Abstract
Nox5, an EF-hand–containing reactive oxygen species (ROS)-generating NADPH oxidase, contains two conserved polybasic regions: one N-terminal (PBR-N), located between the fourth EF-hand and the first transmembrane region, and one C-terminal (PBR-C), between the first and second NADPH-binding subregions. Here, we show that phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)P2], a major phosphoinositide in plasma membrane, binds to human Nox5 causing Nox5 to localize from internal membranes to the plasma membrane. Enzymatic modulation of PtdIns(4,5)P2 levels in intact cells altered cell surface localization of Nox5 in parallel with extracellular ROS generation. Mutations in PBR-N prevented PtdIns(4,5)P2-dependent localization of Nox5 to the plasma membrane and decreased extracellular ROS production. A synthetic peptide corresponding to PBR-N bound to PtdIns(4,5)P2, but not to PtdIns, whereas mutations in the PBR-N peptide abrogated the binding to PtdIns(4,5)P2. Arginine-197 in PBR-N was a key residue to regulate subcellular localization of Nox5 and its interaction with PtdIns(4,5)P2. In contrast, mutation in PBR-C did not affect localization. Thus, extracellular ROS production by Nox5 is modulated by PtdIns(4,5)P2 by localizing Nox5 to the plasma membrane.
INTRODUCTION
The family of reactive oxygen species (ROS)-generating homologues of gp91phox (aka Nox2) consists of seven members in humans including, Nox1-Nox5 and the Dual oxidases, Duox1 and 2 (Vignais, 2002; Bokoch and Knaus, 2003; Lambeth, 2004; Geiszt, 2006; Bedard and Krause, 2007). Nox and Duox enzymes can be broadly classified into those that are regulated by subunit and those that are regulated by calcium (Nauseef, 2004; Lambeth et al., 2007). Nox5 belongs to the calcium-regulated subgroup that is characterized by possessing a domain with single or multiple EF-hand calcium-binding motif(s) N-terminal to the flavocytochrome catalytic domain.
The EF-hand–containing Nox subgroup evolved early and are the most widely distributed of all Nox enzymes in biology (Bedard et al., 2007; Kawahara et al., 2007). Members of this subgroup include animal Nox5 and Duox enzymes, higher plant rboh (respiratory burst oxidase homolog), moss rboh, oomycete rboh, and fungal and amoebal NoxC. Predicted Nox5 genes occur in the genomes of metazoans, such as the sea anemone, Nematostella vectensis; the limpet, Lottia gigantean; the sea urchin, Strongylocentrotus purpuratus; the insects Drosophila melanogaster, Apis mellifera, Anopheles gambiae, and Aedes aegypt; the water flea, Daphnia pulex; and most vertebrates, except for rodents (Kawahara and Lambeth, 2007; Kawahara et al., 2007). The human Nox5 gene is expressed predominantly as two alternatively spliced forms, Nox5α and β (Banfi et al., 2001), as well as relatively minor isoforms, Nox5δ and γ (Banfi et al., 2001), and a short form Nox5 (Nox5-S) that lacks the entire EF-hand region (Cheng et al., 2001). Nox5α is strongly expressed in the spleen, especially in the area rich in mature B- and T-lymphocytes (Banfi et al., 2001). Nox5β mRNA is expressed in the testis especially in pachytene spermatocyte (Banfi et al., 2001). Nox5-S is expressed in most fetal tissues (Cheng et al., 2001), Barrett's esophageal adenocarcinoma cells (Si et al., 2007), and microvascular endothelial cells (BelAiba et al., 2007).
The biological and pathobiological functions of Nox5 in animals have been studied by several groups. In ovarian smooth muscle of D. melanogaster, Nox5-derived ROS participates in calcium signal transduction linked to muscle contraction and ovulation. In this system, a peptide hormone-stimulated cytosolic calcium flux required Nox5-derived ROS as an upstream signal (Ritsick et al., 2007). Recently, the proto-oncogene c-Abl was identified as a Nox5-binding protein, indicating that Nox5 is related to cell growth and signal transduction (El Jamali et al., 2008). In addition, in vitro studies suggest that Nox5-derived ROS enhances cell growth of prostate cancer (Brar et al., 2003), hairy cell leukemia cells (Kamiguti et al., 2005), and human aortic smooth muscle cells (Jay et al., 2008).
Originally, the regulation of Nox5 was not thought to be as complex as current investigation is revealing. Consistent with the presence of EF-hand calcium-binding motifs, human Nox5 is activated by calcium (Banfi et al., 2001, 2004). However, in this cell-free assay, a high concentration of calcium (>7 μM) is required to activate Nox5, which raised the question of whether it can be regulated within the physiological concentration range of intracellular calcium (Banfi et al., 2004). Recently, it was shown that phorbol 12-myristate 13-acetate (PMA)-stimulated cells induced phosphorylation of Nox5 on Thr494 and Ser498, resulting in activation of Nox5 by lower levels of calcium (Jagnandan et al., 2007; Serrander et al., 2007). In addition, calmodulin has been proposed as a modulator of Nox5 activity, because its recombinant addition in vitro also sensitized Nox5 to lower calcium concentrations (Tirone and Cox, 2007).
The present studies were motivated by our previous investigations of the structurally conserved regions specific for each Nox subfamily (Kawahara et al., 2007). Detailed comparisons of 18 Nox5 ortholog sequences from eukaryotic genomes identified two highly conserved polybasic regions: an N-terminal polybasic region (PBR-N) and a C-terminal polybasic region (PBR-C). PBR-N consist of residues 187-197 located between the fourth EF-hand motif and first transmembrane region (GenBank accession no. AAK57338). PBR-C is located in primary sequence between first and second predicted NADPH-binding subregions at residues 578-583 of the human Nox5α sequence (see alignments of Supplemental Data S1 and S2, respectively).
The conservation of these polybasic regions among sequence Nox5 orthologues suggested that they might give important roles in Nox5 enzymatic activity, regulation, or other functions. Recently it was found that short regions of basic residues can bind to phosphorylated forms of phosphatidylinositol (PtdIns) lipids (McLaughlin et al., 2002; Heo et al., 2006), resulting in the protein's localization to the plasma membrane. Such a localization mechanism is used by various proteins including several small GTPases (Ueyama et al., 2005; Heo et al., 2006), Wiskott-Aldrich syndrome protein (Prehoda et al., 2000), phospholipase D (Sciorra et al., 1999), and MyD88 adaptor-like protein (Kagan and Medzhitov, 2006). Herein, we tested the hypothesis that PtdIns phosphate modulates Nox5 activity or localization via these polybasic regions. PBR-N but not PBR-C was found to regulate the localization of Nox5, thereby affecting its production of extracellular ROS.
MATERIALS AND METHODS
Materials
Human embryonic kidney (HEK) 293 cells were obtained from American Type Culture Collection (Manassas, VA). Ionomycin, ferricytochrome c, superoxide dismutase (SOD), and mouse monoclonal anti-β-actin antibody were purchased from Sigma (St. Louis, MO). Diogenes was obtained from National Diagnostics (Atlanta, GA). 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′,-tetraacetic acid acetoxymethyl ester (BAPTA-AM) was obtained from EMD Chemicals (La Jolla, CA). Anti-human Nox5 antibody was made in rabbits immunized with synthetic peptides corresponding to the residues 491-506 and 577-588 of human Nox5α. Because the PBR-C overlapped partially with the antigen, another rabbit anti-human Nox5 antibody, kindly provided by Dr. Karl-Heinz Krause (Geneva Medical Faculty and University Hospitals, Geneva, Switzerland), was also used in Figures 1F and 5B; this antibody was raised against recombinant protein corresponding to residues 1-169 of human Nox5α (Banfi et al., 2001; Serrander et al., 2007). Hexahistidine-tagged peptides corresponding to residues 186-200 of human Nox5α were synthesized by the Emory University Microchemical Facility (Atlanta, GA).
Figure 1.
Effects of mutations in Nox5 polybasic regions on the ROS-generation from Nox5-transfected HEK293 cells. (A) Schematic representation of Nox5 motifs including two polybasic regions that are evolutionarily conserved in animal Nox5 orthologues. EF, EF-hand; I-VI, transmembrane-I to VI; FAD1–2, predicted flavin-binding subregions; VXGPFG, VXGPFG-motif; NADPH1-4, predicted NADPH-binding subregions; PBR-N, N-terminal polybasic region; PBR-C, C-terminal polybasic region. Open circles indicate predicted heme-binding histidine residues. (B and C) Sequences of Nox5 displaying the appropriate mutations in N- and C-terminal polybasic regions (PBR-N and -C, respectively). (D) ROS generation was measured in HEK293 cells transfected with wild-type Nox5 (Nox5 WT) or the Nox5 mutant forms PBR-N-mut-Q, -A, and -E. ROS production was measured by luminol-based luminescence expressed as relative luminescence units (RLU) per 1 × 105 cells. Cells were treated with DMSO (□) or 1 μM ionomycin (■), and the maximum value is shown. Data represent means ± SD (n = 6); *p<0.05, **p<0.001, compared with Nox5 WT. (E) ROS generation was tested using HEK293 cells transfected with either wild-type Nox5 (Nox5 WT) or with the mutant forms of Nox5 PBR-C-mut-Q. ROS production was measured by luminol-based luminescence and is expressed as relative luminescence units (RLU) per 1 × 105 cells. Data represent means ± SD (n = 5); * p < 0.05, ** p < 0.001, compared with Nox5 WT. (F) Protein expression of Nox5 mutations were shown with an immunoblot against Nox5, which was detected as a protein of about 75 kDa. Similar protein loading is confirmed by an immunoblot against β-actin. Similar results were obtained in two independent experiments.
