase activity of Rab11. We then examined the cellular distribution of Gβ1γ2 in the presence or absence of Rab11a mutants (Figure 3C). The constitutively active Rab11a mutant (Rab11Q70L) caused a prominent accumulation of Gβ1γ2 in large vesicles, in which this Rab11a mutant was also detected (Figure 3C, i–iii). In contrast, the dominant-negative Rab11a mutant (Rab11S25N) prevented the formation of large vesicles and did not change the cellular distribution of Gβ1γ2, which remained at the plasma membrane (Figure 3C, iv–vi). These results suggested that Gβ1γ2 trafficking toward endocytic compartments depends on the interaction of this heterodimer with active Rab11a.
Figure 3.
Gβ1γ2 shows a higher affinity for a constitutively active Rab11a Q70L mutant but does not affect the GTPase activity of recombinant Rab11a. HEK-293T cells were transfected with His6-Gβ1γ2 and the indicated amounts of GFP-tagged constructs of wild-type, constitutively active (Q70L), or dominant-negative (S25N) Rab11a; His6-Gβ1γ2 was isolated by pull-down from cells grown in DMEM supplemented with 10% fetal bovine serum by using Talon resin. (A) The GFP-fusion constructs of Rab11a were detected by an anti-GFP antibody in the pull-down (top, left) or in the total lysate (bottom, left), and the presence of His6-Gβ1γ2 in the pull-down was determined by an anti-His antibody. Right graph shows the densitometric values of GFP-Rab11a and its mutants as detected in Gβγ pull-downs, averaged from three independent experiments (means ± SEM). (B) Rab11a GTPase assay. His6-Rab11a was expressed and purified from E. coli BL21. His6-Gβ1γ2 was expressed and isolated from HEK-293T as described in Materials and Methods. Both proteins were incubated to interact overnight followed by incubation with GTP for 30 min. GTPase activity was assessed by a colorimetric method; the samples were read at 620 nm. Insets show isolated His6-Gβ1γ2 (left) and His6-Rab11a (induced or not with 0.1 μM of IPTG; right) used in the experiment. (C) Confocal images showing the distribution of His6-Gβ1γ2 and GFP-Rab11a mutants in HEK-293T cells transiently transfected with His6-Gβ1γ2 and the indicated GFP-Rab11 constructs. i–iii, show the distribution of His6-Gβ1γ2 (i), Rab11a Q70L (ii), and the merged images (iii), whereas (iv–vi) show the equivalent experiments in the presence of Rab11a S25N. Bars, 8 μm.
Effect of PhLP1 and Gαi2 on the Interaction between Gβ1γ2 and Rab11a
Next, we wanted to determine whether Gβ1γ2 interaction with Rab11a required the availability of Gβ1γ2 as a free heterodimer, exposing its Gα-interacting interface. For this, we tested whether the interaction between Gβ1γ2 and Rab11a could be competed with Gαi2 or PhLP1, a ubiquitous protein known to interact with Gβγ at the Gα-interacting interface (Gaudet et al., 1996). In these experiments, either Flag-tagged PhLP1 or Gαi2 were transiently transfected into HEK-293T cells together with His6-Gβ1γ2 and EGFP-Rab11a, and the interaction between His6-Gβ1γ2 and EGFP-Rab11a was analyzed by pull-down assays. Overexpression of PhLP1 completely blocked the interaction between Gβ1γ2 and Rab11a (Figure 4A, top and bottom). Similarly, overexpression of Gαi2 greatly reduced the interaction between Rab11a and Gβ1γ2 (Figure 4B, top and bottom), indicating that interaction between Gβ1γ2 and Rab11a occurs when Gβ1γ2 dissociates from Gα, suggesting that it might be part of the signal transduction process initiated with the release of Gβ1γ2 upon GPCR activation.
Figure 4.
Expression of Gαi2 or PhLP1 prevent the interaction between Gβ1γ2 and Rab11a. HEK-293T were transfected with His6-Gβ1γ2 and GFP-Rab11a either in the presence or absence of Flag-tagged PhLP1 or Gαi2. The interaction between His6-Gβ1γ2 and GFP-Rab11a was determined by pull-down assays using Talon resin. (A) Expression of Flag-tagged PhLP1 inhibits the interaction between Gβ1γ2 and GFP-Rab11a (top). Bottom graph represents the mean densitometric values of GFP-Rab11a bound to Gβγ obtained in three independent experiments (means ± SEM, *p < 0.05 compared with the absence of PhPL1 assessed by t test analysis). (B) Expression of Gαi2 significantly reduces the interaction between His6-Gβ1γ2 and GFP-Rab11a (top). Bottom graph shows the results of densitometric analysis of GFP-Rab11a bound to Gβγ obtained in three independent experiments (means ± SEM, *p < 0.05 compared with the absence of PhPL1 assessed by t test analysis). The presence in the pull-downs of His6-Gβ1γ2 and the competing proteins (Gαi2 or PhLP1) and the presence of transfected GFP-Rab11a in total lysates was demonstrated by Western blot.
Effect of GPCR Stimulation on Gβ1γ2 Signaling Associated with Rab11a-positive Endosomes
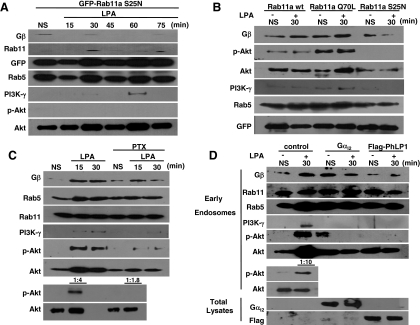
Next, we investigated the effect of G protein-coupled receptor stimulation on the subcellular distribution of Gβ1γ2 and Rab11a. HEK-293T cells were treated with LPA for various times, and then endosomes were isolated on a flotation sucrose gradient (Kobayashi et al., 2002). The identity of the endosomes was verified by their immunoreactivity to Rab5 or EEA1, and Rab11 recognized as early and recycling endosomal markers, respectively (Figure 5A); no Rab7 immunoreactivity was detected in this preparation (Figure 5D). The activation of LPA receptors promoted the association of Gβ1γ2 toward early and recycling endosomes as demonstrated by a significant increase in the presence of Gβ in endosomes obtained from LPA stimulated cells, which increased in a time-dependent manner, being above basal levels at 15 min, reaching a maximum between 30 and 60 min, and starting to decrease at 75 min after LPA stimulation (Figure 5, A and B).
