Abstract
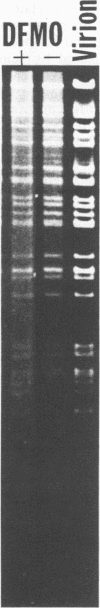
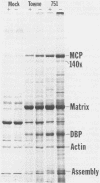
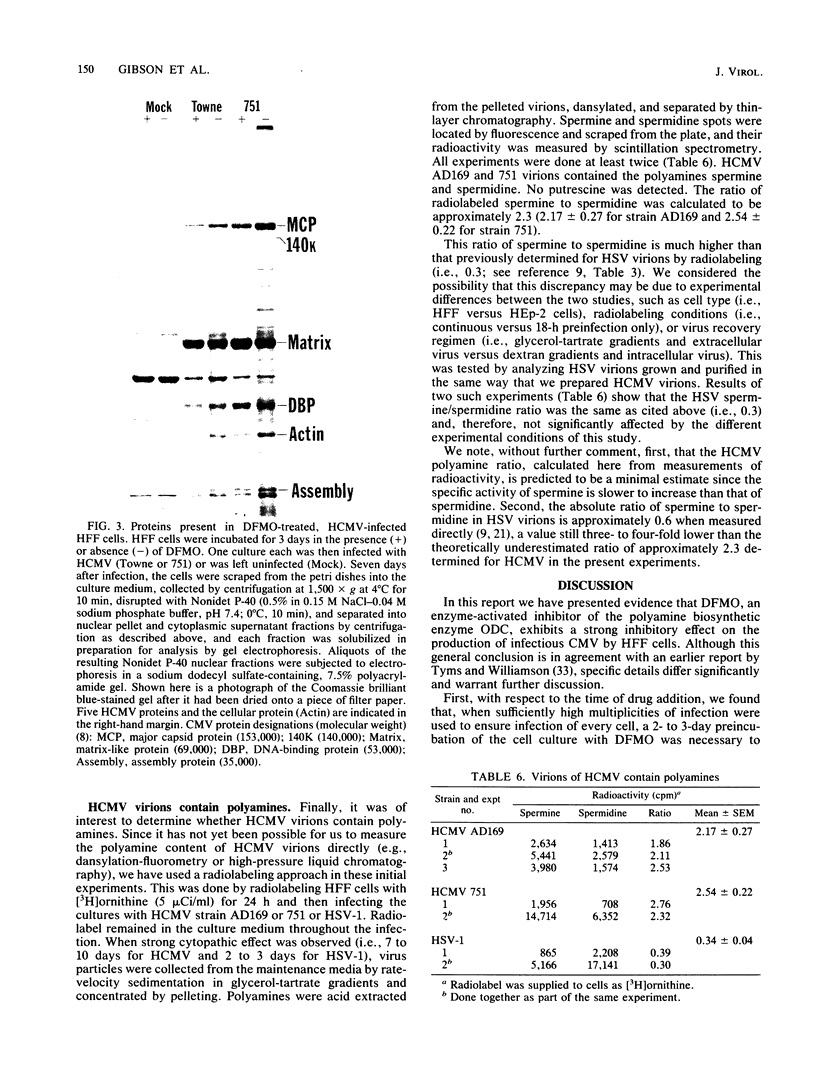
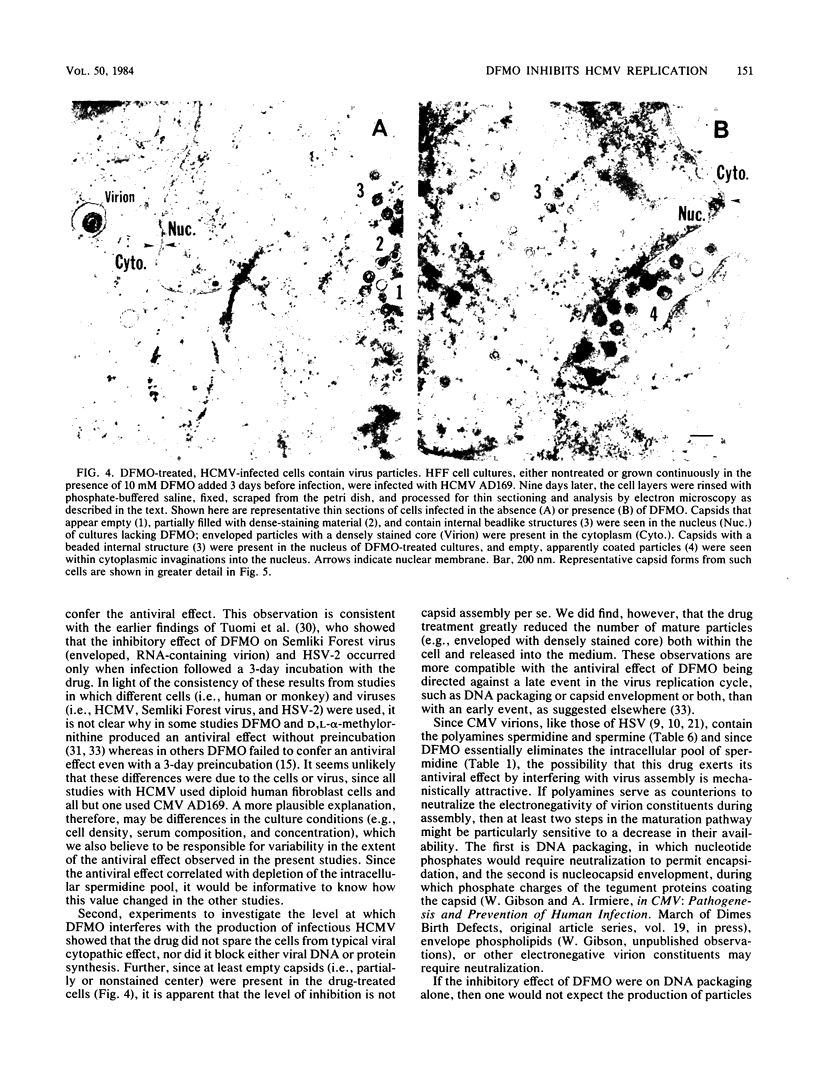
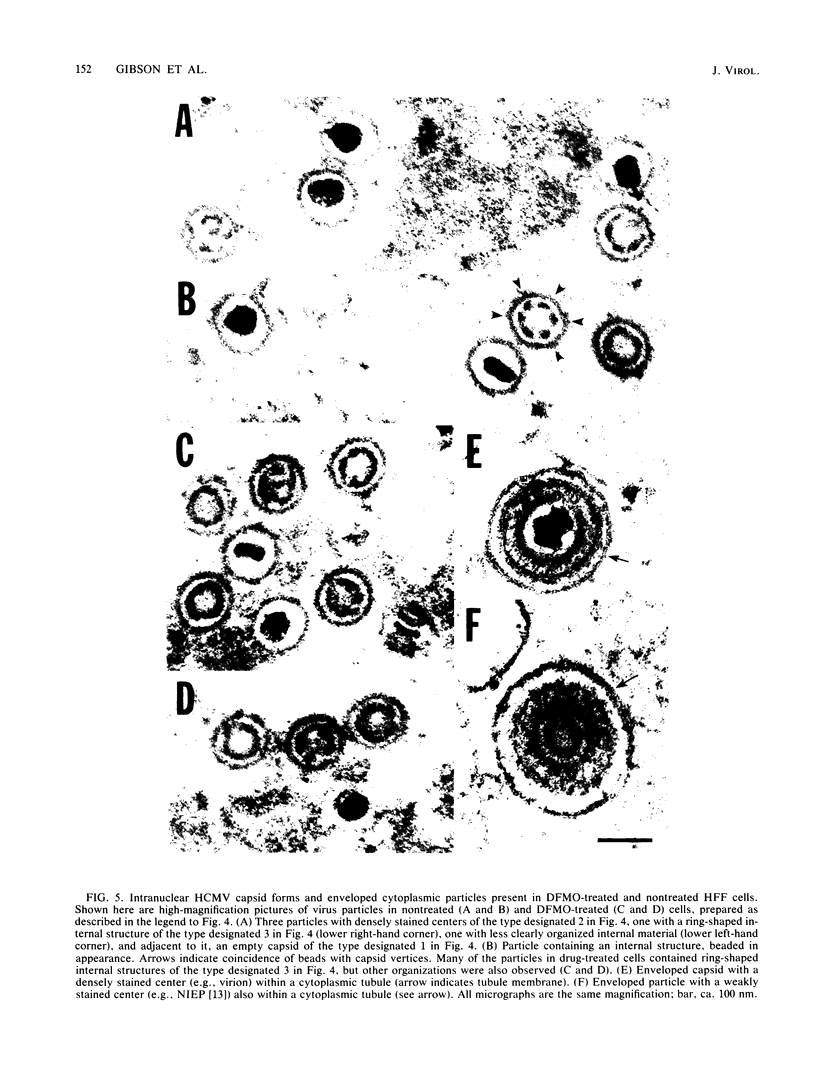
D,L-alpha-Difluoromethylornithine (DFMO) is an inhibitor of ornithine decarboxylase, the first enzyme in the polyamine biosynthetic pathway. Exposure of human foreskin fibroblast cells to DFMO before their infection with human strains of cytomegalovirus (CMV) resulted in a reduction in the amount of infectious virus produced. A 3-day exposure to the drug was required to elicit maximal antiviral effect. Cells exposed to DFMO at the time of infection produced normal amounts of infectious virus. Preexposure to the drug for 1, 2, or 3 days before infection resulted in at least 10-, 100-, or 1,000-fold decreases, respectively, in the amount of infectious virus produced. This decrease paralleled the loss of intracellular spermidine and was partially spared by the addition of exogenous putrescine, spermidine, or spermine (10 microM). When added 3 days before infection, DFMO depressed production of herpes simplex virus and simian CMV, as well as wild-type and laboratory prototype strains of human CMV. Although some antiviral effect was observed at a drug concentration of 1 mM, 10 mM gave a stronger effect and was the amount routinely used. At 30 mM DFMO, growth of noninfected cells was slowed but not arrested. Studies to investigate the level at which DFMO interferes with CMV replication showed that DFMO-treated, infected cells (i) exhibit a typical CMV-specific cytopathic effect, (ii) synthesize both viral proteins and viral DNA, (iii) contain at least some capsid forms, and (iv) shed greatly reduced amounts of virus particles into the growth medium. Since CMV virions, like those of herpes simplex virus, contain the polyamines spermidine and spermine, and since DFMO essentially eliminates the pool of intracellular spermidine, the possibility is suggested that this drug may exert its antiviral effect by interfering with virus assembly, perhaps at the level of DNA packaging or capsid envelopment or both.