Figure 5.
The effect of PtdIns(4)P-5K on Nox5 activity is eliminated by mutations in PBR-N. (A) Wild-type Nox5 (Nox5 WT) and mutations of Nox5 (sequences shown in Figures 2 and 3) were cotransfected along with either empty vector (PtdIns(4)P-5K: −) or PtdIns(4)P-5-kinase α (PtdIns(4)P-5K: +). ROS generation was activated with 1 μM ionomycin and measured by luminol-based luminescence. Data represent means ± SD (n = 6); *p < 0.05, **p < 0.001, compared with empty vector (PtdIns(4)P-5K: −). (B) Protein from HEK293 cells transfected with the indicated Nox5 vectors along with PtdIns(4)P-5K or the mock vector (PtdIns(4)P-5K: + and −, respectively) were surface-biotinylated, and isolated with streptavidin-conjugated resin. The biotinylated fraction (prep: avidin) and total cell lysates (cell lysate) were immunoblotted with an anti-Nox5 antibody. Similar protein loading was confirmed by immunoblots using a β-actin antibody. Similar results were obtained in three independent experiments.
Constructs
Mutations were introduced by PCR-mediated mutagenesis as described previously (Kawahara et al., 2005). Nox5-R190A (Arg190 of human Nox5α substituted with Ala) was constructed by amplifying human Nox5α cDNA (GenBank accession No. AAK57338) using primer set A (primer-1: GCATGGATCCACCATGAACACATCTGGAGA; R190A-primer 2, TGACGGCTCCTGCTCCTGCTCCAAGACCTAGACGTCCT) and primer set B (R190A-primer 3, AGGACGTCTAGGTCTTGGAGCAGGAGCAGGAGCCGTCA, primer-4 GCATAAGCTTCTAGAAATTCTCTTGGAAAAATCTGAAGCCGAAC). Nucleotides encoding the mutated amino acid are underlined in primers 2 and 3. These PCR products contain BamHI (italics) and HindIII (underlined) with primers 1 and 4, respectively. Following a second round of PCR using primers 1 and 4 and digestion by each enzyme, the nucleotide fragments were subcloned into the pCMV-tag 5A (Stratagene, La Jolla, CA). R192A, R194A, R195A, R194A/R195A, R197A, R197E, and P195A of Nox5 were generated using a similar strategy. Nox5-PBR-N-mut-A, PBR-N-mut-E, PBR-N-mut-Q, and PBR-C-mut-Q were generated by specific primers 2 and 3: PBR-N-mut-A-primer 2 (TGGCTGACGGCACCAGCACCAGAACCAGCACCGGCAGCACCGGCACAGCTGACCCGAGCATACTG), PBR-N-mut-A-primer 3 (CAGTATGCTCGGGTCAGCTGTGCCGGTGCTGCCGGTGCTGGTTCTGGTGCTGGTGCCGTCAGCCA), PBR-N-mut-Q-primer 2 (CACCACAACCACAACCGCAACAACCGCAACAGCTGACCCGAGCATACTG), PBR-N-mut-Q-primer 3 (TCAGCTGTTGCGGTTGTTGCGGTTGTGGTTGTGGTGCTGGTGCCGTCAGCCAGTG), PBR-N-mut-E-primer 2 (CACCAGAACCAGAACCGGAAGAACCGGAACAGCTGACCCGAGCATACTG), PBR-N-mut-E-primer 3 (TCAGCTGTTCCGGTTCTTCCGGTTCTGGTTCTGGTGCTGGTGCCGTCAGCCAGTG), PBR-C-mut-Q- primer 2 (AGTATCATGTACCAGCACCAGCAACAACAGCATACTTGCCCCAGCT), and PBR-C-mut-Q-primer 3 (AGCTGGGGCAAGTATGCTGTTGTTGCTGGTGCTGGTACATGATACT).
To visualize the Nox5 protein, an N-terminal GFP fusion of Nox5 was made using the pEGFP-C3 vector (BD Biosciences, San Jose, CA). Human-type I PtdIns-4-phosphate 5-kinase (PtdIns(4)P-5K) and the PH-domain of human PLCδ-1 (residues 1-183) were amplified from the IMAGE clones (GenBank accession No. BC007833 and BC050382, respectively) obtained from Invitrogen (Carlsbad, CA) using primers for PtdIns(4)P-5K (TTTTGAATTCGGCGTCGGCCTCCTCCGGGCCGTCGTCTTC and TTTTGTCGACTTAATGGGTGAACTCTGACTCTGCAAC) and PH-PLCδ-1 (TTTTGTCGACCACCATGGACTCGGGCCGGGACTTCCTG and TTTTGGATCCTTCCGGGCATAGCTGTCGTCCACCTG). The nucleotide fragments were digested by restriction enzymes, EcoRI (italics) and SalI (underlined) for PtdIns(4)P-5K or SalI (italics) and BamHI (underlined) for PH-PLCδ-1, and were subcloned into pCMV-tag 3A (Stratagene) and pDsRed-N1 (BD Biosciences) vectors, respectively. The N-terminally myc-tagged PtdIns(4,5)P2-5-phosphatase domain (residues 214–644) was amplified from its cDNA (GenBank accession No. NM_019892), which was kindly provided by Dr. Balla Tamas (National Institute of Child Health and Human Development, Bethesda, MD), with primer-A, TTTTGAATTCGTCGGATCTTGCAGACTACAAGCT and primer-B, TTTTGTCGACTCAAGAAACGGAGCAGATGGTGCTGGAGTTCT. The PCR fragment was subcloned into the pCMV-tag 3A after digestion with EcoRI (italics in primer-A) and SalI (underlined in primer-B).
Measurement of ROS
HEK293 cells were grown for 24 h in six-well plates and allowed to reach to 50% confluence in 2 ml DMEM with 10% (vol/vol) fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were transfected with vectors encoding indicated genes using FuGENE 6 obtained from Roche (Basel, Switzerland). At 48 h after transfection, cells were washed twice with HBSS containing 1 mM calcium and 1 mM magnesium ions and then harvested by centrifuging at 500 × g for 5 min. Cells (2 × 105) were resuspended in HBSS, and the cells in suspension were mixed with 200 μM luminol with 0.32 U of horseradish peroxidase (HRP) in a 200-μl total volume in each well of a 96-well plate as described previously (Kawahara et al., 2005). Luminescence was quantified using a FluoStar luminometer (BMG Labtech, Durham, NC). Cells were treated with vehicle alone (0.1% DMSO) or with 1 μM ionomycin in DMSO to stimulate calcium-dependent ROS generation. The maximal value was achieved immediately and is the value reported.
Extracellular superoxide was measured using Diogenes (National Diagnostics, Atlanta, GA), a superoxide-enhanced chemiluminescence reagent (Shiose et al., 2001). Resuspended cells (1 × 105) in 50 μl HBSS were mixed with an equal volume of Diogenes in each well of a 96-well plate according to the manufacture's instructions, and luminescence was quantified using a FluoStar luminometer. Superoxide is reported as SOD-inhibitable Diogenes-based luminescence. Cells were treated with vehicle (0.1% DMSO) or 1 μM ionomycin, and the maximum value was reported. Extracellular superoxide was also measured using the SOD-inhibitable increase in absorbance at 550 nm of reduced cytochrome c as previously described (Kawahara et al., 2004). Cells cultured on six-well plates were incubated in 1 ml HBSS containing ferricytochrome c (100 μM) for 20 min at 37°C after treatment with vehicle (0.1% DMSO) or 1 μM ionomycin, and then absorbance of reduced cytochrome c with a Ultraspec spectrophotometer (GE Healthcare, Piscataway, NJ) was measured. SOD-inhibitable reduction of cytochrome c was expressed as an absorbance at 550 nm per 106 cells minus that obtained in the presence of SOD.
Preparation of Permeabilized Cells
Cultured cells were harvested and washed three times with HBSS lacking calcium and magnesium ions. Cells were treated with 5 μM thapsigargin and incubated for 20 min at 37°C to release calcium ion from intracellular stores. Cells were then washed with calcium- and magnesium-free HBSS, and resuspended in phosphate buffer (pH 7.2) containing 135 mM NaCl, 100 μg/ml BSA, and protease inhibitor mixture (Complete EDTA-free, Roche). Reduced Streptolysin-O (final concentration, 5 U/ml) was added to the 107 cells in 10 ml and incubated for 10 min at 37°C as described (Brown et al., 2003). Cells were then chilled to 4°C, centrifuged for 5 min at 1250 × g, and washed three times with phosphate buffer (pH 7.2). Permeabilized cells were stored at 4°C until the experiment was performed.
Measurement of Superoxide Generation in Permeabilized Cells
Permeabilized cells were suspended in 30 mM MOPS buffer (pH 7.2) containing 100 mM KCl and protease inhibitor mixture (Complete EDTA-free, Roche). Buffered calcium with calculated free calcium concentrations ranging from 17 nM to 1 mM, prepared by calcium calibration buffer kits no. 2 (for 17 nM-1.35 μM) and no. 3 (for 5 μM–1 mM; Molecular Probes, Eugene, OR). Permeabilized cells (∼5 × 105 cell equivalents) were aliquoted into 200 μl of buffered calcium in 30 mM MOPS buffer (pH 7.2) containing 100 μM FAD and 100 μM ferricytochrome c. After 5 min at room temperature, 1 μl NADPH solution was added to a final concentration of 200 μM with or without SOD (100 U/ml), and the kinetics of absorbance at 550-nm wavelength was measured for 10 min using a Synergy HT spectrophotometer (Bio-Tek Instruments, Winooski, VT). The permeabilized cells were then lysed with 20 mM HEPES buffer containing 1% Triton X, and the protein was quantified using the BCA protein assay reagent kit (Pierce, Rockford, IL). The rate of superoxide production was expressed as SOD-inhibitable cytochrome c reduction that was normalized to 1 min and 1 mg protein.