Figure 5.
LPA stimulation increases Gβ1γ2 association to Rab11a-positive endosomes. HEK-293T cells grown to 80% confluence were serum starved for 15 h followed by stimulation with 10 μM LPA for the indicated times. Cells were fractionated to isolate early and late endosomes as described in Materials and Methods. (A and D) LPA stimulation induces a time-dependent association of Gβ1γ2 heterodimer with Rab11-positive endosomal fractions as detected by Western blotting using an anti-Gβ1 antibody. Distribution of Rab11, Rab5, early endosomal marker EEA1, and late endosomal marker Rab7 was assessed by Western blot as indicated. NS, nonstimulated cells; TL, total cell lysates. The effect of LPA stimulation on the association of PI3-kinase-γ and the presence and endosomal phosphorylation of AKT was also evaluated in the same endosomal fractions by using specific antibodies. (B) Graph represents the mean densitometric values of Gβ1 present in the endosomal fractions determined in three independent experiments. Means ± SEM, *p < 0.001 compared with nonstimulated cells (analysis of variance and Student-Newman-Keuls test). (C) Results of similar densitometric analysis of AKT phosphorylation (means ± SEM, *p < 0.001 compared with nonstimulated cells. (E) Wortmannin prevented LPA-induced endosomal recruitment of PI3-kinase-γ and phosphorylation of AKT. HEK-293T cells were incubated with 300 nM wortmannin for 1 h before LPA stimulation, and early endosomes were isolated as described, and proteins were detected by Western blot using anti-phospho-Ser473-AKT (top), total AKT (middle), and PI3-kinase-γ catalytic subunit (bottom). (F) Wortmannin does not affect LPA-dependent ERK phosphorylation. Total cell lysates were obtained from HEK-293T cells pretreated with wortmannin before LPA stimulation, and the presence of the indicated proteins was detected by Western blotting using specific antibodies.
Endosomal trafficking of Gβ1γ2 could contribute to the compartmentalization of signaling cascades by the association of effector proteins such as PI3-kinase in endosomal compartments (Seabra and Wasmeier, 2004). Therefore, we wanted to know whether LPA stimulation promoted the association of PI3-kinase to early endosomes and whether Gβ1γ2 had any influence on this process. To assess this question, the preparation of early endosomes obtained from LPA-stimulated cells was analyzed by Western blotting using antibodies against the catalytic subunit of PI3-kinase-γ, the isoform known to be a target of Gβγ subunits (Stephens et al., 1994). As shown in Figure 5A, recruitment of endogenous PI3-kinase-γ to endosomes was induced by LPA in a time-dependent manner, the kinase being present between 15 and 60 min but no longer detectable after 75 min of stimulation. To assess the consequences of the association of PI3-kinase-γ to endosomes, we also examined the recruitment and activation of one of its more relevant downstream effectors, AKT, to this compartment. Unexpectedly, a fraction of AKT was already detected in endosomes obtained from nonstimulated cells and its presence was further increased after LPA stimulation (Figure 5A). Interestingly, the activation of AKT, determined by monitoring its phosphorylation state with antibodies recognizing AKT phosphorylated at Ser-473, was also increased after 15 min and remained so until 75 min of LPA stimulation (Figure 5, A and C), following a pattern similar to the association of Gβγ to this endosomal fraction (Figure 5, A and B). Pretreatment of cells with Wm prevented the association of PI3-kinase-γ and the activation of AKT in response to LPA; however, a fraction of AKT was found associated with endosomes regardless of the inhibition of PI3-kinases with wortmannin (Figure 5E). A further evidence that wortmannin does not globally disrupt the signaling pathways induced by LPA was the activation of extracellular signal-regulated kinase (ERK) 1/2 detected in whole cell lysates from Wm-pretreated LPA stimulated HEK-293T cells, in which the activation of AKT was also affected by Wm (Figure 5F).
To test the question of whether the interaction between Gβ1γ2 and Rab11a was necessary for the association of PI3-kinase-γ to endosomes and the ensuing phosphorylation of AKT, endosomal fractions were obtained from LPA-stimulated HEK-293T cells transfected with dominant-negative Rab11a mutant (Rab11a S25N). In agreement with the results obtained by confocal microscopy (Figure 3C, iv–vi), Gβ1γ2 was barely detectable in endosomes obtained from Rab11a S25N-transfected cells (Figure 6A) and PI3-kinase-γ was practically absent in these endosomes, being detected only at one time point (1 h) (Figure 6A). A fraction of AKT, was still associated with early endosomes isolated from Rab11a S25N-transfected cells, but this fraction corresponded to the nonphosphorylated inactive form of the kinase (Figure 6A). To investigate whether the activation status of Rab11a may affect agonist-dependent Gβγ trafficking to endosomes and the relevant downstream events, we determined the presence of Gβ and its downstream targets in endosomal fractions obtained from control or LPA-stimulated HEK-293T cells expressing either wild-type Rab11a, constitutively active Rab11a mutant (Rab11a Q70L-EGFP), or a dominant-negative Rab11a mutant (Rab11a S25N-EGFP). Figure 6B shows that both wild-type Rab11a and Rab11a Q70L increased the presence of Gβ in endosomes obtained from LPA-stimulated cells. Yet, dominant-negative Rab11a S25N mutant reduced the presence of Gβ in endosomes. Moreover, endosomal AKT activation was blocked in samples obtained from Rab11a S25N-transfected cells, as observed in Figure 6A. In addition, we detected a slight increase in the association of PI3-kinase-γ to endosomes from Rab11a Q70L-transfected cells stimulated with LPA (Figure 6B). Evidence that demonstrated that Rab11 S25N mutant does not globally disrupt the signaling pathways induced by LPA was the activation of ERK 1/2 and AKT detected in whole cell lysates from LPA-stimulated HEK-293T cells expressing Rab11 S25N mutant (Supplemental Figure S1). To investigate whether inhibition of Gi-dependent pathways affects LPA-stimulated trafficking of Gβγ to endosomal compartments and recruitment and activation of AKT, HEK-293T cells were treated with PTX and the effect of LPA on the association of Gβ and AKT to endosomal compartments was determined. Figure 6C shows that PTX reduced the association of Gβ to endosomes as well as the presence and activation of AKT.