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barzilai R., Lazarus L. H., Goldblum N. Viscosity-density gradient for purification of foot-and-mouth disease virus. Arch Gesamte Virusforsch. 1972;36(1):141–146. doi: 10.1007/BF01250304. [DOI] [PubMed] [Google Scholar]
- Boldogh I., Beth E., Huang E. S., Kyalwazi S. K., Giraldo G. Kaposi's sarcoma. IV. Detection of CMV DNA, CMV RNA and CMNA in tumor biopsies. Int J Cancer. 1981 Oct 15;28(4):469–474. doi: 10.1002/ijc.2910280412. [DOI] [PubMed] [Google Scholar]
- Drew W. L., Mintz L., Miner R. C., Sands M., Ketterer B. Prevalence of cytomegalovirus infection in homosexual men. J Infect Dis. 1981 Feb;143(2):188–192. doi: 10.1093/infdis/143.2.188. [DOI] [PubMed] [Google Scholar]
- Fenwick M. L., Walker M. J. Suppression of the synthesis of cellular macromolecules by herpes simplex virus. J Gen Virol. 1978 Oct;41(1):37–51. doi: 10.1099/0022-1317-41-1-37. [DOI] [PubMed] [Google Scholar]
- Gibson W. Protein counterparts of human and simian cytomegaloviruses. Virology. 1983 Jul 30;128(2):391–406. doi: 10.1016/0042-6822(83)90265-9. [DOI] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Compartmentalization of spermine and spermidine in the herpes simplex virion. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2818–2821. doi: 10.1073/pnas.68.11.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W. Structural and nonstructural proteins of strain Colburn cytomegalovirus. Virology. 1981 Jun;111(2):516–537. doi: 10.1016/0042-6822(81)90354-8. [DOI] [PubMed] [Google Scholar]
- Haegele K. D., Alken R. G., Grove J., Schechter P. J., Koch-Weser J. Kinetics of alpha-difluoromethylornithine: an irreversible inhibitor of ornithine decarboxylase. Clin Pharmacol Ther. 1981 Aug;30(2):210–217. doi: 10.1038/clpt.1981.150. [DOI] [PubMed] [Google Scholar]