Biotinylation
HEK293 cells were cultured to 50–60% confluency on 10-cm-diameter dishes. Media was removed and cells were rapidly washed twice with ice-cold HBSS. Cells were biotinylated with sulfo-NHS-SS-Biotin (Pierce) in isotonic labeling buffer (100 mM NaCl, 4 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 30 mM NaHCO3, and 0.1 M sodium phosphate, pH 7.2) at 4°C for 20 min according to the manufacture's instructions (Pierce). The reactions were quenched with quenching buffer containing 50 mM Tris in HBSS buffer, and the cells (4 × 107 cells) were incubated with lysis/washing buffer (50 mM Tris/HCl, pH 8.0, containing 150 mM NaCl, 1% NP40, 0.5% sodium deoxycholate, and protease inhibitor mixture; Complete; Roche) for 1 h at 4°C with gentle shacking. Lysates were centrifuged at 12,000 × g for 5 min at 4°C. After removing an aliquot of the supernatant, streptavidin-conjugated Sepharose beads (GE Healthcare, Pittsburgh, PA) were added to the remainder of supernatant and mixed by gentle rotation for 60 min, followed by washing three times. Laemmli sample buffer (2×, Bio-Rad Laboratories, Hercules, CA) containing protease inhibitor mixture (Complete) and 25 mM Tris(2-carboxyethyl)phosphine (TCEP, Pierce) was added to the beads and incubated at 60°C for 30 min. After centrifuging the beads for 1 min at 1000 × g, the eluted supernatant was carefully taken as the surface fraction. This eluted fraction and an aliquot of the whole cell lysate were immunoblotted with an anti-Nox5 antibody.
Immunoblot
Whole cell extract was obtained from cultured cells lysed in Laemmli sample buffer containing protease inhibitor mixture (Complete), 100 μM diisopropyl fluorophosphate, and 25 mM TCEP. We find that the latter strong reducing agent is preferable to the other common weaker reducing agents dithiothreitol and β-mercaptoethanol (Han and Han, 1994) because it allows visualization of Nox5 protein at 75 kDa, matching the estimated size (82 kDa) better than a previously reported size on gels of 70 kDa (Serrander et al., 2007). Protein extracted from 8 × 104 cell equivalents were resolved by 10% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) filters as previously described (Kawahara et al., 2005). After blocking with bovine serum albumin (3%), proteins were probed using their respective primary antibodies and HRP-conjugated secondary antibodies against rabbit and mouse IgG (Bio-Rad Laboratories). Primary antibodies were used at dilutions of 1:1000 (anti-Nox5 antibody), and 1:20,000 (anti-β-actin antibody). Visualization was carried out using an enhanced chemiluminescent substrate kit (Pierce).
Intracellular Calcium Ion Measurement
HEK293 cells (2 × 104 cells) were cultured in 96-cell plates and transfected with the indicated vectors 48 h before experiments. After removing culture medium, cells were incubated with a Fluo-4-NW (Invitrogen) loading buffer consisting of HBSS containing 20 mM HEPES, 2.5 mM probenecid, and 1.5 mM CaCl2 for 30 min at 37°C and then at room temperature for an additional 30 min, following the manufacturer's instructions. Plates were placed in a Synergy HT fluorometric system (Bio-Tek Instruments), and florescence was measured using filters for excitation at 485 nm and emission at 520 nm. Changes in intracellular Ca2+ concentration were expressed as increase in the florescence intensity (F) over resting levels (F/F0). F0 was the mean of F value of mock vector-transfected cells in absence of ionomycin treatment.
Confocal Fluorescence Imaging
HEK293 cells were seeded on glass coverslips and transfected with the indicated vectors using FuGENE 6. At 24 h after transfection, cells were fixed using 4% methanol-free formaldehyde (Polysciences, Warrington, PA) and mounted with Vectashield medium (Vector Laboratories, Burlingame, CA). Confocal imaging was performed using a LSM510 META confocal laser scanning fluorescence microscope (Carl Zeiss, Oberkochen, Germany). The intensity of each channel of images was analyzed by LSM Image Browser (Carl Zeiss).
Lipid–protein-binding Assay
Hexahistidine-tagged peptides corresponding to residues 186–200 of human Nox5α were synthesized by the Emory University's Microchemical Facility, and sequences of mutations PBR-N-mut-A and R197E are shown in Figure 1B and 2A, respectively. PtdIns or PtdIns(4,5)P2-conjugated agarose beads were obtained from Echelon Bioscience Beads (50 μl; Salt Lake City, UT) and contained 1 nmol PtdIns or PtdIns(4,5)P2. Beads were suspended in an equal volume of binding/wash buffer (10 mM HEPES buffer, pH 7.4, containing 0.5% NP-40, 150 mM NaCl). Two nanomoles of peptide diluted in 20 μl binding/wash buffer were added to the beads. After incubation for 4 h at 4°C, the sample was centrifuged at 100 × g, and the beads were washed five times with 1 ml binding/wash buffer, pelleting the beads between washes by centrifugation at 100 × g. Peptides bound to beads were eluted with an equal volume of 2× Laemmli sample buffer and heated to 95°C for 5 min. Peptides were separated by 17% SDS-PAGE and transferred to the PVDF filters to detect peptides by immunoblot with an anti-hexahistidine-tag antibody.
Liposomes (PolyPIPosomes) containing PtdIns or PtdIns(4,5)P2 were obtained from Echelon Bioscience. Liposomes were composed of 65% phosphatidylcholine, 30% phosphatidylethanolamine, and 5% PtdIns or PtdIns(4,5)P2. Liposome-binding assay was carried out as described (Huang et al., 2004) with minor modifications. Five nanomoles of hexahistidine-tagged peptide, 20 μl of 1 mM liposomes and 200 μl of binding buffer (50 mM Tris/HCl buffer, pH 7.5, 150 mM NaCl) were rotated for 15 min at room temperature and centrifuged at 20,000 × g for 1 h. Collected liposomes were resuspended in 1 ml of binding buffer and then centrifuged, repeating three times. The peptides that bound to liposomes were resolved by 10–20% gradient SDS-PAGE and were transferred to PVDF filters, and peptides were detected by immunoblot with an anti-hexahistidine antibody (Cell Signaling Technology, Danvers, MA).
Statistical Analysis
Data are presented as the maximum oxidase activity, read as relative luminescence units (for luminol and Diogenes chemiluminescence assays) or as absorbance at 550 nm (for cytochrome c reduced) and are expressed as means ± SD. Means were calculated from at least three independent transfection experiments, and each assay was performed in triplicate for each transfection. Statistical significance was determined by t test by the GraphPad Prism software (GraphPad Software, San Diego, CA).
RESULTS
Identification of Conserved Polybasic Regions of Nox5 Ortholog Sequences
PBR-N corresponds to residues 187-197 of human Nox5α (Figure 1A), a region that contains five basic amino acids and five proline residues (Figure 1B). PBR-C consists of another short polybasic sequence located in the region between the first and second NADPH-binding subregions and corresponds to residues 578-588 of human Nox5α (Figure 1A). PBR-C of human Nox5 contains four basic residues (Figure 1C). Human Nox5α was mutated to examine whether amino acid residues in these regions were involved in Nox5 function. The five arginine residues were replaced with alanine (PBR-N-mut-A), glutamine (PBR-N-mut-Q), or glutamic acid (PBR-N-mut-E; Figure 1B). All mutations significantly suppressed both basal and ionomycin-stimulated Nox5 activity using the luminol assay, which detects ROS production (Figure 1D). The PBR-N also contains two prolines (Pro193 and 196) conforming to a canonical PXXP proline-rich motif region (Li, 2005). However, mutation of P193Q did not affect activity (Supplemental Data S3), indicating that a PXXP motif in this region was not relevant to Nox5 function.
The effect of mutations in PBR-C was also investigated. All four basic residues were mutated to glutamine (PBR-C-mut-Q; see Figure 1C). This mutation also decreased basal and ionomycin-stimulated Nox5 activity using the luminol-based assay (Figure 1E). Protein expression was not affected by the mutations in PBR-N or -C (Figure 1F).
Contribution of Individual Arginine Residues in the N-terminal Polybasic Region
Individual point mutations in each arginine residue of PBR-N (Figure 2A) were constructed to determine the relative contributions of each of these basic residues. All mutations except for R190A and R194A produced a statistically significant decrease Nox5 activity (Figure 2B), and the point mutation at residue 197 substituted to alanine residue (R197A) inhibited activity most strongly (Figure 2B). None of the mutations affected Nox5 protein expression levels (Figure 2C). The effect of glutamic acid substitution at residue 197 (R197E) was greater than alanine substitution. Thus, the arginine residue at position 197 of human Nox5 contributes the most to activity among the basic residues in the PBR-N, though all residues except for R190 and R194 are necessary for optimal activity.
Figure 2.