Figure 6.
Expression of dominant-negative Rab11a, Gαi2, PhLP1, or PTX treatment attenuated the effect of LPA on endosomal recruitment of Gβγ, PI3-kinase-γ, and phosphorylation of AKT. (A) HEK-293T cells transiently transfected with dominant-negative GFP-Rab11a mutant (GFP-Rab11a S25N) were serum starved for 15 h followed by stimulation with 10 μM LPA for the indicated times. Cell lysates were obtained and early endosomal fraction was isolated by a flotation step gradient as described in Materials and Methods. The presence of the indicated proteins in the endosomal fraction was detected by Western blotting using specific antibodies. (B) Effect of different Rab11a constructs on LPA-dependent Gβγ and downstream effectors association to endosomes. HEK-293T cells were transiently transfected with GFP-Rab11a, GFP-Rab11a Q70L, or GFP-Rab11a S25N mutants, cells were processed as described, and the presence of the indicated proteins in the endosomal fraction was detected by Western blotting using specific antibodies. NS, nonstimulated cells. The effect of LPA stimulation on the association of PI3-kinase-γ and the presence and endosomal phosphorylation of AKT was also evaluated in the same fractions by using specific antibodies. (C) Effect of PTX on Gβγ and downstream effector association to Rab11-positive endosomes. HEK-293T cells grown to 80% confluence were serum starved for 15 h followed by stimulation with 10 μM LPA for the indicated times or left unstimulated (NS). A group of cells were incubated with 100 ng/ml PTX for 20 h. The presence of the indicated proteins in the endosomal fraction was detected by Western blotting using specific antibodies. NS, nonstimulated cells. The effect of LPA stimulation and PTX treatment on association of PI3-kinase-γ and the presence and endosomal phosphorylation of AKT were also evaluated in the same endosomal fractions by using specific antibodies. To assess the phosphorylation of AKT in samples with similar amounts of endosomal AKT, we diluted the samples obtained from LPA-stimulated cells (1:4 or 1:1.8, as indicated), and then we phosphorylated AKT and total AKT were detected by Western blot. (D) Effect of Gβγ-interacting proteins on LPA-induced recruitment of Gβγ and downstream effectors to Rab11-positive endosomal compartments. HEK-293T cells transiently transfected with Gαi2 or Flag-PhLP1 were serum starved for 15 h followed by stimulation with 10 μM LPA during 30 min or left unstimulated (NS). The presence of Gβ1, PI3-kinase-γ, total or phosphorylated AKT, and the other indicated proteins in the endosomal fraction or total lysates was determined by Western blotting using specific antibodies. To assess the phosphorylation of AKT in samples with similar amounts of endosomal AKT, the sample obtained from control cells stimulated with 10 μM LPA was diluted as indicated, and then phosphorylated AKT and total AKT were detect Western blot. The expression of transfected Gαi2 or PhLP1 in total lysates was demonstrated by Western blot.
To examine whether LPA-induced trafficking of free Gβγ is important for the activation of AKT at endosomes, we tested the effect of PhLP1 or Gαi2, whose overexpression would decrease the availability of free Gβγ. For these experiments, we obtained endosomes from control or LPA-stimulated HEK-293T cells transfected with either Flag-tagged PhdLP1 or Gαi2. As shown in Figure 6D, both PhLP1 and Gαi2 attenuated the agonist-dependent appearance of Gβγ in early endosomes and the association of PI3-kinase-γ to this fraction. Moreover, they interfered with the association of AKT to endosomes (just the agonist-dependent fraction) and prevented its phosphorylation. To confirm that LPA has a dual effect on endosomal AKT (increases its association and promotes its phosphorylation) endosomal samples from LPA-stimulated cells were diluted to obtain a similar amount of total AKT in fractions from nonstimulated cells and LPA-stimulated cells, in these conditions, it was clear that the increase in the phosphorylation detected in endosomal AKT in LPA-stimulated cells was due to phosphorylation of the kinase and that an increase on its association to the endosomal fraction was also occurring (Figure 6D, bottom, early endosomes). Together, these results suggest that upon GPCR activation, Gβγ dissociated from Gα gets engaged in a trafficking path to early and perhaps recycling endosomes mediated by a direct interaction with Rab11a. This interaction is necessary to assemble and activate a PI3K/AKT signaling complex associated to Rab11-positive endosomes. These data establish a new paradigm of endosomal activation of a Gβγ-regulated PI3-kinase cascade.
Trafficking of Gβ1γ2 to Rab11a Endosomes Leads to Cell Survival and Proliferation
To explore the functional consequences of Gβγ trafficking to Rab11a-positive endosomes, we evaluated agonist-dependent survival and proliferation of HEK-293T cells transiently transfected with wild-type Rab11a, constitutively active Rab11a mutant (EGFP-Rab11a Q70L) or a dominant-negative Rab11a mutant (EGFP-Rab11a S25N). As shown in Figure 7A, LPA protected against apoptosis in cells expressing either wild-type Rab11a or Rab11a Q70L; in the Rab11a Q70L-expressing cells an antiapoptotic effect was detected event in the absence of LPA stimulation. However, overexpression of dominant-negative Rab11a S25N mutant blocked the LPA-dependent survival effect, resulting in apoptosis. Additionally, overexpression of Gαi2 or treatment with PTX also interfered with the survival effect promoted by LPA, as shown in Figure 7A.
Figure 7.