- Helgstrand E., Eriksson B., Johansson N. G., Lannerö B., Larsson A., Misiorny A., Norén J. O., Sjöberg B., Stenberg K., Stening G. Trisodium phosphonoformate, a new antiviral compound. Science. 1978 Sep 1;201(4358):819–821. doi: 10.1126/science.210500. [DOI] [PubMed] [Google Scholar]
- Irmiere A., Gibson W. Isolation and characterization of a noninfectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology. 1983 Oct 15;130(1):118–133. doi: 10.1016/0042-6822(83)90122-8. [DOI] [PubMed] [Google Scholar]
- Isom H. C., Pegg A. E. Inhibition of cytomegalovirus-stimulated human cell ornithine decarboxylase by alpha-difluoromethylornithine. Biochim Biophys Acta. 1979 Oct 25;564(3):402–413. doi: 10.1016/0005-2787(79)90031-5. [DOI] [PubMed] [Google Scholar]
- Isom H. C. Stimulation of ornithine decarboxylase by human cytomegalovirus. J Gen Virol. 1979 Feb;42(2):265–278. doi: 10.1099/0022-1317-42-2-265. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaFemina R. L., Hayward G. S. Replicative forms of human cytomegalovirus DNA with joined termini are found in permissively infected human cells but not in non-permissive Balb/c-3T3 mouse cells. J Gen Virol. 1983 Feb;64(Pt 2):373–389. doi: 10.1099/0022-1317-64-2-373. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Duchesne M. C., Grove J., Bey P. Anti-proliferative properties of DL-alpha-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Commun. 1978 Mar 15;81(1):58–66. doi: 10.1016/0006-291x(78)91630-3. [DOI] [PubMed] [Google Scholar]
- McCormick F. P., Newton A. A. Polyamine metabolism in cells infected with herpes simplex virus. J Gen Virol. 1975 Apr;27(1):25–33. doi: 10.1099/0022-1317-27-1-25. [DOI] [PubMed] [Google Scholar]