Effects of mutations of the five arginine residues in the PBR-N on ROS generation in Nox5-transfected cells. (A) Sequences of the specific residues of mutated in the PBR-N of Nox5. (B) ROS generation by mutations of Nox5 listed above were tested in transfected HEK293 cells. ROS production was measured by luminol-based luminescence. Cells were treated with DMSO (□) or 1 μM ionomycin (■), and the maximum value is shown. Data represent mean ± SD (n = 6); *p < 0.05, **p < 0.001, compared with Nox5 WT. (C) Protein expression of wild type and mutant forms of Nox5 was determined with immunoblots. Consistent protein loading was demonstrated with immunoblots of β-actin. Similar results were obtained in two independent experiments.
Comparison of Nox5 Activities between Intact Cells and Permeabilized Cells
The luminol-based ROS measurement used in Figures 1 and 2 detects hydrogen peroxide (Dahlgren and Stendahl, 1983). Because hydrogen peroxide can diffuse through membranes, it was unclear from the initial experiment whether the ROS was generated at the cell surface by plasma membrane Nox5 or originated by internally localized Nox5. Although some of the intracellularly generated ROS is expected to be scavenged by intracellular peroxidases and catalase, the possible diffusibility of hydrogen peroxide made it important to consider this issue. To address this issue, we used SOD-inhibitable cytochrome c reduction, a classical assay that detects extracellular superoxide rather than hydrogen peroxide, because negatively charged superoxide is not expected to cross the cell membrane without a specific ionic channel, unlike hydrogen peroxide. SOD itself is membrane-impermeant, so that SOD-inhibitable activity using the assay system in intact cells primarily detects extracellular but not intracellular superoxide. Mutations in PBR-N or -C significantly suppressed basal and ionomycin-stimulated Nox5 activity of intact cells using the SOD-inhibitable cytochrome c reduction (Figure 3A).
Figure 3.
Effects of mutations in PBR-N or -C on cytochrome c reduction by intact and permeabilized cells. (A) Extracellular superoxide production from HEK293 cells transfected with empty vector (mock), Nox5 (Nox5 WT), or mutant forms of Nox5 (PBN-mut-A, -Q, -E, R197E, and PBC-mut-Q) was measured by SOD-inhibitable cytochrome c reduction as in Materials and Methods. Cells were treated with 0.1% DMSO (□) or 1 μM ionomycin (■) and measured for 20 min. SOD-inhibitable reduction of cytochrome c was expressed as an absorbance at 550 nm per 106 cells per 20 min minus that obtained in the presence of SOD. Data represent the mean ± SD (n = 8–11); *p < 0.05, **p < 0.001, compared with Nox5 WT. (B) Cytochrome c reduction was determined at concentrations of free calcium ranging from 10 nM to 1 mM. HEK293 cells were permeabilized and used 48 h after transfection with Nox5 (●) or empty vector (○). Data represent the mean ± SD (n = 4). (C) HEK293 cells expressing the indicated forms of Nox5 were permeabilized and used 48 h after transfection. Superoxide generation was measured using SOD-inhibitable cytochorome c reduction in the presence of 10 mM K2EGTA (□) or 20 μM free calcium ion (■). Data represent the mean ± SD (n = 8–10); *p < 0.05, **p < 0.005, compared with Nox5 WT.
To further investigate the role of PBR-N or -C on Nox5 localization, we measured NADPH-oxidase activity in Streptolysin O-permeabilized Nox5-expressing HEK293 cells, again using SOD-inhibitable cytochrome c reduction (Brown et al., 2003). The pore size in these cells is large enough to let SOD and cytochrome c pass freely through the cell membrane into the cytoplasm (Brown et al., 2003); consequently this assay is able to measure total superoxide production (both intracellular and extracellular). Because of leakage of coenzymes out of the permeabilized cells, it was necessary to include FAD and NADPH in the assay (Supplemental Data S4). In the permeabilized cells, wild-type Nox5-dependent cytochrome c reduction increased as expected in a calcium-dependent manner (Figure 3B), with a calcium EC50 of 16.2 μM. Calcium did not affect basal cytochrome c reduction in nontransfected HEK293 cells (Figure 3B). In contrast to results in intact cells, the mutations in PBR-N only weakly affect calcium-stimulated cytochrome c reduction. The mutation PBR-N-mut-E suppressed activity by ∼20% in the permeabilized cells (Figure 3C), compared with ∼85% in intact cells (Figure 3A). PBR-N-mut-A and R197E also showed only a modest inhibition in the permeabilized cell system, compared with the intact cell system. On the other hand, a mutation in the PBR-C decreased cytochrome c reduction by ∼40% in both permeabilized (Figure 3C) and intact (Figure 3A) cells. Thus, these data suggested that the PBR-N regulates primarily the extracellular generation of ROS. One explanation could be that mutations in PBR-N might affect the localization of Nox5 at the plasma membrane (see below). In contrast, mutations in PBR-C seem to affect primarily the activity of the enzyme itself. The present study focuses on the role of the PBR-C, whereas a future study will explore the detailed role of the PBR-C.
Effect of In Vivo Manipulation of PtdIns(4,5)P2 Levels on Nox5 Activity and Localization
To test the hypothesis that PtdIns(4,5)P2 regulates Nox5 function, Nox5, or mock vector was cotransfected into HEK293 cells with PtdIns(4)P-5-kinase α (PtdIns(4)P-5Kα) or the catalytic domain of type IV PtdIns(4,5)P2-5-phosphatase [PtdIns(4,5)P2-5Ptase]. PtdIns(4)P-5Kα phosphorylates endogenous PtdIns(4)P to increase cellular levels of PtdIns(4,5)P2 (Balla, 2007; Kanaho et al., 2007). As shown in Figure 4A, coexpression of PtdIns(4)P-5Kα along with Nox5 significantly increased ionomycin-stimulated ROS production. PtdIns(4,5)P2-5Ptase, which is localized to the plasma membrane via a membrane-binding C-terminal CAAX motif, hydrolyzes the phosphate groups from PtdIns(4,5)P2 lowering levels of PtdIns(4,5)P2 (Varnai et al., 2006; Balla, 2007). Expression of this enzyme significantly decreased the ROS production by Nox5 (Figure 4A). Nox5 produced a small amount of ROS (10–15% of ionomycin-stimulated activity) before addition of the calcium ionophore (Figure 4A). This activity also required intracellular calcium (Jagnandan et al., 2007; Serrander et al., 2007), which was confirmed by pretreatment with the intracellular calcium chelator BAPTA-AM (Supplemental Data S5). This basal activity of Nox5 was also significantly increased by coexpression of PtdIns(4)P-5Kα and was decreased with PtdIns(4,5)P2-5Ptase. Moreover, using the SOD-inhibitable Diogenes assay, which detects superoxide (Shiose et al., 2001), PtdIns(4)P-5Kα increased and PtdIns(4,5)P2-5Ptase decreased both basal and ionomycin-stimulated extracellular superoxide production by Nox5 (Figure 4B). A similar trend was seen using the SOD-inhibitable cytochrome c reduction assay, except that statistical significance for ionomycin-stimulated activity by PtdIns(4)P-5Kα was not demonstrated (Figure 4C), possibly because the cytochrome c assay is significantly less sensitive than the chemiluminescence assay.
Figure 4.
PtdIns(4,5)P2 participation in Nox5 activation in intact cells. Nox5 was coexpressed in HEK293 cells along with pCMV-3A empty vector (mock), phosphatidylinositol(4)P-5-kinase α (PtdIns(4)P-5K) or the catalytic domain (residues 214-644) of type IV Phosphatidylinositol(4,5)P2-5-phosphatase (PtdIns(4,5)P2-5Ptase). (A) Forty-eight hours after transfection, ROS production was measured by luminol-based luminescence. Cells were treated with 1 μM ionomycin at 5 min after initiating data collection and then immediately achieved maximum value. Data represent means ± SD (n = 4). (B) Extracellular superoxide was measured using SOD-inhibitable luminescence of Diogenes as described in Materials and Methods. Data represent the mean ± SD (n = 6); *p < 0.05, **p< 0.001, compared with mock transfection. (C) Extracellular superoxide was measured by SOD-inhibitable cytochorome c reduction. Data represent the mean ± SD (n = 4); *p < 0.05, **p < 0.001, compared with mock transfection. (D) HEK293 cells transfected with Nox5 were pretreated with 100 nM wortmannin, 25 μM LY294002, or vehicle (DMSO) for 30-min before measurement. ROS production was measured using luminol-based luminescence, and cells were treated with 1 μM ionomycin at 5 min after initiating data collection. Data represent the mean ± SD (n = 4). (E) HEK293 cells transfected with the indicated vectors were loaded with the intracellular calcium indicator Fluo 4-NW. Ionomycin (+ION) was added immediately before data collection. (F) Protein in HEK293 cells transfected with the indicated vectors was labeled by cell surface biotinylation, followed by isolation using streptavidin-conjugated resin. The biotinylated fraction (prep: avidin) and total cell lysates (cell lysate) were blotted with anti-Nox5 antibody. Equal amounts of each cell lysate were confirmed by immunoblots with an anti-β-actin antibody. Similar results were obtained in three independent experiments.
To test whether PtdIns(3,4,5)P3, which is formed from PtdIns(4,5)P2, might be the relevant phospholipid in the above experiments, cells were treated with the PtdIns-3-kinase (PtdIns-3K) inhibitors wortmannin and LY294002 at low concentrations (100 nM and 25 μM) that do not affect PtdIns-4-kinase (Nakanishi et al., 1995). These inhibitors had no effect on maximum levels of ROS generation and actually increased ROS generation at longer time points (Figure 4D). Thus, PtdIns(3,4,5)P3, a secondary product of PtdIns(4,5)P2 by PtdIns-3K may not contribute to Nox5 maximum activity. Although the mechanism of the sustained activity at later time points by PtdIns-3K inhibitors was not clear, it may have resulted from the preservation and accumulation of PtdIns(4,5)P2 by preventing PtdIns-3K and PLC activation (Falasca et al., 1998; Rameh et al., 1998). Thus, these results support the hypothesis that PtdIns(4,5)P2 increases extracellular ROS generation by Nox5.