Expression of dominant-negative Rab11a, Gαi2, or PTX treatment prevents the antiapoptotic and proliferative effect of LPA. (A) Antiapoptotic effect of 10 μM LPA was assessed by Annexin V binding in HEK-293T cells transfected with wild-type, constitutively active, or dominant-negative GFP-tagged mutants of Rab11a (Rab11a WT, Rab11a Q70L, or GFP-Rab11a S25N, respectively), Gαi2, or PTX treatment. NS, nonstimulated cells. The fraction of apoptotic cells in vector-transfected nonstimulated cells was normalized to 100%. Error bars represent the means ± SEM of three independent experiments (analysis of variance and Student-Newman-Keuls test). *p < 0.001 and **p < 0.05 compared with nonstimulated cells. (B) Proliferative effect of 10 μM LPA was assessed by BrdU labeling in HEK-293T cells transfected with wild-type, constitutively active, or dominant-negative GFP-tagged mutants of Rab11a (WT, Q70L, and S25N, respectively), Gαi2, or PTX treatment. NS, nonstimulated cells. Error bars represent the means ± SEM of four independent experiments done by triplicate (analysis of variance and Student-Newman-Keuls test). *p < 0.01 and **p < 0.001 compared with nonstimulated cells. (C) Effect of LPA and PTX on the recruitment to endosomes and phosphorylation of GSK3-β. Total cell lysates and endosomal fractions from samples shown in the Figure 6C were used to detect the presence and endosomal phosphorylation of GSK3-β by using specific antibodies. NS, nonstimulated cells. (D) Rab11 knockdown interferes with the antiapoptotic effect of LPA. Antiapoptotic effect of 10 μM LPA was assessed by Annexin V binding in HEK-293T cells transfected with Rab11 shRNA. NS, nonstimulated cells. Error bars represent the means ± SEM of four independent experiments (analysis of variance and Student-Newman-Keuls test). *p < 0.05 compared with vector-transfected cells. (E) Rab11 knockdown does not globally disrupt LPA-dependent signaling pathways. HEK-293T cells transfected with Rab11 shRNA were serum starved and stimulated with 10 μM LPA at indicated times. Phosphorylation of AKT and ERK in total cell lysates was detected by Western blotting using phospho-specific antibodies. The expression of AKT, ERK, Rab11, and Rab5 was also detected by Western blot as indicated. A representative blot is presented.
To examine the effect of Rab11a on LPA-dependent cell proliferation, we transiently transfected HEK-293T cells with wild-type Rab11a, constitutively active Rab11a mutant (EGFP-Rab11a Q70L) or a dominant-negative Rab11a mutant (EGFP-Rab11a S25N). As shown in Figure 7B, the proliferative effect of LPA was not affected by wild-type or Q70L mutant Rab11a. However, dominant-negative Rab11a S25N mutant blocked the proliferative effect of LPA. Similarly, the proliferative effect of LPA was blocked by overexpression of Gαi2 or PTX treatment, two strategies known to interfere with Gβγ signaling (Figure 7B). Together, our results indicate that agonist-dependent trafficking of Gβγ to Rab11a endosomes is linked to cell survival and proliferation.
To evaluate the possibility that AKT effectors can be recruited to endosomes and activated as a consequence of Gβγ trafficking and AKT stimulation, we explored the presence of GSK3-β, FKHR-1, and Bad proteins, known substrates of AKT, on endosomes from LPA- and PTX-pretreated cells. As shown in Figure 7C, we detected that LPA promoted the association of GSK3-β to the endosomal fraction; however, we could not reveal its phosphorylation and the treatment with PTX did not show a significant effect on LPA-stimulated association of GSK3-β to endosomes. The lack of phosphorylation of GSK3-β associated to the endosomal fraction makes it difficult to connect its recruitment to the activation of AKT, which could be detected in total cell lysates and where the treatment with PTX attenuated the phosphorylation of this AKT substrate in LPA-stimulated cells (Figure 7C). In contrast, we could not detect the presence of FKHR-1 or Bad proteins at the endosomal fraction (data not shown).
To further examine the functional role of endogenous Rab11 in LPA-dependent cell survival, we assessed the effect of short hairpin RNA-induced Rab11 knockdown on the antiapoptotic effect of LPA. As shown in Figure 7D, Rab11 shRNA blocked the LPA-dependent survival effect, resulting in apoptosis. Additionally, we demonstrated that Rab11 shRNA does not globally disrupt LPA-dependent signaling pathways as evidenced by the ability of LPA to promote the activation of ERK 1/2 and AKT as detected in total lysates from cells transiently transfected with shRNA specific for Rab11 (Figure 7E). Together, our results indicate that Gβγ trafficking to endosomes, in association to Rab11, is linked to cell survival.
DISCUSSION
Heterotrimeric G protein signaling is based on G protein activation/inactivation cycles occurring at the plasma membrane, where GPCRs are stimulated by their cognate ligands present in the extracellular milieu (Gilman, 1987; Dessauer et al., 1996; Offermanns and Simon, 1996; Bourne, 1997; Offermanns, 2003; Bourne, 2006). Phosphorylation and trafficking of GPCRs is commonly recognized as part of a mechanism of desensitization (Ghanouni et al., 2001; Whistler et al., 2002; Prossnitz, 2004; Drake et al., 2006; Reiter and Lefkowitz, 2006; Violin et al., 2006). However, internalization of GPCRs has also been proposed as a means to acquire novel signaling properties via association of the internalized receptors with β-arrestins (Ferguson, 2001; Luttrell and Lefkowitz, 2002; Tohgo et al., 2003; Ahn et al., 2004; Prossnitz, 2004; Johnson et al., 2005; Lefkowitz et al., 2006; Marrari et al., 2007; Moore et al., 2007). The role of internalized heterotrimeric G proteins in vesicular trafficking has been recently recognized in yeast, in which it was demonstrated that endocytosis of Gα is a required step in the signaling to Vps34, the yeast PI3K (Slessareva et al., 2006). This finding has added complexity to the established paradigm of heterotrimeric G protein signaling. Here, we demonstrate that Gβ1γ2 interacts with Rab11a. This interaction occurs upon GPCR activation and promotes the trafficking of Gβγ to endosomal compartments in which it is critical for the assembly and activation of the PI3K/AKT signaling pathway associated with Rab11a-positive endosomes. Gβγ trafficking to endosomal compartments and the activation of its downstream pathways depends on its release from heterotrimeric G proteins and the availability of its effector domain, as demonstrated by the inhibitory effect of overexpressing Gαi or phosducin-like protein, known strategies that prevent Gβγ signaling. In agreement with these findings, it has been recently reported that Gβ1γ7 associates with recycling endosomes upon β2-adrenergic receptor stimulation, following a path different from that of the receptor itself (Hynes et al., 2004). Additional studies documented a rapid internalization of different fluorescent Gβγ heterodimers differing in their composition (Saini et al., 2007), suggesting, among other possibilities, that intracellular trafficking of Gβγ heterodimers might confer previously unrecognized specificities to the their ability to activate specific endosome-associated effectors. This interesting possibility offers a whole array of novel connections between Gβγ signaling and processes associated with specific intracellular compartments, explaining the functional diversity of Gβγ subunits that could not be previously revealed by simple biochemical assays.