- McCormick F. Polyamine turnover and leakage during infection of HeLa and L-cells with herpes simplex virus type 1. Virology. 1978 Dec;91(2):496–503. doi: 10.1016/0042-6822(78)90399-9. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Silverstein S. Requirement of protein synthesis for the degradation of host mRNA in Friend erythroleukemia cells infected wtih herpes simplex virus type 1. J Virol. 1978 Sep;27(3):619–627. doi: 10.1128/jvi.27.3.619-627.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N., Wiechmann M. Die Mikrobestimmung von Spermin und Spermidin als 1-Dimethylamino-naphthalin-5-sulfonsäure-Derivate. Hoppe Seylers Z Physiol Chem. 1967 Oct;348(10):1285–1290. [PubMed] [Google Scholar]
- Sydiskis R. J., Roizman B. Polysomes and protein synthesis in cells infected with a DNA virus. Science. 1966 Jul 1;153(3731):76–78. doi: 10.1126/science.153.3731.76. [DOI] [PubMed] [Google Scholar]
- Talbot P., Almeida J. D. Human cytomegalovirus: purification of enveloped virions and dense bodies. J Gen Virol. 1977 Aug;36(2):345–349. doi: 10.1099/0022-1317-36-2-345. [DOI] [PubMed] [Google Scholar]
- Tuomi K., Mäntyjärvi R., Raina A. Inhibition of Semliki Forest and herpes simplex virus production in alpha-difluoromethylornithine-treated cells: reversal by polyamines. FEBS Lett. 1980 Dec 1;121(2):292–294. doi: 10.1016/0014-5793(80)80365-6. [DOI] [PubMed] [Google Scholar]
- Tyms A. S., Scamans E., Williamson J. D. Polyamine metabolism in MRC5 cells infected with different herpesviruses. Biochem Biophys Res Commun. 1979 Jan 30;86(2):312–318. doi: 10.1016/0006-291x(79)90867-2. [DOI] [PubMed] [Google Scholar]
- Tyms A. S., Williamson J. D. Inhibitors of polyamine biosynthesis block human cytomegalovirus replication. Nature. 1982 Jun 24;297(5868):690–691. doi: 10.1038/297690a0. [DOI] [PubMed] [Google Scholar]
- Tyms A. S., Williamson J. D. Polyamine metabolism in MRC-5 cells infected with human cytomegalovirus. J Gen Virol. 1980 May;48(1):183–191. doi: 10.1099/0022-1317-48-1-183. [DOI] [PubMed] [Google Scholar]
- Wahren B., Oberg B. Reversible inhibition of cytomegalovirus replication by phosphonoformate. Intervirology. 1980;14(1):7–15. doi: 10.1159/000149156. [DOI] [PubMed] [Google Scholar]