To rule out the possibility that PtdIns(4,5)P2-enhanced Nox5 activity is an effect of altered calcium availability, intracellular calcium concentrations were measured in HEK293 cells expressing either PtdIns(4)P-5K or PtdIns(4,5)P2-5Ptase. Intracellular calcium concentrations remained and unchanged by the expression of PtdIns metabolizing enzymes in both resting cells and ionomycin-treated cells (Figure 4E). Thus, effects of PtdIns(4,5)P2 levels on activity are not due to altered calcium levels.
Nox5 is predominantly expressed at the plasma membrane in Nox5-transfected HEK293 cells (Serrander et al., 2007) and prostate cancer cells (Brar et al., 2003) and also at the cytoskeleton-rich region in Nox5-transfected Cos7 cells (Jagnandan et al., 2007). A major pool of PtdIns(4,5)P2 is present in the plasma membrane that are rich in F-actin (Tall et al., 2000; Suh and Hille, 2005). Therefore, we tested the hypothesis that PtdIns(4,5)P2 regulates the localization of Nox5 at the plasma membrane. Cell surface proteins were labeled with a thiol-cleavable amine-reactive biotinylating reagent and isolated using avidin-conjugated resin. The relative amount of Nox5 in the surface protein fraction was then analyzed by immunoblot with an anti-Nox5 antibody. Consistent with our hypothesis, coexpression of the PtdIns(4) P-5Kα along with Nox5 increased amount of Nox5 protein at the cell surface (top panel of Figure 4F), but did not increase the total Nox5 protein level (middle panel of Figure 4F). In contrast, coexpression of PtdIns(4,5)P2-5Ptase decreased Nox5 expressed at the cell surface (top panel of Figure 4F). Thus, these data indicate that differences in extracellular ROS production by Nox5 due to manipulation of PtdIns(4,5)P2 levels are due, in large part, to differences in localization of Nox5 at the plasma membrane.
Effect of Mutations in PBR-N and -C on PtdIns(4)P-5K Enhancement of Nox5 Activity
As shown in Figure 4, PtdIns(4)P-5K enhances extracellular ROS generation by recruiting Nox5 to the plasma membrane. To determine the role of the polybasic regions in PtdIns(4)P-5K-augmented Nox5 activity, the ability of mutations to abrogate stimulation by PtdIns(4)P-5K was investigated. The PtdIns(4)P-5K augmentation of Nox5 activity was completely abolished by PBR-N-mut-A and PBR-N-mut-E mutations (Figure 5A). The activity of Nox5 R197E was only slightly (∼10%) increased by PtdIns(4)P-5K, compared with a ∼35% stimulation of wild-type Nox5. In contrast, PtdIns(4)P-5K was still able to increase ROS generation by Nox5 mutated in the PBR-C (PBR-C-mut-Q) by ∼40% (Figure 5A), similar to the stimulation seen with wild-type Nox5. The cell surface expression of Nox5 was also markedly decreased (Figure 5B) by mutations in the PBR-N (PBR-N-mut-A, mut-E, and R197E), but surface expression the PBR-C-mut-Q mutation was abundant, similar to that of wild type (Figure 5B). Furthermore, PtdIns(4)P-5K was able to increase cell surface expression of both wild-type Nox5 and Nox5-PBR-C-mut-Q, but an increase was not detected in forms of Nox5 mutated in the PBR-N (Figure 5B). Thus, these results indicate that the effect of PtdIns(4)P-5K on Nox5 activity and localization is mediated by the N-terminal (but not the C-terminal) polybasic region.
Interaction of the N-Terminal Polybasic Region with PtdIns(4,5)P2
On the basis of the results in Figures 3–5, we hypothesized that PtdIns(4,5)P2 binds to the PBR-N. Peptides were synthesized corresponding to PBR-N, amino acid residues 186-200 of human Nox5. In an effort to detect interaction between peptide and PtdIns(4,5)P2, we used a pulldown assay with PtdIns(4,5)P2- or PtdIns-conjugated agarose beads. The hexahistidine-tagged peptide corresponding to wild-type Nox5 bound to PtdIns(4,5)P2-conjugated resin (lane 2 of top panel in Figure 6A), but not to PtdIns-conjugated resin (lane 1 of top panel in Figure 6A). Peptides corresponding to the mutations of PBR-N-mut-A and R197E did not bind to PtdIns(4,5)P2-conjugated resin (lanes 3 and 4 of top panel in Figure 6A). We also tested the binding of PtdIns(4,5)P2 to the PBR-N using a liposome-based binding assay (Figure 6B). Liposomes containing PtdIns(4,5)P2 bound wild-type PBR-N to a greater extent than those containing PtdIns (lanes 1 and 2 of Figure 6B). Consistent with data using lipid-conjugated agarose beads, peptides corresponding to the mutations (PBR-N-mut-A and R197E) bound more weakly to PtdIns(4,5)P2-containing liposomes (lanes 3 and 4 of Figure 6B). As an additional attempt, a lipid-overlay assay was carried out. Wild-type PBR-N peptide bound to PtdIns(4,5)P2 and also to several other PtdIns phosphates (PtdIns (3)P, PtdIns (4)P, PtdIns (5)P), and relatively weakly to PtdIns(3,4,5)P3 and PtdIns(3,4)P2 (Supplemental Data S6). Peptides corresponding to the mutations of PBR-N-mut-A and R197E failed to bind PtdIns phosphates including PtdIns(4,5)P2. A control peptide (hexahistidine-fused to three alanine residues) showed weak binding to PtdIns(3)P and PtdIns(3,4,5)P3. Thus, the PBR-N peptide shows a rather broad binding specificity to PtdIns lipids. However, we should not ignore the possibility that the short peptide approach might show result inconsistent to the lipid-binding specificity of the intact protein as previously observed in cytosolic phospholipase A2 (Mosior et al., 1998; Das and Cho, 2002). In this context, it should be pointed out that PtdIns(4,5)P2 is the predominant phosphorylated PtdIns in the cells, so its abundance rather than the precise specificity of this site may be the more important factor in physiological conditions.
Figure 6.
Interaction between peptides corresponding to PBR-N of Nox5 and PtdIns(4,5)P2. (A) Interaction between synthetic peptides corresponding to the polybasic region (186-200 amino acids) of Nox5α and PtdIns(4,5)P2 was shown using a lipid-protein pulldown assay performed using PtdIns or PtdIns(4,5)P2-conjugated agarose beads. Wild-type (Nox5-WT) and mutations (PBR-N-mut-A and R197E) peptides bound PtdIns or PtdIns (4,5)P2-resin (precipitated peptide) and nonbinding peptides in assay buffer (input) were separated by 17% SDS-PAGE, and detected by immunoblots using an anti-hexahistidine-antibody and indicated by arrows. (B) Interaction between synthetic peptides corresponding to the polybasic region of Nox5 and PtdIns(4,5)P2 was confirmed using a liposome segmentation assay. Synthetic peptides bound to liposome containing PtdIns(4,5)P2 or PtdIns (bound peptide) were separated by 10–20% gradient SDS-PAGE gel and detected by immunoblots using an anti-hexahistidine antibody.
Colocalization of Nox5 and PtdIns(4,5)P2 in Intact Cells
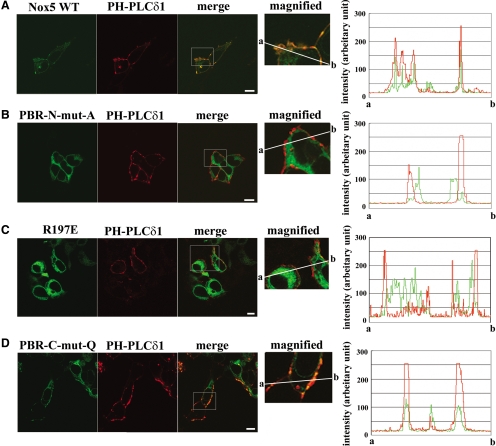
The PH-domain of human PLC-δ1 has a specific affinity for PtdIns(4,5)P2, but not the other PtdIns phosphates (Varnai and Balla, 1998; Varnai et al., 2006; Balla, 2007). A DsRed-fused PH-domain of PLC-δ1 protein (PH-PLCδ1-DsRed) was coexpressed with N-terminally GFP-tagged Nox5 (GFP-Nox5) to test whether Nox5 was colocalized with PtdIns(4,5)P2 in intact cells. Images taken with confocal laser microscopy demonstrated that GFP-Nox5 protein was colocalized with PH-PLCδ1-DsRed (“merge” panel in Figure 7A) and peak intensities of GFP-Nox5 and PH-PLCδ1-DsRed showed almost complete overlap (Figure 7A, right), indicating that PtdIns(4,5)P2 coincides with Nox5 at PtdIns(4,5)P2-rich plasma membrane patches (Huang et al., 2004). Weak GFP florescence was also seen in the cytoplasmic compartment (including internal membranes). In contrast to wild-type Nox5, Nox5-containing mutations in the PBR-N (PBR-N-mut-A and R197E) failed to colocalize with PH-PLCδ1-DsRed (Figures 7, B and C, respectively) because these GFP signals were mainly observed in cytoplasmic organelles. Consistent with the visual appearance, peak intensities of GFP-Nox5 and PH-PLCδ1-DsRed did not significantly overlap (Figures 7, B and C, right panels). Alternatively, mutation in the PBR-C (PBR-C-mut-Q) did not affect colocalization of Nox5 with PH-PLCδ1-DsRed (Figure 7D), and the GFP and DsRed signals showed nearly complete overlap (right graph in Figure 7D). Thus, several lines of evidence indicate that the PBR-N participates in interaction with PtdIns(4,5)P2, and this region is responsible for PtdIns(4,5)P2-mediated localization of Nox5 to plasma membrane.