We used LPA signaling as the model for our studies because this agonist has been known to act through GPCRs that depend on Gβγ to activate PI3K/AKT and ERK signaling associated with receptor internalization (Kou et al., 2002). Considering that Rab11 has been implicated in GPCR trafficking (Hunyady et al., 2002; Volpicelli et al., 2002; Fan et al., 2003; Moore et al., 2004; Theriault et al., 2004) and that direct interaction between Rab proteins and the carboxy terminus of some GPCRs is determinant for the control of receptor internalization (Seachrist et al., 2002; Hamelin et al., 2005), it is presumably that GPCR signaling promotes Rab11 activation, leading to its interaction with Gβγ and intracellular trafficking of the complex.
The mechanism by which AKT gets associated to the endosomal fraction depends in part on the presence of Gβγ in this fraction and the subsequent recruitment and activation of PI3Kγ. This possibility is supported by the finding of AKT in Rab11 immunoprecipitates, where Gβγ is detected in an agonist dependent way, also by the inhibitory action of Gβγ-interfering agents (Gαi overexpression and PTX treatment) and the effect of inhibiting PI3K with wortmannin. In addition, as observed in nonstimulated cells, a fraction of AKT was constitutively found associated with recycling endosomes, we speculate that this fraction of AKT associates to endosomes due to the presence there of AKT-binding proteins such as ProF or APPL1 that has been recently characterized as an endosomal AKT-recruiting protein (Mitsuuchi et al., 1999; Miaczynska et al., 2004; Fritzius et al., 2006). In agreement with this possibility, Zerial and colleagues just demonstrated that APPL1 mediates AKT substrate specificity at endosomal compartments (Schenck et al., 2008).
Based on the current results, we propose a model depicting the intracellular trafficking of Gβγ and its functional consequences to endosomal signaling; as shown in Figure 8, agonist–GPCR activation induces dissociation of Gα and Gβγ subunits. Gβγ, at the plasma membrane, acts on its multiple effectors, including PI3-kinase/AKT pathway (step 1). In contrast, Gβ1γ2 and Rab11a interaction allows Gβ1γ2 trafficking to early endosomes and then to slowly recycling endosomes (step 2). PI3-kinase-γ is then recruited by Gβγ to promote AKT association and activation initiating signaling from endosomes (step 3). A fraction of AKT is located at the endosomal compartment of nonstimulated cells and is further recruited after receptor stimulation (steps 4 and 5). The assembly of signaling complexes at the endosome includes the recruitment of AKT substrates such as GSK-3-β (step 6) and very likely others. Inhibition of PI3-kinase-γ with Wm prevents the phosphorylation of AKT at endosomes but does not affect its original presence, suggesting the existence of a Wm-resistant AKT-binding protein, which anchors a fraction of AKT independently of its activation state. Once Gβ1γ2 has promoted AKT activation, it may be recycled to the plasma membrane (step 7). The relevance of the assembly of endosomal Gβγ signaling complexes is demonstrated by the absence of proliferative and cell survival effects of LPA receptors in cells in which the availability of free Gβγ or its Rab11a-dependent trafficking is compromised.
Figure 8.
Model depicting the redistribution and endosomal signaling of Gβγ. GPCR activation induces the dissociation of heterotrimeric G proteins into Gα and Gβγ subunits. The liberated Gβγ heterodimer activates its effectors such as PI3-kinase-γ at the plasma membrane, leading to the activation of AKT (1). Gβγ also interacts with Rab11a, promoting Gβγ trafficking to early and slowly recycling endosomes (as defined by the presence of Rab11a) (2). The assembly of a signaling complex occurs at endosomes after recruitment of PI3-kinase-γ followed by activation of AKT (steps 3 and 4). A fraction of AKT is associated with this endosomal fraction even in nonstimulated cells, but the association of this protein is further increased in response to receptor stimulation leading to the phosphorylation of endosomal AKT (5). GSK-3-β is recruited in response to receptor stimulation (step 6). Inhibition of PI3-kinase by Wm prevents the association of PI3-kinase-γ to endosomes and the phosphorylation of AKT. To complete the cycle, presumably Gβγ is recycled to the plasma membrane to start a new cycle of signaling (7).
In conclusion, our studies demonstrate a novel role of Gβγ in the assembly of an endosomal signaling complex through direct interactions with Rab11a, which, upon receptor activation promotes the intracellular trafficking of Gβγ and the endosomal activation of the PI3-kinase/AKT signaling pathway to promote cell survival and proliferation. We propose that Rab11a directs the intracellular trafficking of Gβγ into endosomal pathways, thereby switching GPCR signaling from the plasma membrane to endosomal compartments.
Supplementary Material
ACKNOWLEDGMENTS
We thank Margarita Valadéz Sánchez for expert technical assistance, Omar Hernández García and Jaime Estrada Trejo for assistance, and Blanca E. Reyes for confocal microscopy assistance. This work was supported by Consejo Nacional de Ciencia y Tecnologia (CONACyT) grants 45957 (to G.R.C.) and 61127 (to J.V.P.) and National Institutes of Health grant R01-TW006664 (to J.V.P). A.G.-R., M.L.G.-H., I.R.-R., and E.R.-M. are graduate students supported by fellowships from CONACyT. The research of T. B. was supported in part by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Abbreviations used:
- EEA1
early endosomal antigen 1
- GPCR
G protein coupled receptor
- LPA
lysophosphatidic acid
- PhLP1
phosducin-like protein 1
- PI3-kinase
phosphoinositide 3-kinase
- Wm
wortmannin.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-10-1089) on August 13, 2008.