Figure 7.
Colocalization of wild-type Nox5 or Nox5 with mutations in PBR and PH-domain of PLC-δ1. Fluorescence imaging of GFP-fused wild-type Nox5 (A) or the indicated mutations (B-D) and DsRed-fused PH-domain of PLC-δ1 (PH-PLCδ1; A-D) in HEK293 cells is shown. The three large panels show GFP fluorescence (left panel), DsRed-fluorescence (middle panel), and merged fluorescence (merge, right panel). The forth smaller panels show magnified images from the overlay image. The original region magnified was indicated by a frame in each overlay panel. The intensity of each fluorescent signal on a solid line from point a to point b is shown in the graphs in the right panels: green and red lines indicate GFP and DsRed signals, respectively. Bar, 5 μm.
DISCUSSION
Lipid-binding domains in Nox regulatory subunits were previously shown to be associated with the regulation of Nox enzymatic activity. The PX-domain was identified in the phagocyte NADPH oxidase (PX)-related “organizer” subunits, p47phox and NOXO1, and in the adaptor protein, p40phox. The PX domain of p47phox binds with highest affinity to PtdIns(3,4)P2, and with lower, but significant, affinity to PtdIns(4,5)P2, PtdIns(3,4,5)P3, PtdIns(3,5)P2, and PtdIns(3)P (Kanai et al., 2001; Karathanassis et al., 2002). The PX domains of human NOXO1 and p40phox show a different lipid-binding specificity, with NOXO1 having highest affinity for PtdIns(3,5)P2, PtdIns(5)P, PtdIns(4)P, and p40phox exhibiting a preference for PtdIns(3)P (Cheng and Lambeth, 2005; Ellson et al., 2006; Takeya et al., 2006; Ueyama et al., 2007). Interaction between PtdIns phosphates and the PX domain is essential for membrane-targeting of these regulatory subunits, resulting in the formation of the active complex with Nox1 (through the PX-domain of NOXO1) or with Nox2 (through the PX-domains of p47phox and p40phox). However, it was previously unknown whether PtdIns phosphates were involved in the regulation of other Nox subunits or isozymes.
In the present study, we demonstrated a relationship between PtdIns(4,5)P2 levels and extracellular ROS production by Nox5. The PBR-N, conserved among Nox5 orthologues, was shown to mediate this regulatory effect by binding to PtdIns(4,5)P2, resulting in the localization of Nox5 to plasma membrane regions rich in this lipid. PtdIns(4,5)P2 is the most common isomer of PtdInsP2 (Suh and Hille, 2005), and a major pool of PtdIns(4,5)P2 is present in the plasma membrane. Within the plasma membrane, PtdIns(4,5)P2 is also colocalized with F-actin (Tall et al., 2000) and is enriched in cholesterol- and caveolin-rich subregions such as lipid rafts, and focal adhesions (Downes et al., 2005; Ling et al., 2006). Smaller pools are also present in intracellular membranes including those of the Golgi apparatus and endoplasmic reticulum, and in the nuclear matrix, where it is associated, not with membranes, but with dense regions of heterochromatin (Downes et al., 2005).
After the discovery of enzymes that metabolize PtdIns(4,5)P2 (e.g., phospholipase C and PtdIns-3 kinases), PtdIns(4,5)P2 has been studied in three regards: 1) as a precursor of second messengers [e.g., Ins(1,4,5)P3 and PtdIns(3,4,5)P3], 2) as a membrane targeting signal for proteins (e.g., the small GTPases), and 3) as a direct regulator of various protein functions (e.g., voltage-gated K+ channels; Huang et al., 2004; Li et al., 2005; Rohacs et al., 2005; Suh and Hille, 2005; Heo et al., 2006; Kanaho et al., 2007). The present study demonstrates that the second of these functions is relevant to Nox5. PtdIns(4,5)P2, by binding to PBR-N, functions to localize Nox5 to the plasma membrane, thereby directing superoxide generation to the extracellular space. Once there, dismutation of superoxide can occur, catalyzed by extracellular superoxide dismutase and the product, hydrogen peroxide, can act locally or diffuse back across the membrane.
Nox enzymes including Nox5 have been implicated in specific regulatory oxidations that modulate the activity of downstream target enzymes such as protein tyrosine phosphatases (Mahadev et al., 2004; Kamiguti et al., 2005; Ushio-Fukai, 2006; Lee et al., 2007; Shinohara et al., 2007). However, because ROS can be rather indiscriminant in the protein targets that they oxidize (Bedard and Krause, 2007; Fomenko et al., 2007; Lambeth, 2007), it has not been clear how (or whether) specificity for selective oxidation of certain target proteins might be achieved. One such mechanism would be to colocalize the target protein in close proximity to the Nox enzyme, resulting in exposure of the target to high local concentrations of ROS. Because PtdIns(4,5)P2 also recruits other proteins to the plasma membrane, this lipid may participate in colocalization of Nox5 with protein targets of Nox5-derived ROS. For example, PtdIns(4,5)P2 localizes to the same plasma membrane subdomains of several ion channels (e.g., transient receptor potential [TRP] C3, TRPM7, TRPV1, voltage-gated K+ channel Kv, Cl− channel cystic fibrosis transmembrane conductance regulator; Aarts et al., 2003; Suh and Hille, 2005; van Rossum et al., 2005). Interestingly, these channels have been previously described as being redox-sensitive (Schultz and Ustinova, 1998; Cowley and Linsdell, 2002; Cogolludo et al., 2006; Massullo et al., 2006; Poteser et al., 2006; Ruan et al., 2006), suggesting that Nox5-derived ROS might participate in their regulation. Indeed, D. melanogaster Nox5-derived ROS markedly up-regulates the proctolin-stimulated cytosolic calcium flux in ovarian smooth muscle, resulting in muscle contraction (Ritsick et al., 2007). In addition, PtdIns(4,5)P2 functions in the localization of membrane receptors (e.g., G-protein–coupled receptors) and signal transducers. Among these include, redox-sensitive enzymes such as protein tyrosine phosphatases (Tonks, 2006) in lipid rafts in the plasma membrane (Gamper and Shapiro, 2007), and colocalization with Nox5 might facilitate oxidative regulation of these target proteins.
Phosphorylated PtdIns including PtdIns(4,5)P2 is present only in eukaryotes (Michell, 2007), whereas PtdIns occurs in eukaryotes and rarely in a few bacteria such as actinomycetes (Michell, 2007). Nox genes are also seen in eukaryotes, but not in prokaryotes. The PBR-N is broadly conserved in most Nox5 orthologues in vertebrates and other metazoans (Supplemental Data S1). Human Duox1 and Duox2, as well as most other animal Duox sequences, also contain multiple basic residues at or near regions corresponding to the PBR-N of Nox5 (alignment shown in Supplemental Data S7). In preliminary data, we observed that PtdIns(4,5)P2-5Ptase significantly inhibited ionomycin-induced ROS generation by Duox1 in HEK293 cells coexpressing its chaperon protein DuoxA1 (T. Kawahara, unpublished observation). Furthermore, Nox proteins of a higher green plant (Arabidopsis thaliana), moss (Physcomitrella patens), oomycete (Phytophthora sojae), and fungal NoxC, which all possess EF-hand motifs, show conserved polybasic regions between the EF-hand motif and the first transmembrane region (Supplemental Data S8). Fungal NoxB proteins contain a short extension (∼70–120 amino acids long) in their N-termini but lack the EF-hand domain (Kawahara et al., 2007). Most of these NoxB proteins also possess multiple basic residues in this N-terminal extension (Supplemental Data S9), although the relationship between NoxB and PtdIns phosphates has not yet been investigated. The similarity among polybasic sequences at a region corresponding to PBR-N of Nox5 suggests a similar role for PtdIns(4,5)P2 or other PtdIns phosphates among other calcium-regulated Noxes/Duoxes and fungal NoxB.
Because subunit-regulated Nox orthologues do not possess an extension containing an PBR-N (Lambeth, 2004), other mechanisms appear to be responsible for localization of these enzymes to PtdInsP2-rich membranes. Previous immunohistochemical analysis demonstrated that Nox1 is predominantly localized at the plasma membrane of the apical side of human colon epithelial cells (Kawahara et al., 2005). In vascular smooth muscle, Nox1 colocalizes with caveolin, a marker of lipid rafts (Hilenski et al., 2004). Nox4 protein is localized to the plasma membrane of kidney tubules (Kawahara et al., 2005) and to focal adhesion of vascular smooth muscle cells (Hilenski et al., 2004). Consistent with these observations, GFP-tagged Nox1 and Nox4 proteins were able to colocalize with the PH-domain of PLC-δ1 in HEK293 cells without coexpression of the PX-domain–containing cytoplasmic regulators (T. Kawahara, unpublished observation). These data indicate that these Nox enzymes or, alternatively, their membrane-associated binding partner, p22phox, have a different mechanism that directs their subcellular localization, perhaps involving a different PtdIns(4,5)P2- binding region.