REFERENCES
- Ahn S., Shenoy S. K., Wei H., Lefkowitz R. J. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J. Biol. Chem. 2004;279:35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- Albert P. R., Robillard L. G protein specificity: traffic direction required. Cell Signal. 2002;14:407–418. doi: 10.1016/s0898-6568(01)00259-5. [DOI] [PubMed] [Google Scholar]
- Barber M. A., Donald S., Thelen S., Anderson K. E., Thelen M., Welch H. C. Membrane translocation of P-Rex1 is mediated by G protein beta gamma subunits and phosphoinositide 3-kinase. J. Biol. Chem. 2007;282:29967–29976. doi: 10.1074/jbc.M701877200. [DOI] [PubMed] [Google Scholar]
- Bourne H. R. How receptors talk to trimeric G proteins. Curr. Opin. Cell Biol. 1997;9:134–142. doi: 10.1016/s0955-0674(97)80054-3. [DOI] [PubMed] [Google Scholar]
- Bourne H. R. G-proteins and GPCrs: from the beginning. Ernst Schering Found. Symp. Proc. 2006:1–21. doi: 10.1007/2789_2006_001. [DOI] [PubMed] [Google Scholar]
- Brock C., Schaefer M., Reusch H. P., Czupalla C., Michalke M., Spicher K., Schultz G., Nurnberg B. Roles of G beta gamma in membrane recruitment and activation of p110 gamma/p101 phosphoinositide 3-kinase gamma. J. Cell Biol. 2003;160:89–99. doi: 10.1083/jcb.200210115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D. E., Neer E. J. G protein beta gamma subunits. Annu. Rev. Pharmacol. Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- Daaka Y., Luttrell L. M., Lefkowitz R. J. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997a;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- Daaka Y., Pitcher J. A., Richardson M., Stoffel R. H., Robishaw J. D., Lefkowitz R. J. Receptor and G betagamma isoform-specific interactions with G protein-coupled receptor kinases. Proc. Natl. Acad. Sci. USA. 1997b;94:2180–2185. doi: 10.1073/pnas.94.6.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessauer C. W., Posner B. A., Gilman A. G. Visualizing signal transduction: receptors, G-proteins, and adenylate cyclases. Clin. Sci. 1996;91:527–537. doi: 10.1042/cs0910527. [DOI] [PubMed] [Google Scholar]
- Diaz Anel A. M., Malhotra V. PKCeta is required for beta1gamma2/beta3gamma2- and PKD-mediated transport to the cell surface and the organization of the Golgi apparatus. J. Cell Biol. 2005;169:83–91. doi: 10.1083/jcb.200412089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake M. T., Shenoy S. K., Lefkowitz R. J. Trafficking of G protein-coupled receptors. Circ. Res. 2006;99:570–582. doi: 10.1161/01.RES.0000242563.47507.ce. [DOI] [PubMed] [Google Scholar]
- Evanko D. S., Thiyagarajan M. M., Siderovski D. P., Wedegaertner P. B. Gβγ isoforms selectively rescue plasma membrane localization and palmitoylation of mutant Gαs and Gαq. J. Biol. Chem. 2001;276:23945–23953. doi: 10.1074/jbc.M101154200. [DOI] [PubMed] [Google Scholar]
- Fan G. H., Lapierre L. A., Goldenring J. R., Richmond A. Differential regulation of CXCR2 trafficking by Rab GTPases. Blood. 2003;101:2115–2124. doi: 10.1182/blood-2002-07-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S. S. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol. Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- Ford C. E., et al. Molecular basis for interactions of G protein betagamma subunits with effectors. Science. 1998;280:1271–1274. doi: 10.1126/science.280.5367.1271. [DOI] [PubMed] [Google Scholar]
- Fritzius T., et al. A WD-FYVE protein binds to the kinases Akt and PKCzeta/lambda. Biochem. J. 2006;399:9–20. doi: 10.1042/BJ20060511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet R., Bohm A., Sigler P. B. Crystal structure at 2.4 angstroms resolution of the complex of transducin betagamma and its regulator, phosducin. Cell. 1996;87:577–588. doi: 10.1016/s0092-8674(00)81376-8. [DOI] [PubMed] [Google Scholar]
- Gautam N., Downes G. B., Yan K., Kisselev O. The G-protein betagamma complex. Cell Signal. 1998;10:447–455. doi: 10.1016/s0898-6568(98)00006-0. [DOI] [PubMed] [Google Scholar]
- Ghanouni P., Steenhuis J. J., Farrens D. L., Kobilka B. K. Agonist-induced conformational changes in the G-protein-coupling domain of the beta 2 adrenergic receptor. Proc. Natl. Acad. Sci. USA. 2001;98:5997–6002. doi: 10.1073/pnas.101126198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu. Rev. Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. Transmembrane signaling, G proteins, and adenylyl cyclase. Harvey Lect. 1989;85:153–172. [PubMed] [Google Scholar]
- Gorvel J. P., Chavrier P., Zerial M., Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Hamelin E., Theriault C., Laroche G., Parent J. L. The intracellular trafficking of the G protein-coupled receptor TPβ depends on a direct interaction with Rab11. J. Biol. Chem. 2005;280:36195–36205. doi: 10.1074/jbc.M503438200. [DOI] [PubMed] [Google Scholar]
- Houle S., Marceau F. Wortmannin alters the intracellular trafficking of the bradykinin B2 receptor: role of phosphoinositide 3-kinase and Rab5. Biochem. J. 2003;375:151–158. doi: 10.1042/BJ20030872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunyady L., Baukal A. J., Gaborik Z., Olivares-Reyes J. A., Bor M., Szaszak M., Lodge R., Catt K. J., Balla T. Differential PI 3-kinase dependence of early and late phases of recycling of the internalized AT1 angiotensin receptor. J. Cell Biol. 2002;157:1211–1222. doi: 10.1083/jcb.200111013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes T. R., Mervine S. M., Yost E. A., Sabo J. L., Berlot C. H. Live cell imaging of Gs and the beta2-adrenergic receptor demonstrates that both alphas and beta1gamma7 internalize upon stimulation and exhibit similar trafficking patterns that differ from that of the beta2-adrenergic receptor. J. Biol. Chem. 2004;279:44101–44112. doi: 10.1074/jbc.M405151200. [DOI] [PubMed] [Google Scholar]
- Jamora C., Yamanouye N., Van Lint J., Laudenslager J., Vandenheede J. R., Faulkner D. J., Malhotra V. Gbetagamma-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell. 1999;98:59–68. doi: 10.1016/S0092-8674(00)80606-6. [DOI] [PubMed] [Google Scholar]
- Johnson E. E., Christie M. J., Connor M. The role of opioid receptor phosphorylation and trafficking in adaptations to persistent opioid treatment. Neurosignals. 2005;14:290–302. doi: 10.1159/000093044. [DOI] [PubMed] [Google Scholar]
- Kalia M., Kumari S., Chadda R., Hill M. M., Parton R. G., Mayor S. Arf6-independent GPI-anchored protein-enriched early endosomal compartments fuse with sorting endosomes via a Rab5/phosphatidylinositol-3′-kinase-dependent machinery. Mol. Biol. Cell. 2006;17:3689–3704. doi: 10.1091/mbc.E05-10-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Beuchat M. H., Chevallier J., Makino A., Mayran N., Escola J. M., Lebrand C., Cosson P., Gruenberg J. Separation and characterization of late endosomal membrane domains. J. Biol. Chem. 2002;277:32157–32164. doi: 10.1074/jbc.M202838200. [DOI] [PubMed] [Google Scholar]
- Kou R., Igarashi J., Michel T. Lysophosphatidic acid and receptor-mediated activation of endothelial nitric-oxide synthase. Biochemistry. 2002;41:4982–4988. doi: 10.1021/bi016017r. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Pierce K. L., Luttrell L. M. Dancing with different partners: protein kinase a phosphorylation of seven membrane-spanning receptors regulates their G protein-coupling specificity. Mol. Pharmacol. 2002;62:971–974. doi: 10.1124/mol.62.5.971. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Rajagopal K., Whalen E. J. New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol. Cell. 2006;24:643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Lin H. C., Duncan J. A., Kozasa T., Gilman A. G. Sequestration of the G protein beta gamma subunit complex inhibits receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA. 1998;95:5057–5060. doi: 10.1073/pnas.95.9.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell L. M., Lefkowitz R. J. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- Marrari Y., Crouthamel M., Irannejad R., Wedegaertner P. B. Assembly and trafficking of heterotrimeric G proteins. Biochemistry. 2007;46:7665–7677. doi: 10.1021/bi700338m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaczynska M., Christoforidis S., Giner A., Shevchenko A., Uttenweiler-Joseph S., Habermann B., Wilm M., Parton R. G., Zerial M. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell. 2004;116:445–456. doi: 10.1016/s0092-8674(04)00117-5. [DOI] [PubMed] [Google Scholar]
- Miaczynska M., Zerial M. Mosaic organization of the endocytic pathway. Exp. Cell Res. 2002;272:8–14. doi: 10.1006/excr.2001.5401. [DOI] [PubMed] [Google Scholar]
- Michaelson D., Ahearn I., Bergo M., Young S., Philips M. Membrane trafficking of heterotrimeric G proteins via the endoplasmic reticulum and Golgi. Mol. Biol. Cell. 2002;13:3294–3302. doi: 10.1091/mbc.E02-02-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuuchi Y., Johnson S. W., Sonoda G., Tanno S., Golemis E. A., Testa J. R. Identification of a chromosome 3p14.3-21.1 gene, APPL, encoding an adaptor molecule that interacts with the oncoprotein-serine/threonine kinase AKT2. Oncogene. 1999;18:4891–4898. doi: 10.1038/sj.onc.1203080. [DOI] [PubMed] [Google Scholar]
- Moore C. A., Milano S. K., Benovic J. L. Regulation of receptor trafficking by GRKs and arrestins. Annu. Rev. Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- Moore R. H., Millman E. E., Alpizar-Foster E., Dai W., Knoll B. J. Rab11 regulates the recycling and lysosome targeting of beta2-adrenergic receptors. J. Cell Sci. 2004;117:3107–3117. doi: 10.1242/jcs.01168. [DOI] [PubMed] [Google Scholar]
- Novotny J., Kvapil P., Bokoch G. M., Ransnas L. A. Isoproterenol-induced subcellular redistribution of G-protein beta subunits in S49 lymphoma cells demonstrated by a novel competitive ELISA. Arch. Physiol. Biochem. 1995;103:202–210. doi: 10.3109/13813459508996134. [DOI] [PubMed] [Google Scholar]
- Offermanns S. G-proteins as transducers in transmembrane signalling. Prog. Biophys. Mol. Biol. 2003;83:101–130. doi: 10.1016/s0079-6107(03)00052-x. [DOI] [PubMed] [Google Scholar]
- Offermanns S., Simon M. I. Organization of transmembrane signalling by heterotrimeric G proteins. Cancer Surv. 1996;27:177–198. [PubMed] [Google Scholar]
- Pierce K. L., Premont R. T., Lefkowitz R. J. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Preininger A. M., Hamm H. E. G protein signaling: insights from new structures. Sci. STKE. 2004;2004:re3. doi: 10.1126/stke.2182004re3. [DOI] [PubMed] [Google Scholar]
- Prossnitz E. R. Novel roles for arrestins in the post-endocytic trafficking of G protein-coupled receptors. Life Sci. 2004;75:893–899. doi: 10.1016/j.lfs.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Reiter E., Lefkowitz R. J. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol. Metab. 2006;17:159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Reuveny E., Slesinger P. A., Inglese J., Morales J. M., Iniguez-Lluhi J. A., Lefkowitz R. J., Bourne H. R., Jan Y. N., Jan L. Y. Activation of the cloned muscarinic potassium channel by G protein beta gamma subunits. Nature. 1994;370:143–146. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- Rhee S. G., Bae Y. S. Regulation of phosphoinositide-specific phospholipase C isozymes. J. Biol. Chem. 1997;272:15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- Rosenfeldt H., Vazquez-Prado J., Gutkind J. S. P-REX2, a novel PI-3-kinase sensitive Rac exchange factor. FEBS Lett. 2004;572:167–171. doi: 10.1016/j.febslet.2004.06.097. [DOI] [PubMed] [Google Scholar]