The PBR-N has a major effect on cellular localization of Nox5, but the possibility that it also might have a minor effect on Nox5 enzymatic activity should not be dismissed. Using the permeabilized cell assay, mutations in the PBR-N decreased the Nox5 activity by 15–20% (Figure 3B). The mechanism for this decrease in activity is not known, but is significantly smaller than effects on the PBR-C. In contrast to the PBR-N, the PBR-C was not implicated in localization of Nox5, but was implicated in the intrinsic activity of the enzyme itself. A future study will explore the detailed role of the PBR-C in Nox5 activation.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Dr. Tamas Balla for the human type IV PtdIns(4,5)P2-5Ptase clone and for commenting on the manuscript. We express our appreciation to Dr. Karl-Heinz Krause for providing anti-Nox5 antibody and to Ms. Heather Jackson for reading and commenting on the manuscript. This work was supported by National Institutes of Health Grants CA105116, CA084138, and GM067717.
Abbreviations used:
- Nox
NADPH oxidase
- Duox
dual oxidase
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- PBR-N
N-terminal polybasic region
- PBR-C
C-terminal polybasic region
- WT
wild type
- PLC
phospholipase C
- PtdIns(4,5)P2
Phosphatidylinositol (4,5)-bisphosphate
- PtdIns-3K
Phosphatidylinositol 3-kinase
- PtdIns(4,5)P2-5Ptase
Phosphatidylinositol (4,5)-bisphosphate 5-phoshatase
- PtdIns(4)P-5K
Phosphatidylinositol 4-phosphate 5-kinase.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-12-1223) on July 9, 2008.
REFERENCES
- Aarts M., Iihara K., Wei W. L., Xiong Z. G., Arundine M., Cerwinski W., MacDonald J. F., Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;26:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- Balla T. Imaging and manipulating phosphoinositides in living cells. J. Physiol. 2007;582:927–937. doi: 10.1113/jphysiol.2007.132795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfi B., Molnar G., Maturana A., Steger K., Hegedus B., Demaurex N., Krause K. H. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 2001;276:37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- Banfi B., Tirone F., Durussel I., Knisz J., Moskwa P., Molnar G. Z., Krause K. H., Cox J. A. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5) J. Biol. Chem. 2004;279:18583–18591. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- Bedard K., Krause K. H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bedard K., Lardy B., Krause K. H. NOX family NADPH oxidase: not just in mammals. Biochimie. 2007;89:1107–1112. doi: 10.1016/j.biochi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- BelAiba R. S., Djordjevic T., Petry A., Diemer K., Bonello S., Banfi B., Hess J., Pogrebniak A., Bickel C., Gorlach A. NOX5 variants are functionally active in endothelial cells. Free Radic. Biol. Med. 2007;42:446–459. doi: 10.1016/j.freeradbiomed.2006.10.054. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Knaus U. G. NADPH oxidases: not just for leukocytes anymore! Trends Biochem. Sci. 2003;28:502–508. doi: 10.1016/S0968-0004(03)00194-4. [DOI] [PubMed] [Google Scholar]
- Brar S. S., et al. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am. J. Physiol. Cell Physiol. 2003;285:C353–C369. doi: 10.1152/ajpcell.00525.2002. [DOI] [PubMed] [Google Scholar]
- Brown G. E., Stewart M. Q., Liu H., Ha V. L., Yaffe M. B. A novel assay system implicates PtdIns(3,4)P(2), PtdIns(3)P, and PKC delta in intracellular production of reactive oxygen species by the NADPH oxidase. Mol. Cell. 2003;11:35–47. doi: 10.1016/s1097-2765(03)00005-4. [DOI] [PubMed] [Google Scholar]
- Cheng G., Cao Z., Xu X., van Meir E. G., Lambeth J. D. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Cheng G., Lambeth J. D. Alternative mRNA splice forms of NOXO1, Differential tissue expression and regulation of Nox1 and Nox3. Gene. 2005;356:118–126. doi: 10.1016/j.gene.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Cogolludo A., Frazziano G., Cobeno L., Moreno L., Lodi F., Villamor E., Tamargo J., Perez-Vizcaino F. Role of reactive oxygen species in Kv channel inhibition and vasoconstriction induced by TP receptor activation in rat pulmonary arteries. Ann. NY Acad. Sci. 2006;1091:41–51. doi: 10.1196/annals.1378.053. [DOI] [PubMed] [Google Scholar]
- Cowley E. A., Linsdell P. Oxidant stress stimulates anion secretion from the human airway epithelial cell line Calu-3, implications for cystic fibrosis lung disease. J. Physiol. 2002;543:201–209. doi: 10.1113/jphysiol.2002.022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren C., Stendahl O. Role of myeloperoxidase in luminol-dependent chemiluminescence of polymorphonuclear leukocytes. Infect. Immun. 1983;39:736–741. doi: 10.1128/iai.39.2.736-741.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Cho W. Roles of catalytic domain residues in interfacial binding and activation of group IV cytosolic phospholipase A2. J. Biol. Chem. 2002;277:23838–23846. doi: 10.1074/jbc.M202322200. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Gray A., Lucocq J. M. Probing phosphoinositide functions in signaling and membrane trafficking. Trends Cell Biol. 2005;15:259–268. doi: 10.1016/j.tcb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- El Jamali A., Valente A. J., Lechleiter J. D., Gamez M. J., Pearson D. W., Nauseef W. M., Clark R. A. Novel redox-dependent regulation of NOX5 by the tyrosine kinase c-Abl. Free Radic. Biol. Med. 2008;44:868–881. doi: 10.1016/j.freeradbiomed.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellson C., Davidson K., Anderson K., Stephens L. R., Hawkins P. T. PtdIns3P binding to the PX domain of p40phox is a physiological signal in NADPH oxidase activation. EMBO J. 2006;25:4468–4478. doi: 10.1038/sj.emboj.7601346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falasca M., Logan S. K., Lehto V. P., Baccante G., Lemmon M. A., Schlessinger J. Activation of phospholipase C gamma by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomenko D. E., Xing W., Adair B. M., Thomas D. J., Gladyshev V. N. High-throughput identification of catalytic redox-active cysteine residues. Science. 2007;315:387–389. doi: 10.1126/science.1133114. [DOI] [PubMed] [Google Scholar]
- Gamper N., Shapiro M. S. Target-specific PIP2 signaling: how might it work? J. Physiol. 2007;582:967–975. doi: 10.1113/jphysiol.2007.132787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiszt M. NADPH oxidases: new kids on the block. Cardiovasc. Res. 2006;71:289–299. doi: 10.1016/j.cardiores.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Han J. C., Han G. Y. A procedure for quantitative determination of tris(2-carboxyethyl)phosphine, an odorless reducing agent more stable and effective than dithiothreitol. Anal. Biochem. 1994;220:5–10. doi: 10.1006/abio.1994.1290. [DOI] [PubMed] [Google Scholar]
- Heo W. D., Inoue T., Park W. S., Kim M. L., Park B. O., Wandless T. J., Meyer T. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilenski L. L., Clempus R. E., Quinn M. T., Lambeth J. D., Griendling K. K. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- Huang S., Lifshitz L., Patki-Kamath V., Tuft R., Fogarty K., Czech M. Phosphatidylinositol-4,5-bisphosphate-rich plasma membrane patches organize active zones of endocytosis and ruffling in cultured adipocytes. Mol. Cell. Biol. 2004;24:9102–9123. doi: 10.1128/MCB.24.20.9102-9123.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagnandan D., Church J. E., Banfi B., Stuehr D. J., Marrero M. B., Fulton D. J. Novel mechanism of activation of NADPH oxidase 5. Calcium sensitization via phosphorylation. J. Biol. Chem. 2007;282:6494–6507. doi: 10.1074/jbc.M608966200. [DOI] [PubMed] [Google Scholar]
- Jay D. B., Papaharalambus C. A., Seidel-Rogol B., Dikalova A. E., Lassègue B., Griendling K. K. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic. Biol. Med. 2008;45:329–335. doi: 10.1016/j.freeradbiomed.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J. C., Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Kamiguti A. S., Serrander L., Lin K., Harris R. J., Cawley J. C., Allsup D. J., Slupsky J. R., Krause K. H., Zuzel M. Expression and activity of NOX5 in the circulating malignant B cells of hairy cell leukemia. J. Immunol. 2005;175:8424–8430. doi: 10.4049/jimmunol.175.12.8424. [DOI] [PubMed] [Google Scholar]
- Kanaho Y., Kobayashi-Nakano A., Yokozeki T. The phosphoinositide kinase PIP5K that produces the versatile signaling phospholipid PI4,5P(2) Biol. Pharmac. Bull. 2007;30:1605–1609. doi: 10.1248/bpb.30.1605. [DOI] [PubMed] [Google Scholar]
- Kanai F., Liu H., Field S. J., Akbary H., Matsuo T., Brown G. E., Cantley L. C., Yaffe M. B. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat. Cell Biol. 2001;3:675–678. doi: 10.1038/35083070. [DOI] [PubMed] [Google Scholar]
- Karathanassis D., Stahelin R. V., Bravo J., Perisic O., Pacold C. M., Cho W., Williams R. L. Binding of the PX domain of p47(phox) to phosphatidylinositol 3,4-bisphosphate and phosphatidic acid is masked by an intramolecular interaction. EMBO J. 2002;21:5057–5068. doi: 10.1093/emboj/cdf519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara T., Kuwano Y., Teshima-Kondo S., Takeya R., Sumimoto H., Kishi K., Tsunawaki S., Hirayama T., Rokutan K. Role of nicotinamide adenine dinucleotide phosphate oxidase 1 in oxidative burst response to Toll-like receptor 5 signaling in large intestinal epithelial cells. J. Immunol. 2004;172:3051–3058. doi: 10.4049/jimmunol.172.5.3051. [DOI] [PubMed] [Google Scholar]
- Kawahara T., Lambeth J. D. Molecular evolution of Phox-related regulatory subunits for NADPH oxidase enzymes. BMC Evol. Biol. 2007;7:178. doi: 10.1186/1471-2148-7-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara T., Quinn M. T., Lambeth J. D. Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol. Biol. 2007;7:109. doi: 10.1186/1471-2148-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara T., Ritsick D., Cheng G., Lambeth J. D. Point mutations in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J. Biol. Chem. 2005;280:31859–31869. doi: 10.1074/jbc.M501882200. [DOI] [PubMed] [Google Scholar]
- Lambeth J. D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Lambeth J. D. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic. Biol. Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth J. D., Kawahara T., Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic. Biol. Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. K., Edderkaoui M., Truong P., Ohno I., Jang K. T., Berti A., Pandol S. J., Gukovskaya A. S. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology. 2007;133:1637–1648. doi: 10.1053/j.gastro.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Li S. Specificity and versatility of SH3 and other proline-recognition domains: structural basis and implication for cellular signal transduction. Biochem. J. 2005;390:641–653. doi: 10.1042/BJ20050411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Gamper N., Hilgemann D. W., Shapiro M. S. Regulation of Kv7 (KCNQ) K+ channel open probability by phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 2005;25:9825–9835. doi: 10.1523/JNEUROSCI.2597-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling K., Schill N. J., Wagoner M. P., Sun Y., Anderson R. A. Movin' on up; the role of PtdIns(4,5)P2 in cell migration. Trends Cell Biol. 2006;16:276–284. doi: 10.1016/j.tcb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Mahadev K., Motoshima H., Wu X., Ruddy J. M., Arnold R. S., Cheng G., Lambeth J. D., Goldstein B. J. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol. Cell. Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massullo P., Sumoza-Toledo A., Bhagat H., Partida-Sanchez S. TRPM channels, calcium and redox sensors during innate immune responses. Semin. Cell Dev. Biol. 2006;17:654–666. doi: 10.1016/j.semcdb.2006.11.006. [DOI] [PubMed] [Google Scholar]
- McLaughlin S., Wang J., Gambhir A., Murray D. PIP(2) and proteins: interactions, organization, and information flow. Annu. Rev. Biophys. Biomol. Struct. 2002;31:151–175. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Evolution of the diverse biological roles of inositols. Biochem. Soc. Symp. 2007;74:223–246. doi: 10.1042/BSS0740223. [DOI] [PubMed] [Google Scholar]
- Mosior M., Six D. A., Dennis E. A. Group IV cytosolic phospholipase A2 binds with high affinity and specificity to phosphatidylinositol 4,5-bisphosphate resulting in dramatic increases in activity. J. Biol. Chem. 1998;273:2184–2191. doi: 10.1074/jbc.273.4.2184. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Catt K. J., Balla T. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc. Natl. Acad. Sci. USA. 1995;92:5317–5321. doi: 10.1073/pnas.92.12.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauseef W. M. Assembly of the phagocyte NADPH oxidase. Histochem. Cell Biol. 2004;122:277–291. doi: 10.1007/s00418-004-0679-8. [DOI] [PubMed] [Google Scholar]
- Poteser M., Graziani A., Rosker C., Eder P., Derler I., Kahr H., Zhu M. X., Romanin C., Groschner K. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J. Biol. Chem. 2006;281:13588–13595. doi: 10.1074/jbc.M512205200. [DOI] [PubMed] [Google Scholar]
- Prehoda K. E., Scott J. A., Dyche Mullins R., Lim W. A. Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science. 2000;290:801–806. doi: 10.1126/science.290.5492.801. [DOI] [PubMed] [Google Scholar]
- Rameh L. E., Rhee S. G., Spokes K., Kazlauskas A., Cantley L. C., Cantley L. G. Phosphoinositide 3-kinase regulates phospholipase Cgamma-mediated calcium signaling. J. Biol. Chem. 1998;273:23750–23757. doi: 10.1074/jbc.273.37.23750. [DOI] [PubMed] [Google Scholar]
- Ritsick D. R., Edens W. A., Finnerty V., Lambeth J. D. Nox regulation of smooth muscle contraction. Free Radic. Biol. Med. 2007;43:31–38. doi: 10.1016/j.freeradbiomed.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs T., Lopes C.M.B., Michaelidis I., Logothetis D. E. PI(4,5)P2 regulates the activation and desensitization of TPRM8 channels through the TRP domain. Nat. Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- Ruan T., Lin Y. S., Lin K. S., Kou Y. R. Mediator mechanisms involved in TRPV1 and P2X receptor-mediated, ROS-evoked bradypneic reflex in anesthetized rats. J. Appl. Physiol. 2006;101:644–654. doi: 10.1152/japplphysiol.00192.2006. [DOI] [PubMed] [Google Scholar]
- Schultz H. D., Ustinova E. E. Capsaicin receptors mediate free radical-induced activation of cardiac afferent endings. Cardiovasc. Res. 1998;38:348–355. doi: 10.1016/s0008-6363(98)00031-5. [DOI] [PubMed] [Google Scholar]
- Sciorra V. A., Rudge S. A., Prestwich G. D., Frohman M. A., Engebrecht J., Morris A. J. Identification of a phosphoinositide binding motif that mediates activation of mammalian and yeast phospholipase D isoenzymes. EMBO J. 1999;18:5911–5921. doi: 10.1093/emboj/18.21.5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrander L., Jaquet V., Bedard K., Plastre O., Hartley O., Arnaudeau S., Demaurex N., Schlegel W., Krause K. H. NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie. 2007;89:1159–1167. doi: 10.1016/j.biochi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Shinohara M., Shang W. H., Kubodera M., Harada S., Mitsushita J., Kato M., Miyazaki H., Sumimoto H., Kamata T. Nox1 redox signaling mediates oncogenic Ras-induced disruption of stress fibers and focal adhesions by down-regulating Rho. J. Biol. Chem. 2007;282:17640–17648. doi: 10.1074/jbc.M609450200. [DOI] [PubMed] [Google Scholar]
- Shiose A., Kuroda J., Tsuruya K., Hirai M., Hirakata H., Naito S., Hattori M., Sakaki Y., Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J. Biol. Chem. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- Si J., Behar J., Wands J., D. G., B., Lambeth D., Y. E., C., Cao W. STAT5 mediates platelet-activating factor (PAF)-induced NADPH oxidase NOX5-S expression in Barrett's esophageal adenocarcinoma cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007 doi: 10.1152/ajpgi.00291.2007. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Suh B. C., Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr. Opin. Neurobiol. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Takeya R., Taura M., Yamasaki T., Naito S., Sumimoto H. Expression and function of Noxo1gamma, an alternative splicing form of the NADPH oxidase organizer 1. FEBS J. 2006;273:3663–3677. doi: 10.1111/j.1742-4658.2006.05371.x. [DOI] [PubMed] [Google Scholar]
- Tall E. G., Spector I., Pentyala S. N., Bitter I., Rebecchi M. J. Dynamics of phosphatidylinositol 4,5-bisphosphate in actin-rich structures. Curr. Biol. 2000;10:743–746. doi: 10.1016/s0960-9822(00)00541-8. [DOI] [PubMed] [Google Scholar]
- Tirone F., Cox J. A. NADPH oxidase 5 (NOX5) interacts with and is regulated by calmodulin. FEBS Lett. 2007;581:1202–1208. doi: 10.1016/j.febslet.2007.02.047. [DOI] [PubMed] [Google Scholar]
- Tonks N. K. Protein tyrosine phosphatases: from gene, to function, to disease. Nat. Rev. Mol. Cell. Biol. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- Ueyama T., Eto M., Kami K., Tatsuno T., Kobayashi T., Shirai Y., Lennartz M. R., Takeya R., Sumimoto H., Saito N. Isoform-specific membrane targeting mechanism of Rac during Fc gamma R-mediated phagocytosis: positive charge-dependent and independent targeting mechanism of Rac to the phagosome. J. Immunol. 2005;175:2381–2390. doi: 10.4049/jimmunol.175.4.2381. [DOI] [PubMed] [Google Scholar]
- Ueyama T., Lekstrom K., Tsujibe S., Saito N., Leto T. L. Subcellular localization and function of alternatively spliced Noxo1 isoforms. Free Radic. Biol. Med. 2007;42:180–190. doi: 10.1016/j.freeradbiomed.2006.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Science's STKE: Signal Transduction Knowledge Environment 2006. 2006:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- van Rossum D. B., Patterson R. L., Sharma S., Barrow R. K., Kornberg M., Gill D. L., Snyder S. H. Phospholipase Cgamma1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature. 2005;434:99–104. doi: 10.1038/nature03340. [DOI] [PubMed] [Google Scholar]
- Varnai P., Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositides pools. J. Cell Biol. 1998;143:501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnai P., Thyagarajan B., Rohacs T., Balla T. Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J. Cell Biol. 2006;175:377–382. doi: 10.1083/jcb.200607116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignais P. V. The superoxide-generating NADPH oxidase; structural aspects and activation mechanism. Cell. Mol. Life Sci. 2002;59:1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.