- Sadja R., Alagem N., Reuveny E. Gating of GIRK channels: details of an intricate, membrane-delimited signaling complex. Neuron. 2003;39:9–12. doi: 10.1016/s0896-6273(03)00402-1. [DOI] [PubMed] [Google Scholar]
- Saini D. K., Kalyanaraman V., Chisari M., Gautam N. A family of G protein betagamma subunits translocate reversibly from the plasma membrane to endomembranes on receptor activation. J. Biol. Chem. 2007;282:24099–24108. doi: 10.1074/jbc.M701191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck A., Goto-Silva L., Collinet C., Rhinn M., Giner A., Habermann B., Brand M., Zerial M. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell. 2008;133:486–497. doi: 10.1016/j.cell.2008.02.044. [DOI] [PubMed] [Google Scholar]
- Seabra M. C., Wasmeier C. Controlling the location and activation of Rab GTPases. Curr. Opin. Cell Biol. 2004;16:451–457. doi: 10.1016/j.ceb.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Seachrist J. L., Ferguson S. S. Regulation of G protein-coupled receptor endocytosis and trafficking by Rab GTPases. Life Sci. 2003;74:225–235. doi: 10.1016/j.lfs.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Seachrist J. L., Laporte S. A., Dale L. B., Babwah A. V., Caron M. G., Anborgh P. H., Ferguson S. S. Rab5 association with the angiotensin II type 1A receptor promotes Rab5 GTP binding and vesicular fusion. J. Biol. Chem. 2002;277:679–685. doi: 10.1074/jbc.M109022200. [DOI] [PubMed] [Google Scholar]
- Slessareva J. E., Routt S. M., Temple B., Bankaitis V. A., Dohlman H. G. Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein alpha subunit at the endosome. Cell. 2006;126:191–203. doi: 10.1016/j.cell.2006.04.045. [DOI] [PubMed] [Google Scholar]
- Sonnichsen B., De Renzis S., Nielsen E., Rietdorf J., Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L., Smrcka A., Cooke F. T., Jackson T. R., Sternweis P. C., Hawkins P. T. A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein beta gamma subunits. Cell. 1994;77:83–93. doi: 10.1016/0092-8674(94)90237-2. [DOI] [PubMed] [Google Scholar]
- Takida S., Wedegaertner P. B. Heterotrimer formation, together with isoprenylation, is required for plasma membrane targeting of Gbetagamma. J. Biol. Chem. 2003;278:17284–17290. doi: 10.1074/jbc.M213239200. [DOI] [PubMed] [Google Scholar]
- Tang W. J., Gilman A. G. Type-specific regulation of adenylyl cyclase by G protein beta gamma subunits. Science. 1991;254:1500–1503. doi: 10.1126/science.1962211. [DOI] [PubMed] [Google Scholar]
- Theriault C., Rochdi M. D., Parent J. L. Role of the Rab11-associated intracellular pool of receptors formed by constitutive endocytosis of the beta isoform of the thromboxane A2 receptor (TP beta) Biochemistry. 2004;43:5600–5607. doi: 10.1021/bi036268v. [DOI] [PubMed] [Google Scholar]
- Tohgo A., Choy E. W., Gesty-Palmer D., Pierce K. L., Laporte S., Oakley R. H., Caron M. G., Lefkowitz R. J., Luttrell L. M. The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J. Biol. Chem. 2003;278:6258–6267. doi: 10.1074/jbc.M212231200. [DOI] [PubMed] [Google Scholar]
- Ullrich O., Reinsch S., Urbe S., Zerial M., Parton R. G. Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Prado J., Basile J., Gutkind J. S. Modular architecture and novel protein-protein interactions regulating the RGS-containing Rho guanine nucleotide exchange factors. Methods Enzymol. 2004;390:259–285. doi: 10.1016/S0076-6879(04)90017-1. [DOI] [PubMed] [Google Scholar]
- Violin J. D., Dewire S. M., Barnes W. G., Lefkowitz R. J. G protein-coupled receptor kinase and beta-arrestin-mediated desensitization of the angiotensin II type 1A receptor elucidated by diacylglycerol dynamics. J. Biol. Chem. 2006;281:36411–36419. doi: 10.1074/jbc.M607956200. [DOI] [PubMed] [Google Scholar]
- Volpicelli L. A., Lah J. J., Fang G., Goldenring J. R., Levey A. I. Rab11a and myosin Vb regulate recycling of the M4 muscarinic acetylcholine receptor. J. Neurosci. 2002;22:9776–9784. doi: 10.1523/JNEUROSCI.22-22-09776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zastrow M., Sorkin A. Signaling on the endocytic pathway. Curr. Opin. Cell Biol. 2007;19:436–445. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi Y., Dutt P., Lippincott-Schwartz J., Arias I. M. Rab11a and myosin Vb are required for bile canalicular formation in WIF-B9 cells. Proc. Natl. Acad. Sci. USA. 2005;102:15087–15092. doi: 10.1073/pnas.0503702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch H. C., Coadwell W. J., Ellson C. D., Ferguson G. J., Andrews S. R., Erdjument-Bromage H., Tempst P., Hawkins P. T., Stephens L. R. P-Rex1, a PtdIns(3,4,5)P3- and Gbetagamma-regulated guanine-nucleotide exchange factor for Rac. Cell. 2002;108:809–821. doi: 10.1016/s0092-8674(02)00663-3. [DOI] [PubMed] [Google Scholar]
- Whistler J. L., Enquist J., Marley A., Fong J., Gladher F., Tsuruda P., Murray S. R., Von Zastrow M. Modulation of postendocytic sorting of G protein-coupled receptors. Science. 2002;297:615–620. doi: 10.1126/science.1073308. [DOI] [PubMed] [Google Scholar]
- Willoughby D., Cooper D. M. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol. Rev. 2007;87:965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- Zerial M., McBride H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.